Meningococcal ACWY immunisation programme for adolescents An update

























- Slides: 41

Meningococcal ACWY immunisation programme for adolescents An update for healthcare professionals Updated September 2016

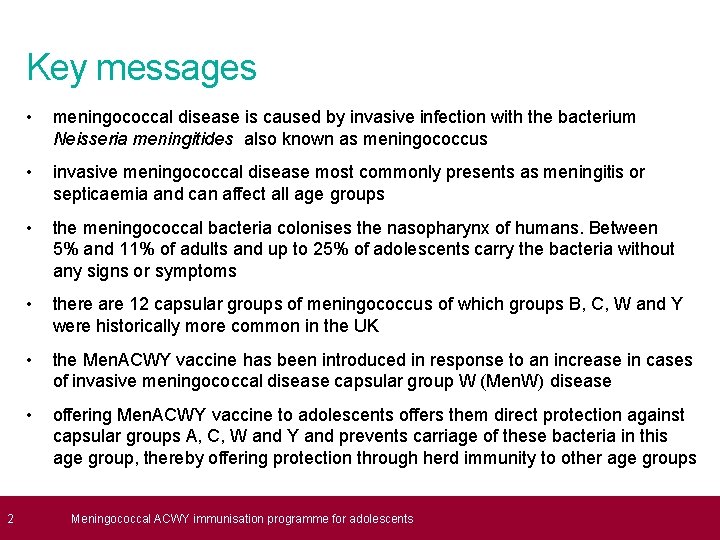
2 Key messages • meningococcal disease is caused by invasive infection with the bacterium Neisseria meningitides also known as meningococcus • invasive meningococcal disease most commonly presents as meningitis or septicaemia and can affect all age groups • the meningococcal bacteria colonises the nasopharynx of humans. Between 5% and 11% of adults and up to 25% of adolescents carry the bacteria without any signs or symptoms • there are 12 capsular groups of meningococcus of which groups B, C, W and Y were historically more common in the UK • the Men. ACWY vaccine has been introduced in response to an increase in cases of invasive meningococcal disease capsular group W (Men. W) disease • offering Men. ACWY vaccine to adolescents offers them direct protection against capsular groups A, C, W and Y and prevents carriage of these bacteria in this age group, thereby offering protection through herd immunity to other age groups Meningococcal ACWY immunisation programme for adolescents

3 Aim of resource • to raise awareness of invasive meningococcal disease (IMD) epidemiology and the impact of IMD on infants and adolescents • to support and educate healthcare professionals involved in discussing immunisation against meningococcal ACWY disease with patients, parents or carers • to promote the uptake of meningococcal ACWY vaccine through increasing awareness in healthcare professionals involved in immunisation Meningococcal ACWY immunisation programme for adolescents

4 Learning outcomes After completing this training, healthcare professionals will be able to: • describe the aetiology and epidemiology of meningococcal disease • be aware of the most common types of meningococci in the UK and their relationship in causing invasive meningococcal disease • advise and inform patients or parents/carers of those who are eligible about the importance of receiving Men. ACWY vaccine and provide evidence-based information where required • identify sources of additional information and resources Meningococcal ACWY immunisation programme for adolescents

5 Contents • What is meningococcal disease? • Why are adolescents being offered Men. ACWY vaccine? • Who should be offered Men. ACWY vaccine and when? • Immunisation against meningococcal ACWY disease and the use of Menveo® or Nimenrix® vaccines • The role of healthcare professionals • Resources Meningococcal ACWY immunisation programme for adolescents

6 What is meningococcal disease? Meningococcal ACWY immunisation programme for adolescents

7 What is meningococcal disease? • meningococcal disease occurs as a result of an invasive bacterial infection caused by Neisseria meningitidis also known as meningococci • there are 12 identified capsular groups of meningococcus, groups B, C, W and Y are the most common in the UK • meningococcal infection most commonly presents as either meningitis or septicaemia, or a combination of both • highest rates of disease are in children under 2 years, particularly infants aged 5 months although invasive disease can occur at any age Meningococcal ACWY immunisation programme for adolescents

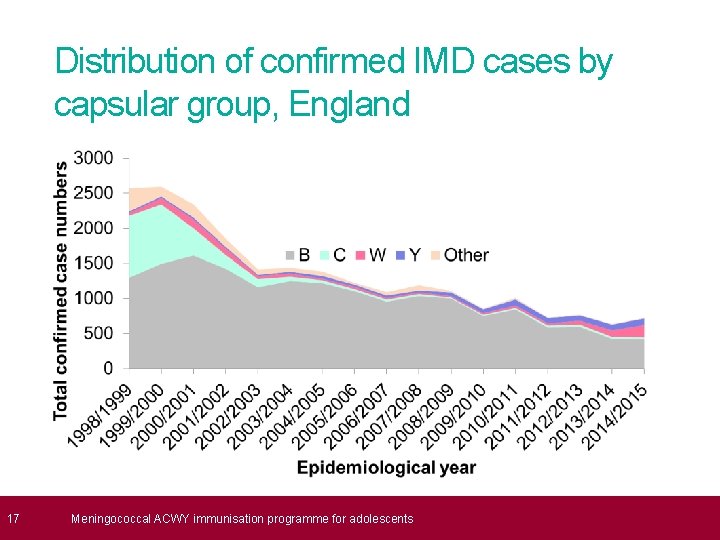
8 What is meningococcal disease? (Cont’d) • since the introduction of the routine meningococcal C conjugate immunisation programme, cases of invasive meningococcal disease (IMD) in the UK from group C have reduced dramatically • capsular group B now accounts for approximately 80% of all laboratory confirmed cases reported to Public Health England • although cases of meningococcal disease overall have been in decline since 2000, cases of capsular group W were first observed in previously healthy adults in 2009 • in 2011, cases of meningococcal W had extended across all age groups and across all regions in England, indicating the strain is now endemic Meningococcal ACWY immunisation programme for adolescents

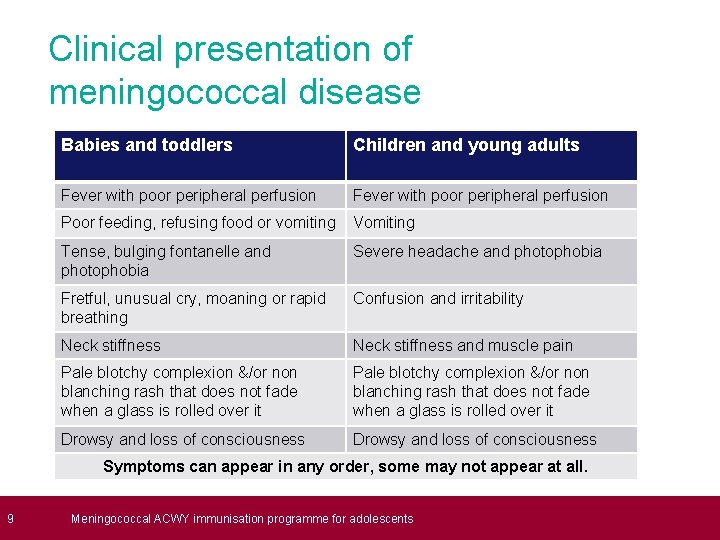
9 Clinical presentation of meningococcal disease Babies and toddlers Children and young adults Fever with poor peripheral perfusion Poor feeding, refusing food or vomiting Vomiting Tense, bulging fontanelle and photophobia Severe headache and photophobia Fretful, unusual cry, moaning or rapid breathing Confusion and irritability Neck stiffness and muscle pain Pale blotchy complexion &/or non blanching rash that does not fade when a glass is rolled over it Drowsy and loss of consciousness Neck stiffness & muscle pain Drowsy and loss of consciousness Symptoms can appear in any order, some may not appear at all. Meningococcal ACWY immunisation programme for adolescents

10 The meningococcal rash • a distinctive red rash can appear anywhere on the body • the rash is formed of tiny ‘pinpricks’ also known as petechiae and appears red in colour. The rash may later develop into purple bruising of the skin • the meningococcal rash can be distinguished from other rashes by pressing a glass tumbler against it • a meningococcal rash will not fade when a glass tumbler is rolled over it • a febrile illness and rash that does not fade is a sign of meningococcal septicaemia Meningococcal ACWY immunisation programme for adolescents The ‘tumbler’ test picture courtesy of Meningitis Research Foundation http: //www. meningitis. org/symptoms

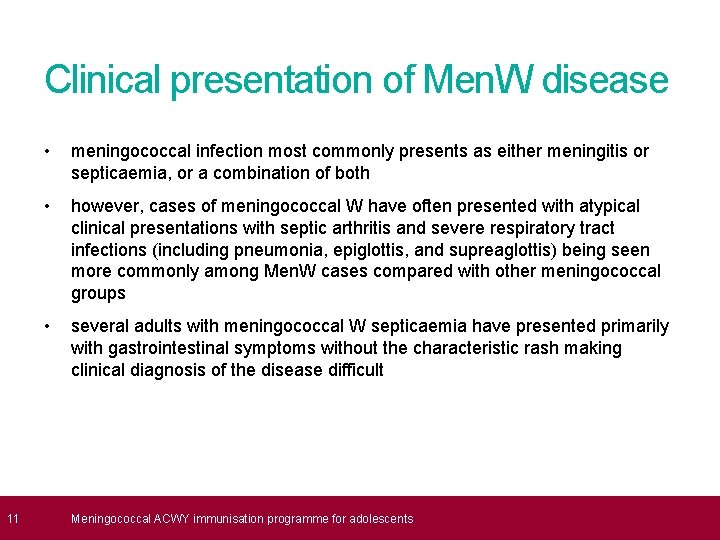
11 Clinical presentation of Men. W disease • meningococcal infection most commonly presents as either meningitis or septicaemia, or a combination of both • however, cases of meningococcal W have often presented with atypical clinical presentations with septic arthritis and severe respiratory tract infections (including pneumonia, epiglottis, and supreaglottis) being seen more commonly among Men. W cases compared with other meningococcal groups • several adults with meningococcal W septicaemia have presented primarily with gastrointestinal symptoms without the characteristic rash making clinical diagnosis of the disease difficult Meningococcal ACWY immunisation programme for adolescents

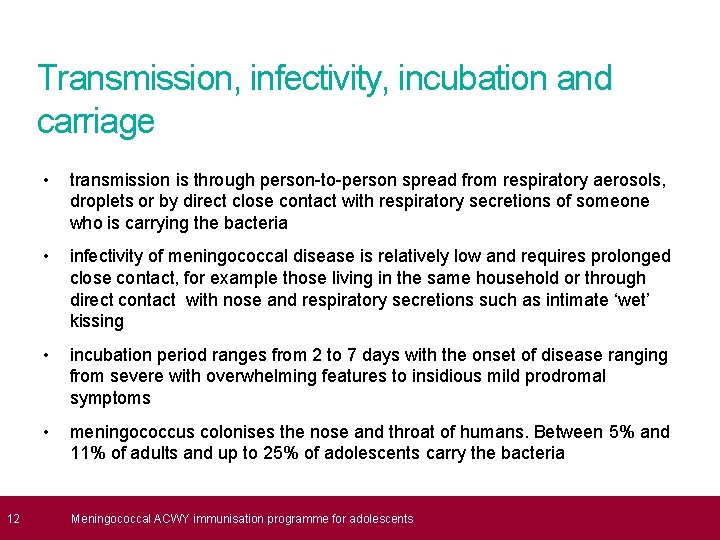
12 Transmission, infectivity, incubation and carriage • transmission is through person-to-person spread from respiratory aerosols, droplets or by direct close contact with respiratory secretions of someone who is carrying the bacteria • infectivity of meningococcal disease is relatively low and requires prolonged close contact, for example those living in the same household or through direct contact with nose and respiratory secretions such as intimate ‘wet’ kissing • incubation period ranges from 2 to 7 days with the onset of disease ranging from severe with overwhelming features to insidious mild prodromal symptoms • meningococcus colonises the nose and throat of humans. Between 5% and 11% of adults and up to 25% of adolescents carry the bacteria Meningococcal ACWY immunisation programme for adolescents

13 Potential complications of meningococcal disease • it is estimated that approximately one quarter of those diagnosed with meningococcal disease caused by Neisseria meningitides will suffer complications as a result • complications can vary in severity and can either be temporary or permanent. The more severe the disease, the greater the risk of complications Complications can include loss of hearing, loss of vision, loss of memory and/or concentration, difficulties in co-ordination and balance, epilepsy, cerebral palsy and limb amputations, and may result in death. Meningococcal ACWY immunisation programme for adolescents

14 Why was the Men C vaccine previously given to adolescents replaced with Men. ACWY? Meningococcal ACWY immunisation programme for adolescents

15 Why was the Men C vaccine previously given to adolescents replaced with Men. ACWY vaccine? • although cases of meningococcal disease overall have been in decline since 2000, cases of meningococcal W were first observed in previously healthy adults in 2009 • in 2011, cases had extended across all age groups and across all regions in England, indicating the strain had become endemic • for the first time in a decade, meningococcal W related deaths were observed in young infants, particularly those under 2 years of age • additionally, an increase in Men. W cases among university students across the country suggested that carriage and transmission of bacteria had become established Meningococcal ACWY immunisation programme for adolescents

16 Why was the Men C vaccine previously given to adolescents replaced with Men. ACWY vaccine? (Cont’d) • in February 2015, the JCVI agreed that the current increase in meningococcal W cases in England Wales constituted an outbreak • as a control measure, an immunisation programme was recommended for all adolescents aged 14 -18 years and included the replacement of the adolescent dose of Men. C with Men. ACWY • this will ensure direct protection against meningococcal capsular group C as previously recommended, and will also ensure direct protection against capsular groups A, W and Y • operationally, adolescents aged between 13 -18 years to be offered the vaccine as part of routine and catch up programmes, including ‘freshers’ up to the age of 25 years • JCVI recommended as best option to generate population level herd immunity, providing protection across all other age groups, including infants at highest risk Meningococcal ACWY immunisation programme for adolescents

17 Distribution of confirmed IMD cases by capsular group, England Meningococcal ACWY immunisation programme for adolescents

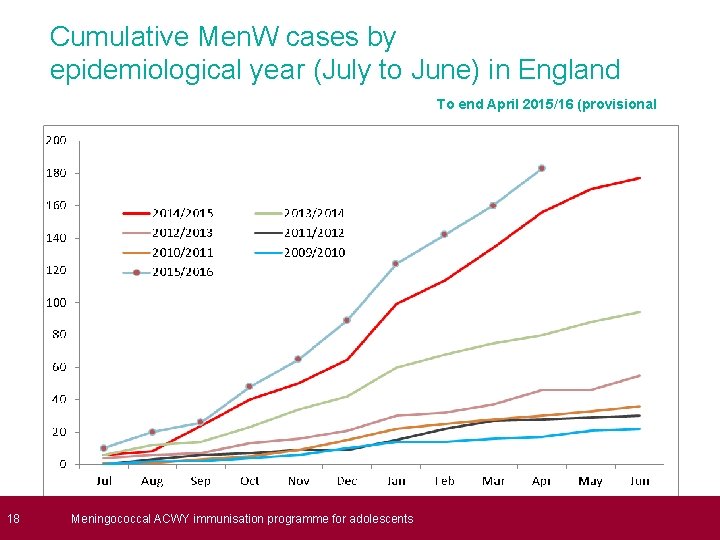
18 Cumulative Men. W cases by epidemiological year (July to June) in England To end April 2015/16 (provisional Meningococcal ACWY immunisation programme for adolescents )

19 Who should receive the Men. ACWY vaccine and when? Meningococcal ACWY immunisation programme for adolescents

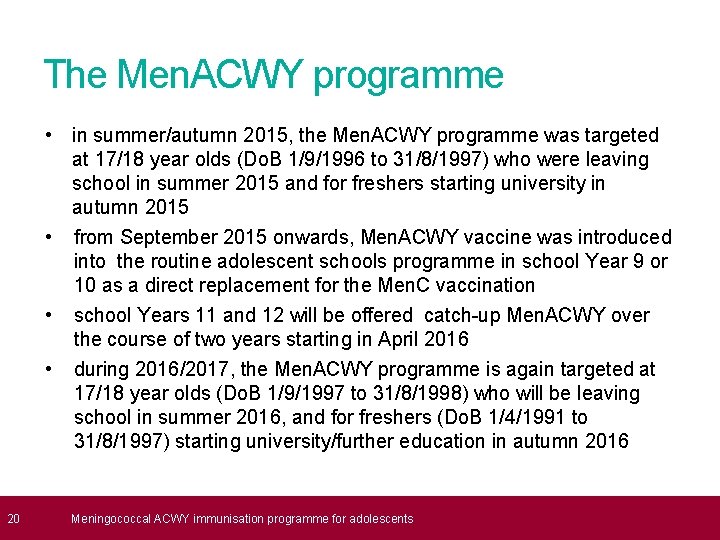
20 The Men. ACWY programme • in summer/autumn 2015, the Men. ACWY programme was targeted at 17/18 year olds (Do. B 1/9/1996 to 31/8/1997) who were leaving school in summer 2015 and for freshers starting university in autumn 2015 • from September 2015 onwards, Men. ACWY vaccine was introduced into the routine adolescent schools programme in school Year 9 or 10 as a direct replacement for the Men. C vaccination • school Years 11 and 12 will be offered catch-up Men. ACWY over the course of two years starting in April 2016 • during 2016/2017, the Men. ACWY programme is again targeted at 17/18 year olds (Do. B 1/9/1997 to 31/8/1998) who will be leaving school in summer 2016, and for freshers (Do. B 1/4/1991 to 31/8/1997) starting university/further education in autumn 2016 Meningococcal ACWY immunisation programme for adolescents

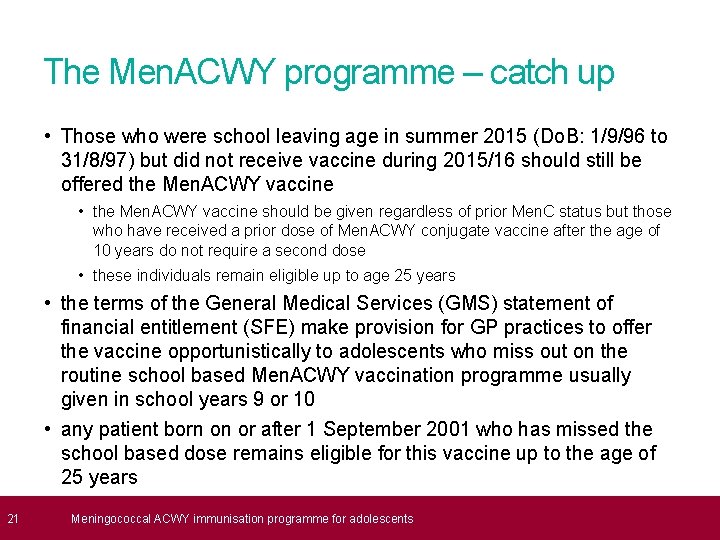
21 The Men. ACWY programme – catch up • Those who were school leaving age in summer 2015 (Do. B: 1/9/96 to 31/8/97) but did not receive vaccine during 2015/16 should still be offered the Men. ACWY vaccine • the Men. ACWY vaccine should be given regardless of prior Men. C status but those who have received a prior dose of Men. ACWY conjugate vaccine after the age of 10 years do not require a second dose • these individuals remain eligible up to age 25 years • the terms of the General Medical Services (GMS) statement of financial entitlement (SFE) make provision for GP practices to offer the vaccine opportunistically to adolescents who miss out on the routine school based Men. ACWY vaccination programme usually given in school years 9 or 10 • any patient born on or after 1 September 2001 who has missed the school based dose remains eligible for this vaccine up to the age of 25 years Meningococcal ACWY immunisation programme for adolescents

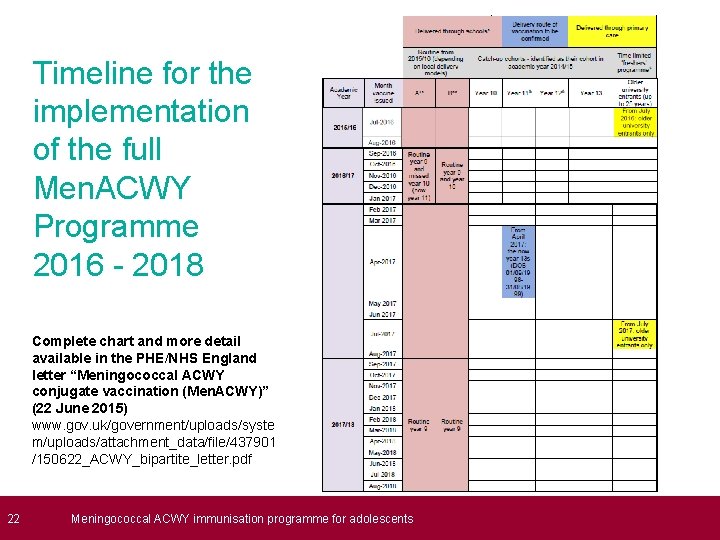
22 Timeline for the implementation of the full Men. ACWY Programme 2016 - 2018 Complete chart and more detail available in the PHE/NHS England letter “Meningococcal ACWY conjugate vaccination (Men. ACWY)” (22 June 2015) www. gov. uk/government/uploads/syste m/uploads/attachment_data/file/437901 /150622_ACWY_bipartite_letter. pdf Meningococcal ACWY immunisation programme for adolescents

23 Other eligible groups • all individuals under 25 years who have never received a dose of Men. C conjugate vaccine • if a prospective students immunisation history cannot be confirmed before attending university, it is acceptable to offer a dose of Men. ACWY conjugate vaccine. Ideally the dose should be administered at least two weeks before attending university to ensure timely protection Changes to Men. C conjugate vaccine schedule

24 Immunisation against meningococcal ACWY disease – the recommended vaccines The use of Menveo® and Nimenrix® vaccines Meningococcal ACWY immunisation programme for adolescents

Brand name: Menveo ® Generic Name: meningococcal group A oligosaccharide; meningococcal group C; meningococcal group W 135 oligosaccharide; meningococcal group Y oligosaccharide. Multi-component group A, C, W 135 and Y conjugate vaccine marketed by Glaxo. Smith. Kline. 25 • licensed for use in children from 2 years, adolescents and adults at risk of invasive disease from Neisseria meningitidis A, C, W and Y and can safely be given with other routine adolescent vaccines • recommended for adolescents and adults as part of a routine and catchup immunisation programme Meningococcal ACWY immunisation programme for adolescents

26 Menveo® • Menveo® is one of two vaccines recommended for the routine Men. ACWY immunisation programme for adolescents • Menveo® will be centrally supplied through Immform • it is important immunisers familiarise themselves with the vaccine and its product information to avoid administration errors Image courtesy of GSK Meningococcal ACWY immunisation programme for adolescents

27 Administration of Menveo® • Menveo® should be administered via intramuscular injection (IM) into the arm (deltoid muscle) • the vaccine is supplied containing two separate vials – one vial containing Men A (powder) and the second vial containing Men. CWY (solution) • the Men. CWY solution should be injected into the Men. A powder to reconstitute • invert and shake the solution and powder vigorously and withdraw 0. 5 ml of reconstituted product. It is normal for a small amount of liquid to remain in the vial following withdrawal of the dose • one dose is 0. 5 ml Meningococcal ACWY immunisation programme for adolescents

28 Administration of Menveo® (Cont’d) • after reconstitution, the solution should be clear, colourless to light yellow and free from visible foreign particles • prior to administration, healthcare professionals should change the needle ensuring that they use one which is suitable for IM administration into the deltoid muscle • the vaccine should not be administered where there are variations in physical appearance or signs of foreign particulate are observed after shaking Meningococcal ACWY immunisation programme for adolescents

Brand name: Nimenrix® Generic name: Neisseria meningitidis group A polysaccharide, Neisseria meningitidis group C polysaccharide, Neisseria meningitidis group W-135 polysaccharide, Neisseria meningitidis group Y polysaccharide. Multi-component group A, C, W 135 and Y conjugate vaccine marketed by Glaxo. Smith. Kline. 29 • licensed for use in children from 12 months, adolescents and adults at risk of invasive disease from Neisseria meningitidis A, C, W and Y and can safely be given with other routine adolescent vaccines • recommended for adolescents and adults as part of a routine and catch-up immunisation programme Meningococcal ACWY immunisation programme for adolescents

30 Nimenrix® • Nimenrix® is one of two vaccines recommended for the routine Men. ACWY immunisation programme for adolescents • Nimenrix® is centrally supplied through Immform • it is important immunisers familiarise themselves with the vaccine and its product information to avoid administration errors Image courtesy of GSK Meningococcal ACWY immunisation programme for adolescents

31 Administration of Nimenrix® • Nimenrix® should be administered via intramuscular injection (IM) into the arm (deltoid muscle) • the vaccine is supplied as one vial of powder and one pre-filled syringe • the contents of the pre-filled syringe should be vigorously mixed with the contents of the vial prior to administration providing one dose (0. 5 ml) • after reconstitution, the solution should be clear, colourless to light yellow and free from visible foreign particles • the vaccine should not be administered where there are variations in physical appearance or signs of foreign particulate are observed after shaking Meningococcal ACWY immunisation programme for adolescents

32 How many doses? • Men. ACWY vaccine should be administered as a single dose only • the need for, and the timing of a booster dose of Men. ACWY vaccine has not yet been determined and is therefore not currently recommended • those who have already received a Men. C vaccine over the age of 10 years should still be offered Men. ACWY conjugate vaccine as part of a catch-up programme to ensure protection against the additional capsular groups A, W and Y • the Men. ACWY conjugate vaccine can be administered at any interval after Men. C vaccine and at any interval before, after or at the same time as other vaccines eg Td/IPV, MMR, travel vaccines • those who have already received a Men. ACWY conjugate vaccine at the age of 10 years or over do not require an additional dose (with the exception of close contacts of a confirmed case of Men. ACWY infection) Meningococcal ACWY immunisation programme for adolescents

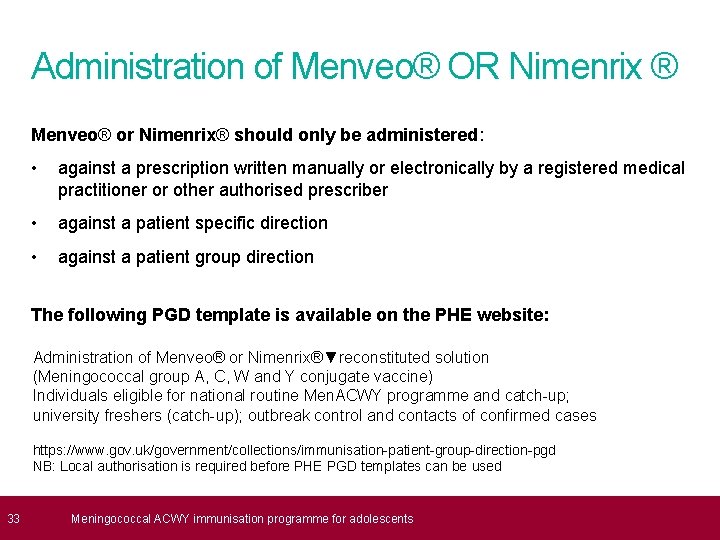
33 Administration of Menveo® OR Nimenrix ® Menveo® or Nimenrix® should only be administered: • against a prescription written manually or electronically by a registered medical practitioner or other authorised prescriber • against a patient specific direction • against a patient group direction The following PGD template is available on the PHE website: Administration of Menveo® or Nimenrix®▼reconstituted solution (Meningococcal group A, C, W and Y conjugate vaccine) Individuals eligible for national routine Men. ACWY programme and catch-up; university freshers (catch-up); outbreak control and contacts of confirmed cases https: //www. gov. uk/government/collections/immunisation-patient-group-direction-pgd NB: Local authorisation is required before PHE PGD templates can be used Meningococcal ACWY immunisation programme for adolescents

34 Contraindications Menveo® OR Nimenrix® should not be administered to those who have had: 1. A confirmed anaphylaxis to a previous dose of the vaccine OR 2. A confirmed anaphylaxis to any constituent or excipient of the vaccine • there are very few individuals who cannot receive meningococcal vaccines • where there is doubt, appropriate advice should be sought rather than withholding immunisation Meningococcal ACWY immunisation programme for adolescents

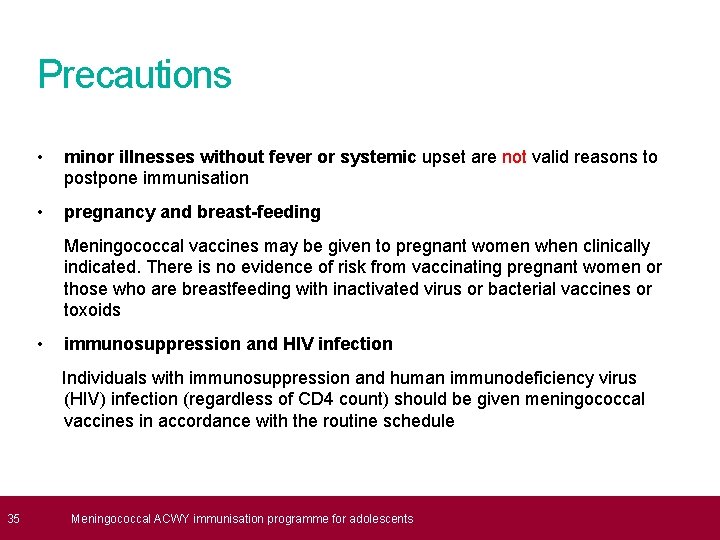
35 Precautions • minor illnesses without fever or systemic upset are not valid reasons to postpone immunisation • pregnancy and breast-feeding Meningococcal vaccines may be given to pregnant women when clinically indicated. There is no evidence of risk from vaccinating pregnant women or those who are breastfeeding with inactivated virus or bacterial vaccines or toxoids • immunosuppression and HIV infection Individuals with immunosuppression and human immunodeficiency virus (HIV) infection (regardless of CD 4 count) should be given meningococcal vaccines in accordance with the routine schedule Meningococcal ACWY immunisation programme for adolescents

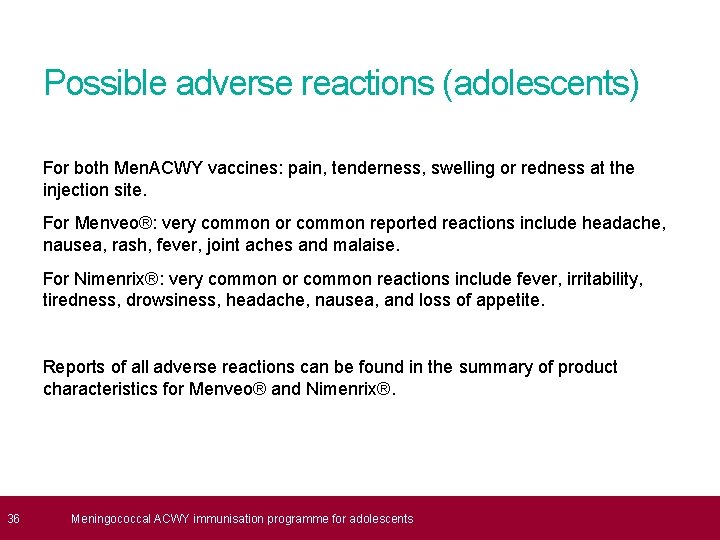
36 Possible adverse reactions (adolescents) For both Men. ACWY vaccines: pain, tenderness, swelling or redness at the injection site. For Menveo®: very common or common reported reactions include headache, nausea, rash, fever, joint aches and malaise. For Nimenrix®: very common or common reactions include fever, irritability, tiredness, drowsiness, headache, nausea, and loss of appetite. Reports of all adverse reactions can be found in the summary of product characteristics for Menveo® and Nimenrix®. Meningococcal ACWY immunisation programme for adolescents

37 Reporting suspected adverse reactions Yellow card scheme • all suspected adverse reactions should be reported to the MHRA using the yellow card scheme • success depends on early, complete and accurate reporting • report even if uncertain about whether vaccine caused condition • http: //mhra. gov. uk/yellowcard • see chapter 8 of Green Book for details Meningococcal ACWY immunisation programme for adolescents

38 The role of healthcare professionals To provide clear, concise and accurate information to patients, parents or carers of those receiving the Men. ACWY vaccine as part of the routine adolescent immunisation programme. Every effort should be made by healthcare professionals to maximise the uptake of the meningococcal ACWY vaccine in adolescents and to ensure that patients, parents or carers are fully informed about the importance of ensuring protection against meningococcal ACWY disease. Meningococcal ACWY immunisation programme for adolescents

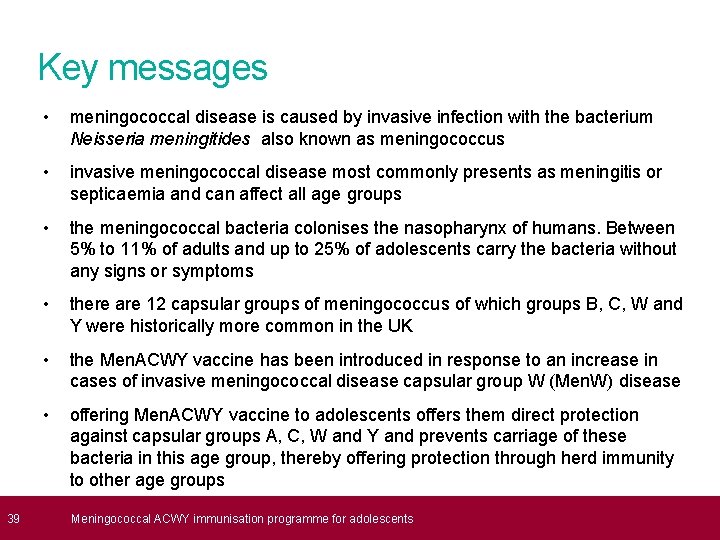
39 Key messages • meningococcal disease is caused by invasive infection with the bacterium Neisseria meningitides also known as meningococcus • invasive meningococcal disease most commonly presents as meningitis or septicaemia and can affect all age groups • the meningococcal bacteria colonises the nasopharynx of humans. Between 5% to 11% of adults and up to 25% of adolescents carry the bacteria without any signs or symptoms • there are 12 capsular groups of meningococcus of which groups B, C, W and Y were historically more common in the UK • the Men. ACWY vaccine has been introduced in response to an increase in cases of invasive meningococcal disease capsular group W (Men. W) disease • offering Men. ACWY vaccine to adolescents offers them direct protection against capsular groups A, C, W and Y and prevents carriage of these bacteria in this age group, thereby offering protection through herd immunity to other age groups Meningococcal ACWY immunisation programme for adolescents

40 Useful links • Public Health England/ NHS England. Meningococcal ACWY conjugate vaccination (Men. ACWY). 22 June 2015 https: //www. gov. uk/government/publications/menacwy-vaccine-introduction • Public Health England. Immunisation against infectious diseases (The Green Book) Meningococcal chapter 22 https: //www. gov. uk/government/publications/meningococcal-the-green-bookchapter-22 • Public Health England. Meningococcal leaflets, consent forms, PGD, information for healthcare practitioners and other Men. ACWY-specific materials: https: //www. gov. uk/government/collections/meningococcal-acwymenacwy-vaccination-programme • Meningitis Research Foundation: http: //www. meningitis. org/ • Meningitis Now. https: //www. meningitisnow. org/ • NHS Choices. http: //www. nhs. uk/conditions/Meningitis/Pages/Introduction. aspx Meningococcal ACWY immunisation programme for adolescents

41 About Public Health England exists to protect and improve the nation's health and wellbeing, and reduce health inequalities. It does this through world-class science, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. PHE is an operationally autonomous executive agency of the Department of Health. Public Health England Wellington House 133 -155 Waterloo Road London SE 1 8 UG Tel: 020 7654 8000 www. gov. uk/phe Twitter: @PHE_uk Facebook: www. facebook. com/Public. Health. England © Crown copyright 2016 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v 3. 0. To view this licence, visit OGL or email psi@nationalarchives. gsi. gov. uk. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. Published July 2016 PHE publications gateway number: 2016155 Vaccination against shingles (Herpes Zoster)