Medication Assisted Treatment For Opioid Use Disorders Shannon






























- Slides: 48

Medication Assisted Treatment For Opioid Use Disorders Shannon Allen, MD Jade Wellness Center July 19, 2018



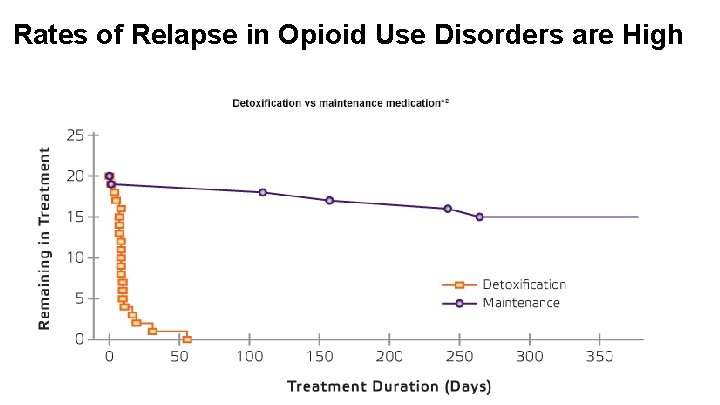
Rates of Relapse in Opioid Use Disorders are High



Benefits of Medication Assisted Treatments • Reduce risk of overdose and death • Improve social functioning • Reduce risk of infectious disease transmission • Reduce engagement in criminal activities • Improved maternal and fetal outcomes • Increased retention in treatment • Safe


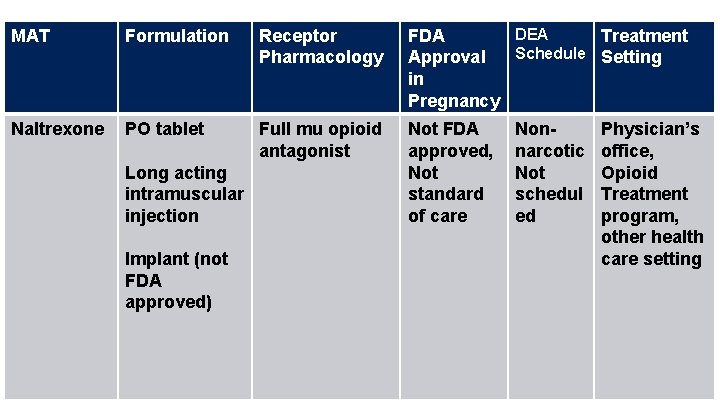
MAT Formulation Receptor Pharmacology DEA FDA Treatment Schedule Setting Approval in Pregnancy Naltrexone PO tablet Full mu opioid antagonist Not FDA approved, Not standard of care Long acting intramuscular injection Implant (not FDA approved) Nonnarcotic Not schedul ed Physician’s office, Opioid Treatment program, other health care setting

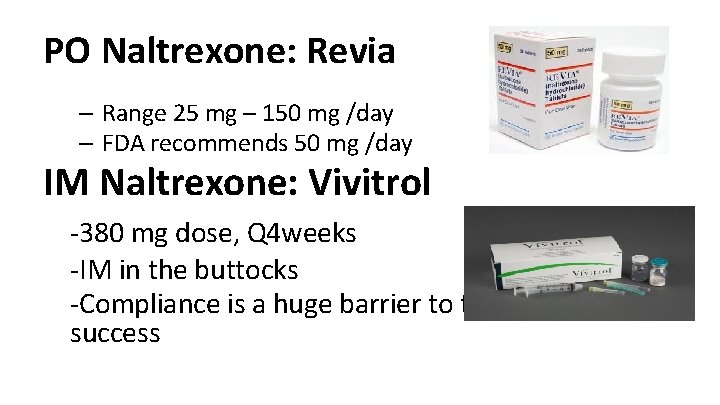
PO Naltrexone: Revia – Range 25 mg – 150 mg /day – FDA recommends 50 mg /day IM Naltrexone: Vivitrol -380 mg dose, Q 4 weeks -IM in the buttocks -Compliance is a huge barrier to treatment success


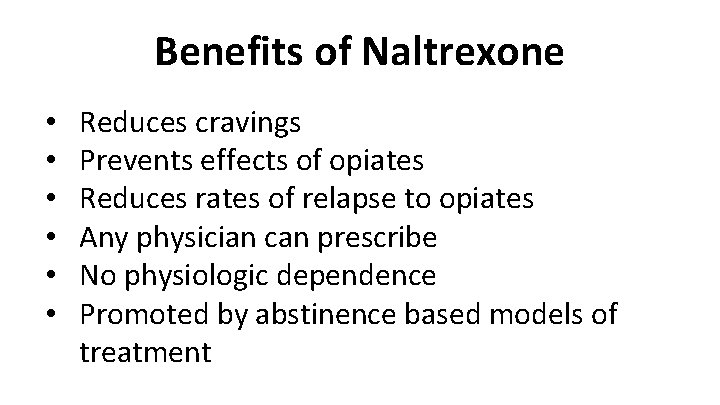
Benefits of Naltrexone • • • Reduces cravings Prevents effects of opiates Reduces rates of relapse to opiates Any physician can prescribe No physiologic dependence Promoted by abstinence based models of treatment

Lee, J. D. , Friedmann, P. D. , Kinlock, T. W. , Nunes, E. V. , Boney, T. Y. , Hoskinson Jr, R. A. , … & Gordon, M. (2016). Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. New England Journal of Medicine, 374(13), 1232 -1242. Improved Opioid Outcomes with XR-NTX: Lower relapse rates Greater percentage of opioid-negative UDS Longer time to relapse Zero overdoses

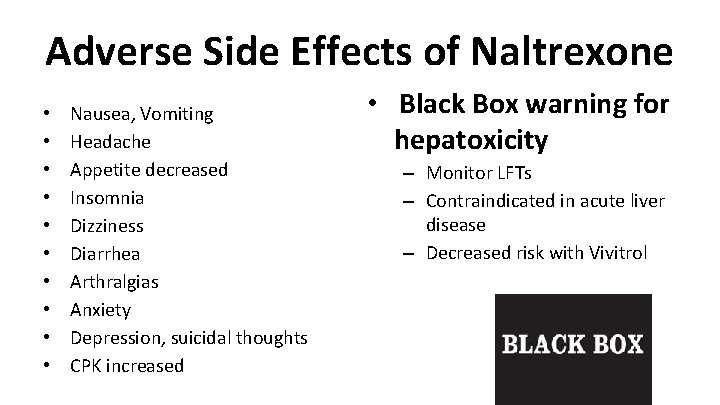
Adverse Side Effects of Naltrexone • • • Nausea, Vomiting Headache Appetite decreased Insomnia Dizziness Diarrhea Arthralgias Anxiety Depression, suicidal thoughts CPK increased • Black Box warning for hepatoxicity – Monitor LFTs – Contraindicated in acute liver disease – Decreased risk with Vivitrol

Injection Site Reaction with Vivitrol • Increased risk if injection occurs in SC adipose layer • Redness, induration, swelling, tenderness • Can lead to cellulitis, abscess, necrosis

Precipitated Opioid Withdrawal with Naltrexone • Recommendation is to be opioid free for minimum 710 days • Naloxone Challenge • Can be a barrier to starting antagonist treatment

Risk of Opioid Overdose with Naltrexone Accidental overdoses occur: • After detox from opioids, before first dose is administered • With attempts to overcome the opioid receptor blockade • Using opioids after discontinuation of treatment • Using opioids after missing a dose

Barriers to Naltrexone • • • Lack of insurance or finances to pay for it Patient lack of awareness Physician lack of knowledge Incarceration Complexity of ordering XR-NTX Complexity of administering

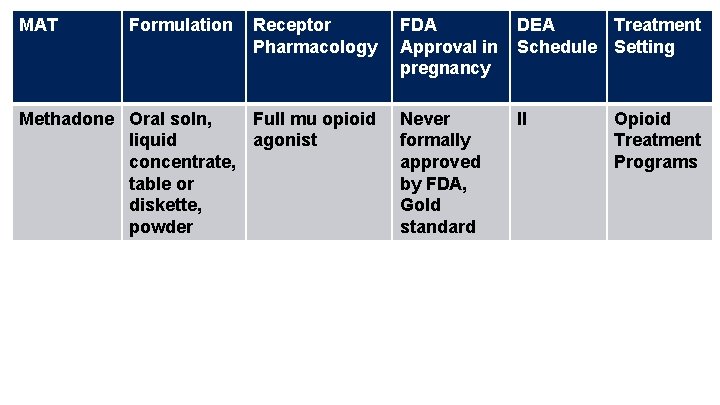
MAT Formulation Receptor Pharmacology Methadone Oral soln, Full mu opioid liquid agonist concentrate, table or diskette, powder FDA Approval in pregnancy DEA Treatment Schedule Setting Never formally approved by FDA, Gold standard II Opioid Treatment Programs

Historical Context of Methadone Maintenance • Dr. Vincent Dole, becomes chair of Narcotics Committee of the Health Research Council of NYC. Joins with Dr. Marie Nyswander, an addiction psychiatrist, and together develop methadone as a maintenance therapy in 1964 – “Methadone maintenance provides a safe and effective way to normalize the function of otherwise intractable opiate addiction” • 1973 – Methadone Control Act Establishes federally funded clinics for prevention and treatment of opioid dependence. Regulates licensing for dispensing methadone.

Methadone Pharmacology • Extensive bioavailability – Up to 80% orally bioavailable • Long elimination half life, ranging from 22 -36 hours – Allows for daily dosing • Peak serum levels in 2 -4 hours – Highest rates of sedation, euphoria, analgesia • Tolerance remains stable – No need to escalate the dose (vs. short acting opioids) • Hepatic metabolism through CYP 450 enzymes – Major substrate of CYP 3 A 4 isozyme

Opioid Treatment Programs SAMHSA-certified program, that engages in supervised assessment and treatment for individuals who are addicted to opioids. • Can include IOP, residential programs, hospital settings • Variety of on-site services – medically supervised withdrawal – maintenance treatment – medical, psychiatric, psychosocial and other types of supportive care. • High level of regulations (federal, state, county, etc. )

Eligibility For Methadone Maintenance – Must be 18 years old • some states make exception for 16 -18 yo – Must have at least 12 months of physiologic dependence to opioids • Exceptions: pregnancy • Exceptions: high risk populations such as HIV positive, recently released from incarceration – Must meet requirements for moderate to severe Opioid use disorder

Methadone Maintenance Goals Of Treatment • • relieve narcotic craving suppress the abstinence syndrome block the euphoric effects associated with opiates Minimize side effect profile Dosing • Doses > 60 mg per day are recommended • Most people stabilize between 60 – 120 mg daily

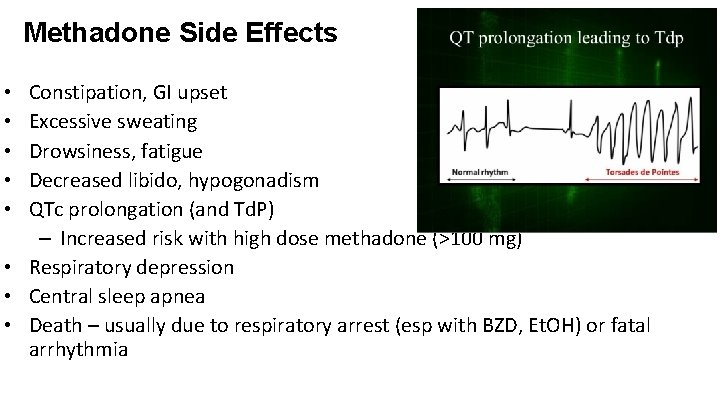
Methadone Side Effects Constipation, GI upset Excessive sweating Drowsiness, fatigue Decreased libido, hypogonadism QTc prolongation (and Td. P) – Increased risk with high dose methadone (>100 mg) • Respiratory depression • Central sleep apnea • Death – usually due to respiratory arrest (esp with BZD, Et. OH) or fatal arrhythmia • • •

Barriers to Methadone Maintenance Treatment • • • Lack of insurance or finances to pay for treatment Incarceration – Only 55% of prison systems allow for prescription of methadone Logistics of frequent dosing – Transportation, childcare, time off from work, etc Legal involvement Must meet state eligibility

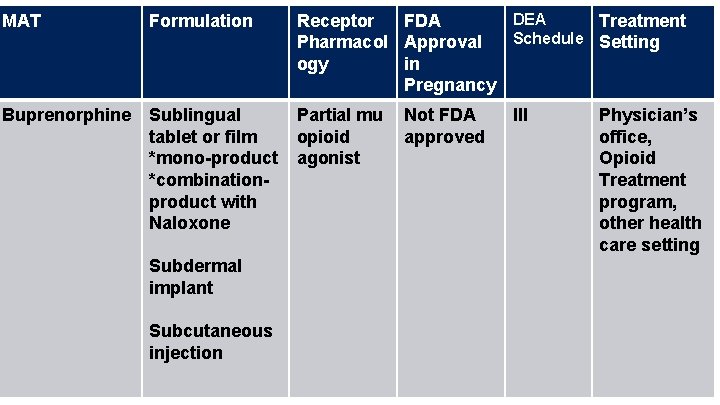
MAT Formulation DEA Receptor FDA Treatment Schedule Setting Pharmacol Approval ogy in Pregnancy Buprenorphine Sublingual tablet or film *mono-product *combinationproduct with Naloxone Partial mu opioid agonist Subdermal implant Subcutaneous injection Not FDA approved III Physician’s office, Opioid Treatment program, other health care setting

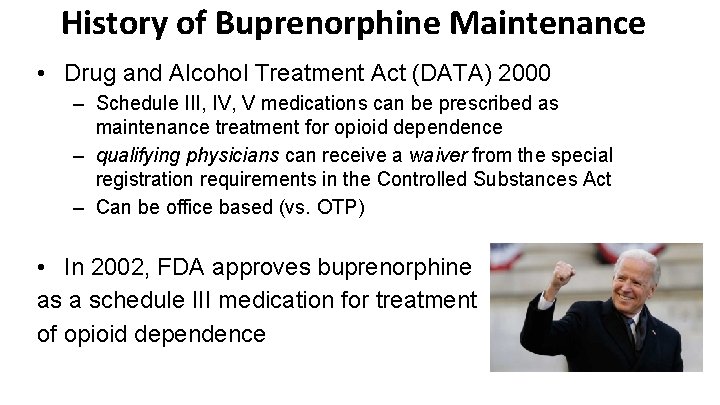
History of Buprenorphine Maintenance • Drug and Alcohol Treatment Act (DATA) 2000 – Schedule III, IV, V medications can be prescribed as maintenance treatment for opioid dependence – qualifying physicians can receive a waiver from the special registration requirements in the Controlled Substances Act – Can be office based (vs. OTP) • In 2002, FDA approves buprenorphine as a schedule III medication for treatment of opioid dependence

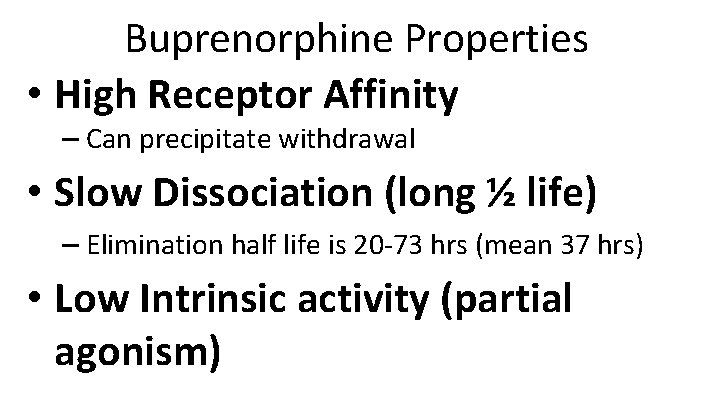
Buprenorphine Properties • High Receptor Affinity – Can precipitate withdrawal • Slow Dissociation (long ½ life) – Elimination half life is 20 -73 hrs (mean 37 hrs) • Low Intrinsic activity (partial agonism)

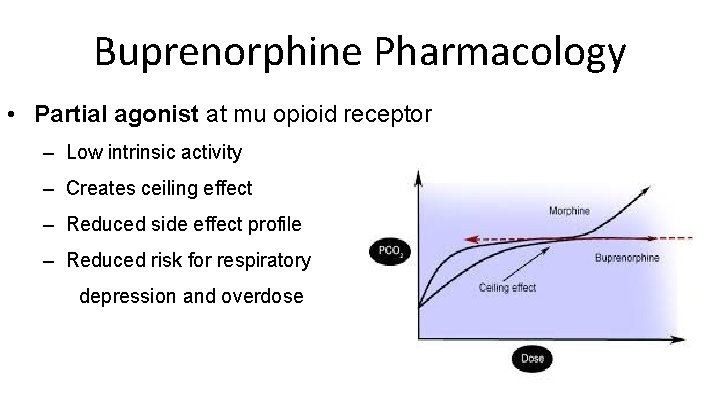
Buprenorphine Pharmacology • Partial agonist at mu opioid receptor – Low intrinsic activity – Creates ceiling effect – Reduced side effect profile – Reduced risk for respiratory depression and overdose


Buprenorphine Induction • Patients are advised to abstain from short-acting opiates for >24 hours, long acting opiates for >48 -72 hours • Goal: Moderate Withdrawal – COWS >10 – CINA >10 • Buprenorphine is initiated at low dose – 2 -4 mg – If no precipitated withdrawal within 2 hours, can repeat dose (up to 8 mg on day 1)

Buprenorphine Formulations • Poor oral bioavailability • <40% of SL administration • 2 sublingual formulations – Buprenorphine (Subutex) • Preferred formulation in pregnancy and breastfeeding – Buprenorphine with naloxone (Suboxone, Zubsolv) • 4: 1 buprenorphine to naloxone • Naloxone, only active if IV or IM administration • Added to limit diversion

Buprenorphine Formulations • Buccal formulation (Bunavail) -- daily --4: 1 buprenorphine to naloxone

Buprenorphine Formulations • Subdermal implant (Probuphine) --buprenorphine mono-product --8 mg equivalent --Remove and reinsert at 6 months --Must complete training to prescribe and surgically insert/remove

Buprenorphine Formulations • Subcutaneous injection (Sublocade) --Buprenorphine mono-product --Repeat injections every 4 weeks 2 x 300 mg, then 100 mg maintenance dose --corresponds to 100% mu receptor occupancy --Must be REMS certified --Blackbox warning: intravenous administration can result in potentially fatal thrombo-embolic events

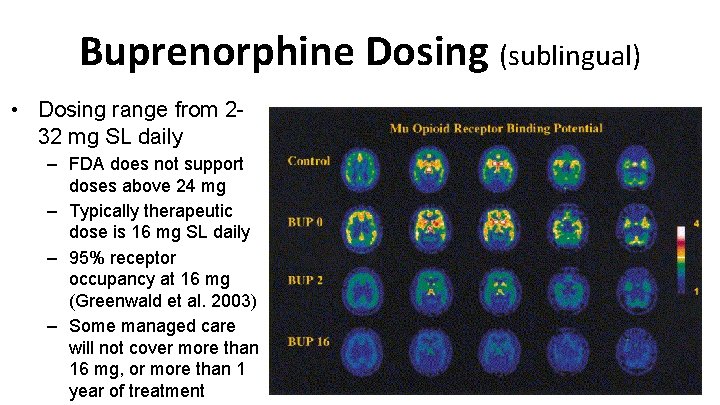
Buprenorphine Dosing (sublingual) • Dosing range from 232 mg SL daily – FDA does not support doses above 24 mg – Typically therapeutic dose is 16 mg SL daily – 95% receptor occupancy at 16 mg (Greenwald et al. 2003) – Some managed care will not cover more than 16 mg, or more than 1 year of treatment

Buprenorphine Side Effects • • Constipation, GI upset Excessive sweating Drowsiness, fatigue Decreased libido, hypogonadism QTc prolongation? ? Central sleep apnea Respiratory Depression (especially with BZD) – Children are vulnerable

Diversion of Buprenorphine is Uncommon In 2014, Buprenorphine made up United States Reasons: To manage withdrawal To self treat pain To self treat depression <1% of all reported drugs diverted in the

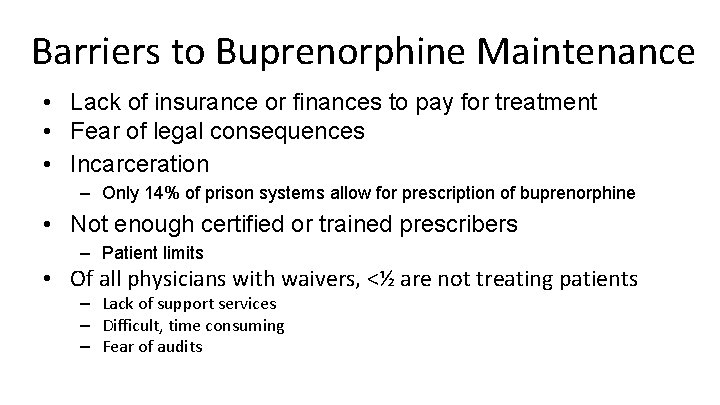
Barriers to Buprenorphine Maintenance • Lack of insurance or finances to pay for treatment • Fear of legal consequences • Incarceration – Only 14% of prison systems allow for prescription of buprenorphine • Not enough certified or trained prescribers – Patient limits • Of all physicians with waivers, <½ are not treating patients – Lack of support services – Difficult, time consuming – Fear of audits

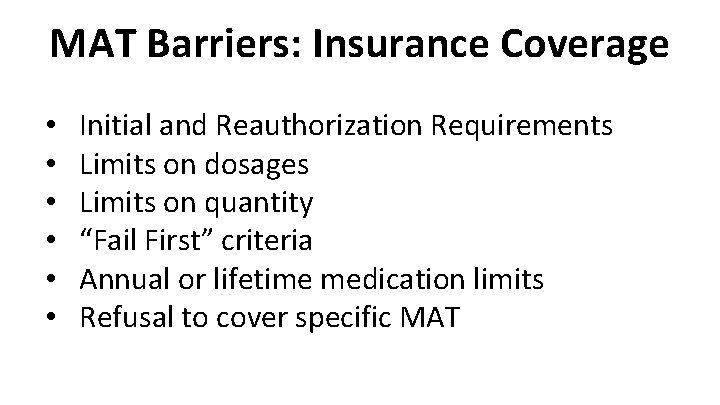
MAT Barriers: Insurance Coverage • • • Initial and Reauthorization Requirements Limits on dosages Limits on quantity “Fail First” criteria Annual or lifetime medication limits Refusal to cover specific MAT

MAT Barriers: Provider • Inadequate Training – Treatment of addiction is limited in medical school and residencies – Therapists are not trained about MAT • • Negative attitude, Stigma Follow Abstinence Base Philosophy Suboptimal Dosing Preference for detox or tapering off

MAT Barriers: Pharmacy • • Refusal to carry MAT Negative attitudes, Stigma Additional Requirements Refusal to fill prescriptions

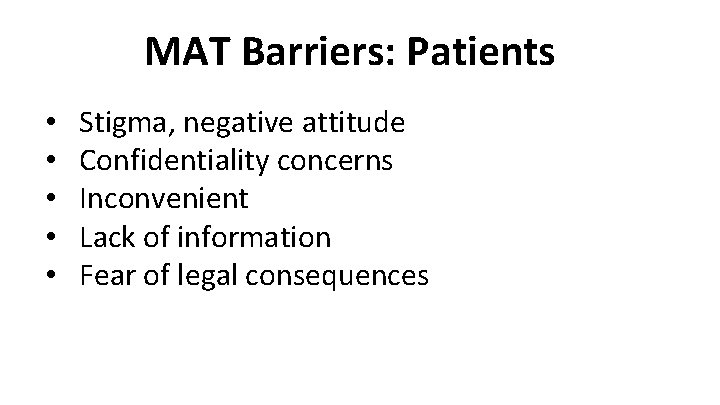
MAT Barriers: Patients • • • Stigma, negative attitude Confidentiality concerns Inconvenient Lack of information Fear of legal consequences



Which MAT Should I Choose?

Treatment Works! But no single treatment is right for everyone
 Medication assisted treatment colorado
Medication assisted treatment colorado Rass vs poss
Rass vs poss Opiyatlar grubu
Opiyatlar grubu Bromazepam umrechnungstabelle
Bromazepam umrechnungstabelle Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Types of pain
Types of pain Mechanism of action of opioid analgesics
Mechanism of action of opioid analgesics Children's pain scale
Children's pain scale Opioid receptors location
Opioid receptors location Brian erickson md
Brian erickson md Opioid settlement calculator
Opioid settlement calculator Poker chip pain scale
Poker chip pain scale Kentucky opioid response effort
Kentucky opioid response effort Cjktos
Cjktos Apa itu computer assisted language learning
Apa itu computer assisted language learning Hidden costs of assisted living
Hidden costs of assisted living Robot assisted surgery
Robot assisted surgery Microwave assisted extraction
Microwave assisted extraction Laser assisted bonding
Laser assisted bonding Derive logically necessary conclusion from given premises.
Derive logically necessary conclusion from given premises. Font finder
Font finder Advantages of call
Advantages of call Contoh computer assisted instruction
Contoh computer assisted instruction Chapter 27 nutritional therapy and assisted feeding
Chapter 27 nutritional therapy and assisted feeding Assisted picking
Assisted picking Manual assisted cough
Manual assisted cough Single point service
Single point service What part
What part Park place seattle assisted living
Park place seattle assisted living Information is lost gradually but very slowly
Information is lost gradually but very slowly Nih assisted referral tool
Nih assisted referral tool Virtualization techniques in cloud computing
Virtualization techniques in cloud computing Eno pointe assisted living
Eno pointe assisted living Define isometric exercise
Define isometric exercise Computer assisted passenger prescreening system
Computer assisted passenger prescreening system Small party assisted rescue
Small party assisted rescue Uvulopalatoplasty
Uvulopalatoplasty Computer assisted guidance systems
Computer assisted guidance systems Peer assisted
Peer assisted Online proctored exams candidate guidelines peoplecert
Online proctored exams candidate guidelines peoplecert Audit techniques training
Audit techniques training Georgia senior living association
Georgia senior living association Healthfinder florida
Healthfinder florida Hydrogen assisted cracking
Hydrogen assisted cracking Computer aided teaching
Computer aided teaching Protea senior living
Protea senior living Base case system evaluation
Base case system evaluation