Max Quant Summer School Martinsried June 26 2013


![Refinement of Aurora kinase target sequence Currently accepted target sequences: [RK]-X-[ST]-[ILV] (S. serevisiae) [RKN]-R-X-[ST]-[ILVM] Refinement of Aurora kinase target sequence Currently accepted target sequences: [RK]-X-[ST]-[ILV] (S. serevisiae) [RKN]-R-X-[ST]-[ILVM]](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-29.jpg)

![Kinase of interest must be active during experiment [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+ Kinase of interest must be active during experiment [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-37.jpg)
![Importance of normalization by protein ratio [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+ Overexpressed PKDkd Importance of normalization by protein ratio [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+ Overexpressed PKDkd](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-38.jpg)
![Refinement of PKD target sequence Currently accepted target sequence: [LVI]-X-[RK]-X-X-[ST] [LV]-K-K-K-L-[ST] Depletion of Pro! Refinement of PKD target sequence Currently accepted target sequence: [LVI]-X-[RK]-X-X-[ST] [LV]-K-K-K-L-[ST] Depletion of Pro!](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-40.jpg)

- Slides: 41

Max. Quant Summer School Martinsried, June 26, 2013 Sample preparation and measurement strategies in phosphoproteomics Boris Maček Proteome Center Tübingen 1

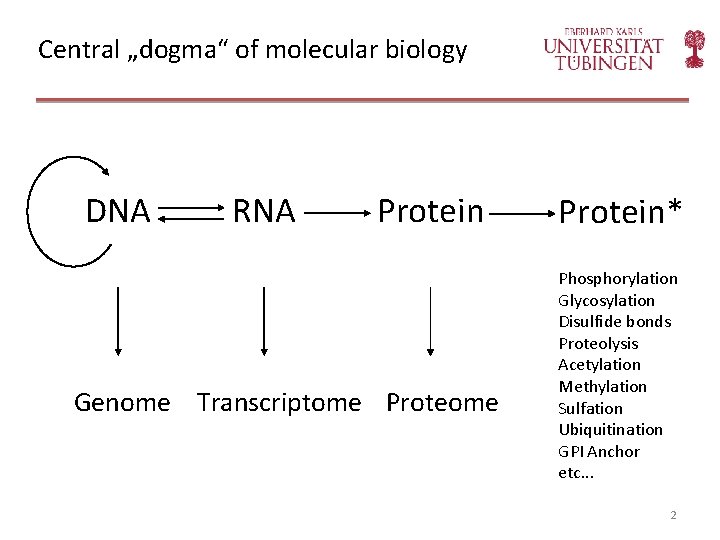
Central „dogma“ of molecular biology DNA RNA Protein Genome Transcriptome Protein* Phosphorylation Glycosylation Disulfide bonds Proteolysis Acetylation Methylation Sulfation Ubiquitination GPI Anchor etc. . . 2

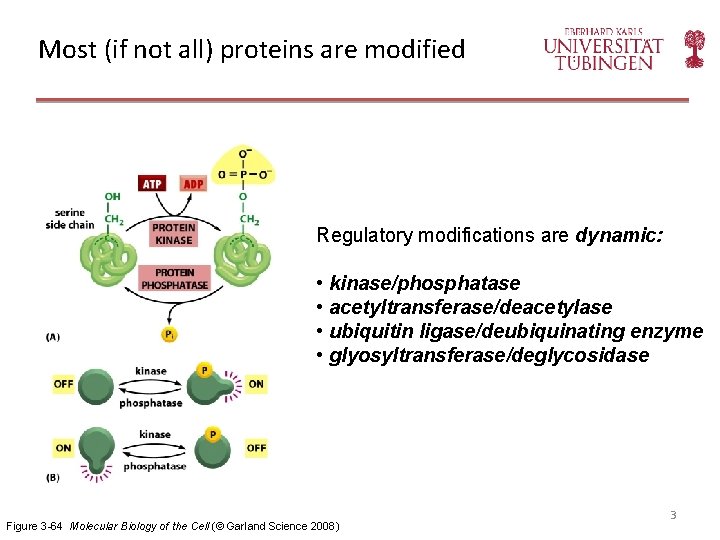
Most (if not all) proteins are modified Regulatory modifications are dynamic: • kinase/phosphatase • acetyltransferase/deacetylase • ubiquitin ligase/deubiquinating enzyme • glyosyltransferase/deglycosidase Figure 3 -64 Molecular Biology of the Cell (© Garland Science 2008) 3

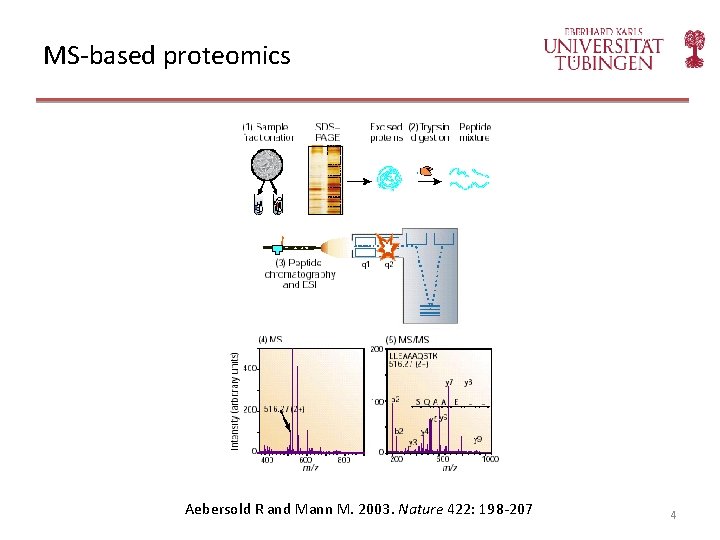
MS-based proteomics Aebersold R and Mann M. 2003. Nature 422: 198 -207 4

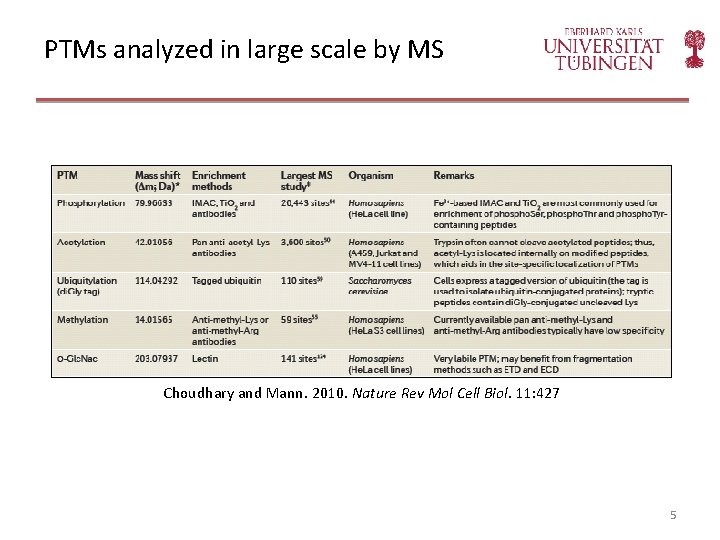
PTMs analyzed in large scale by MS Choudhary and Mann. 2010. Nature Rev Mol Cell Biol. 11: 427 5

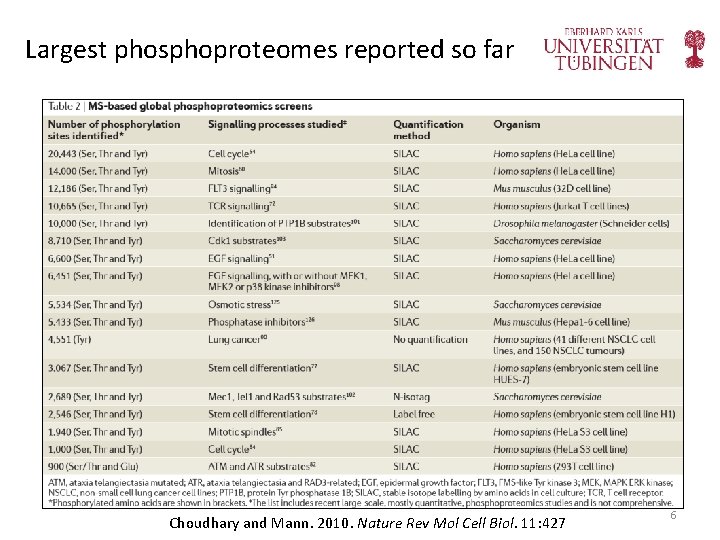
Largest phosphoproteomes reported so far Choudhary and Mann. 2010. Nature Rev Mol Cell Biol. 11: 427 6

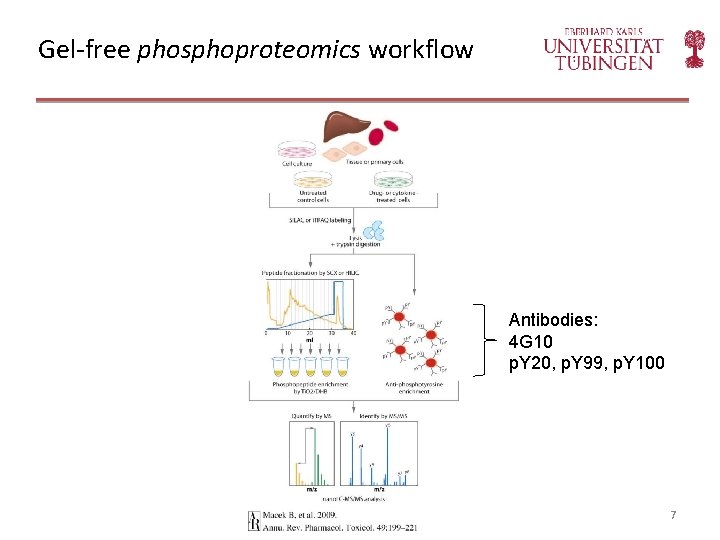
Gel-free phosphoproteomics workflow Antibodies: 4 G 10 p. Y 20, p. Y 99, p. Y 100 7

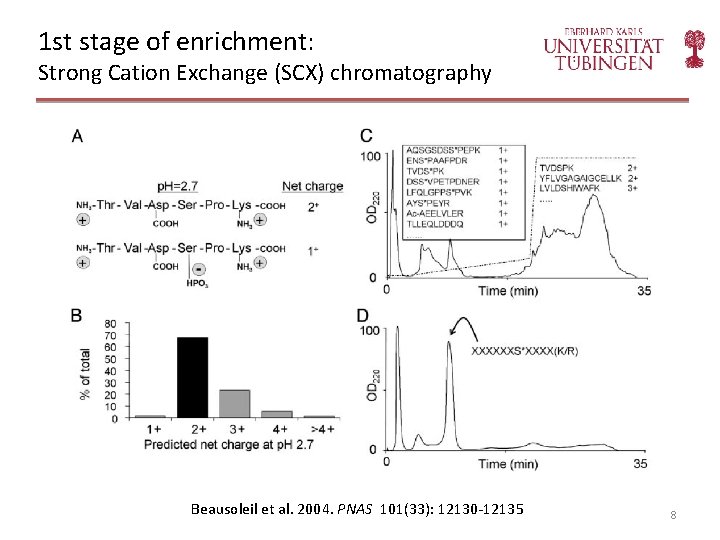
1 st stage of enrichment: Strong Cation Exchange (SCX) chromatography Beausoleil et al. 2004. PNAS 101(33): 12130 -12135 8

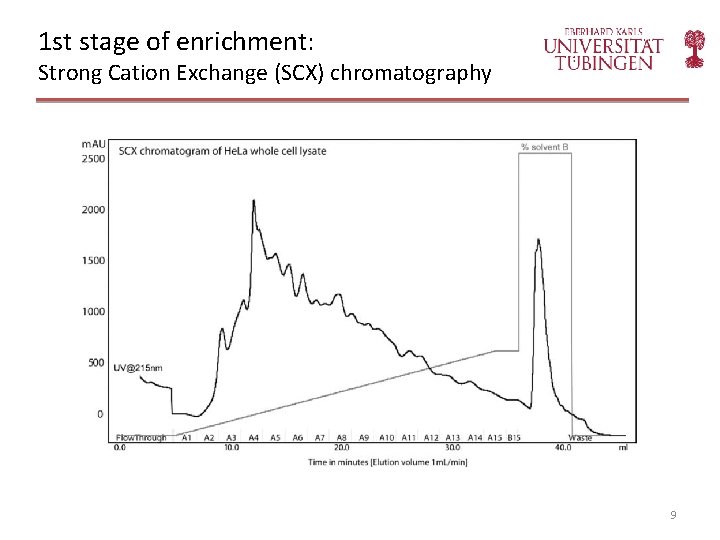
1 st stage of enrichment: Strong Cation Exchange (SCX) chromatography 9

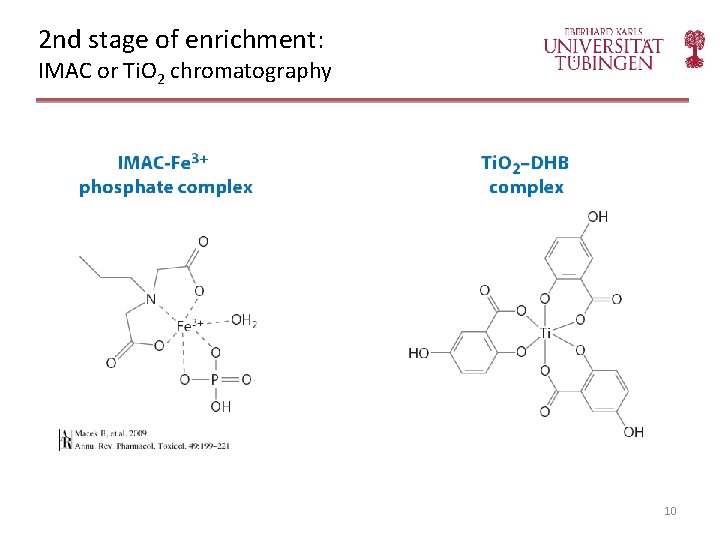
2 nd stage of enrichment: IMAC or Ti. O 2 chromatography 10

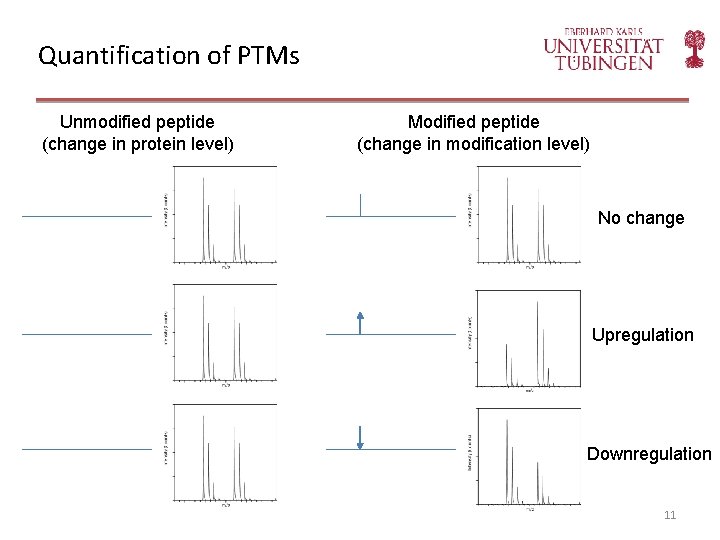
Quantification of PTMs Unmodified peptide (change in protein level) Modified peptide (change in modification level) No change Upregulation Downregulation 11

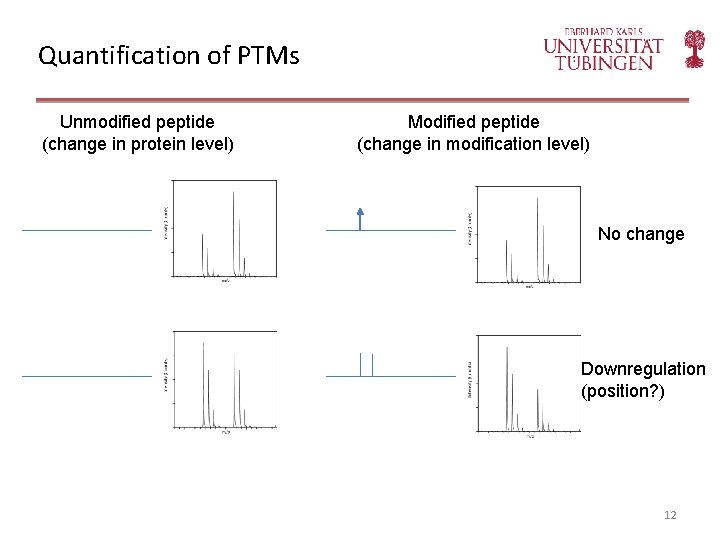
Quantification of PTMs Unmodified peptide (change in protein level) Modified peptide (change in modification level) No change Downregulation (position? ) 12

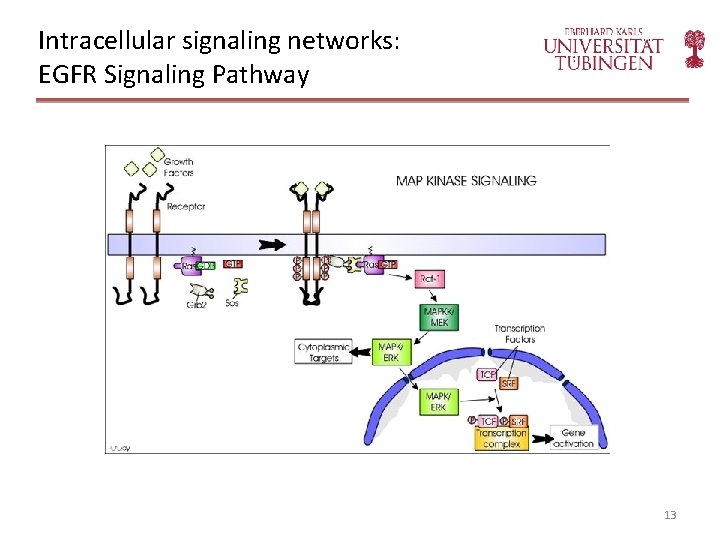
Intracellular signaling networks: EGFR Signaling Pathway 13

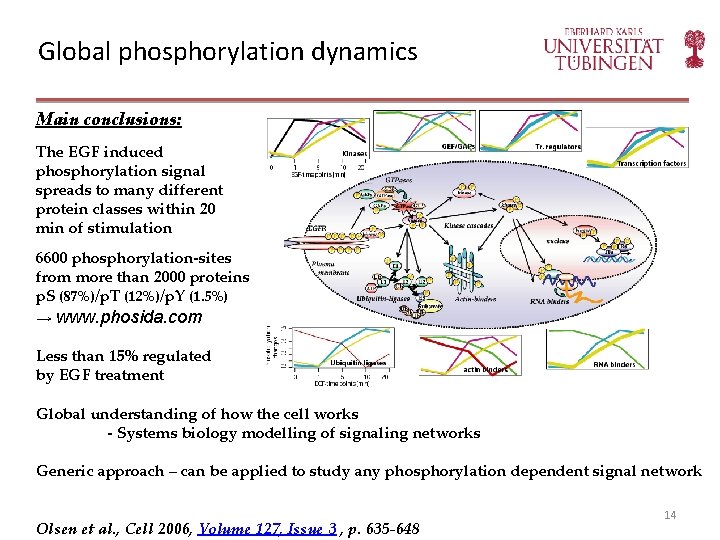
Global phosphorylation dynamics Main conclusions: The EGF induced phosphorylation signal spreads to many different protein classes within 20 min of stimulation 6600 phosphorylation-sites from more than 2000 proteins p. S (87%)/p. T (12%)/p. Y (1. 5%) → www. phosida. com Less than 15% regulated by EGF treatment Global understanding of how the cell works - Systems biology modelling of signaling networks Generic approach – can be applied to study any phosphorylation dependent signal network Olsen et al. , Cell 2006, Volume 127, Issue 3 , p. 635 -648 14

Detection of kinase substrate candidates • targets of Aurora kinase in S. pombe (with S. Hauf, FMI/MPI) • Koch et al. 2011. Science Signaling 4(179): rs 6 • targets of Polo and Fin 1 in S. pombe (with I. Hagan, Paterson Institute) • targets of protein kinase D in human cells (with A. Hausser, Uni Stuttgart) • Franz-Wachtel et al. 2012. MCP • targets of S/T kinases and phosphatases in model bacteria

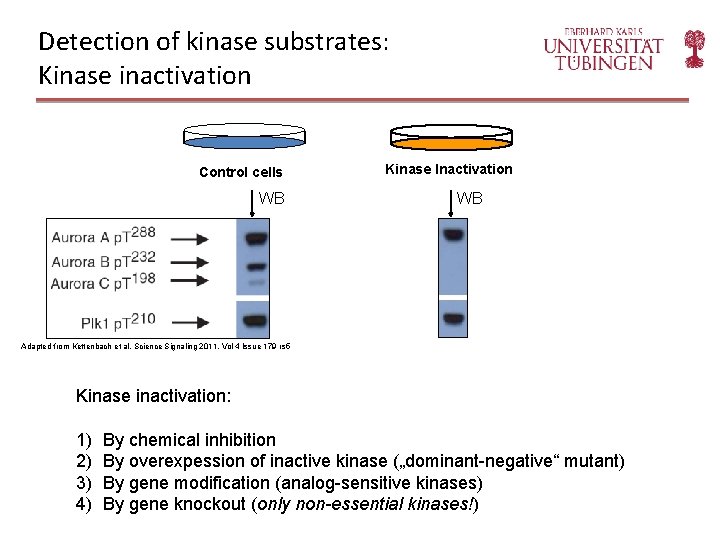
Detection of kinase substrates: Kinase inactivation Control cells WB Kinase Inactivation WB Adapted from Kettenbach et al. Science Signaling 2011, Vol 4 Issue 179 rs 5 Kinase inactivation: 1) 2) 3) 4) By chemical inhibition By overexpession of inactive kinase („dominant-negative“ mutant) By gene modification (analog-sensitive kinases) By gene knockout (only non-essential kinases!)

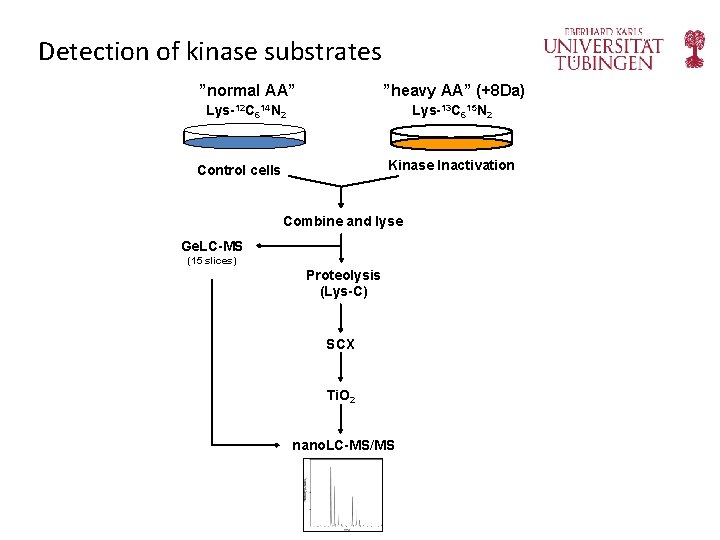
Detection of kinase substrates ”normal AA” ”heavy AA” (+8 Da) Lys-12 C 614 N 2 Lys-13 C 615 N 2 Kinase Inactivation Control cells Combine and lyse Ge. LC-MS (15 slices) Proteolysis (Lys-C) SCX Ti. O 2 nano. LC-MS/MS

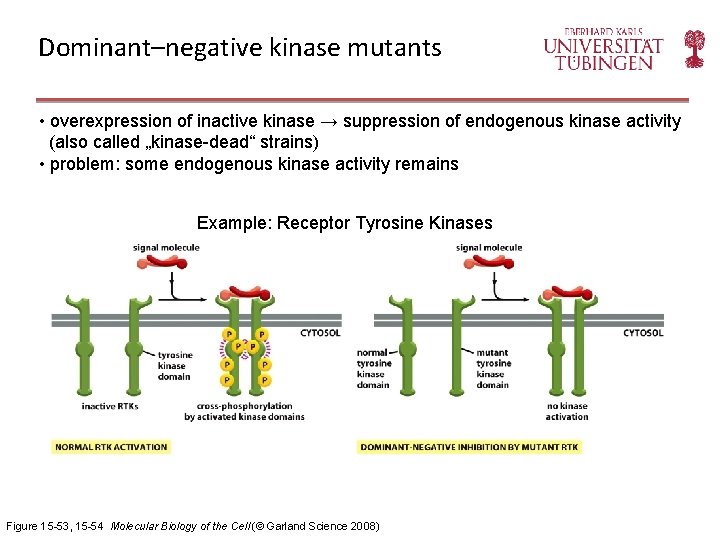
Dominant–negative kinase mutants • overexpression of inactive kinase → suppression of endogenous kinase activity (also called „kinase-dead“ strains) • problem: some endogenous kinase activity remains Example: Receptor Tyrosine Kinases Figure 15 -53, 15 -54 Molecular Biology of the Cell (© Garland Science 2008)

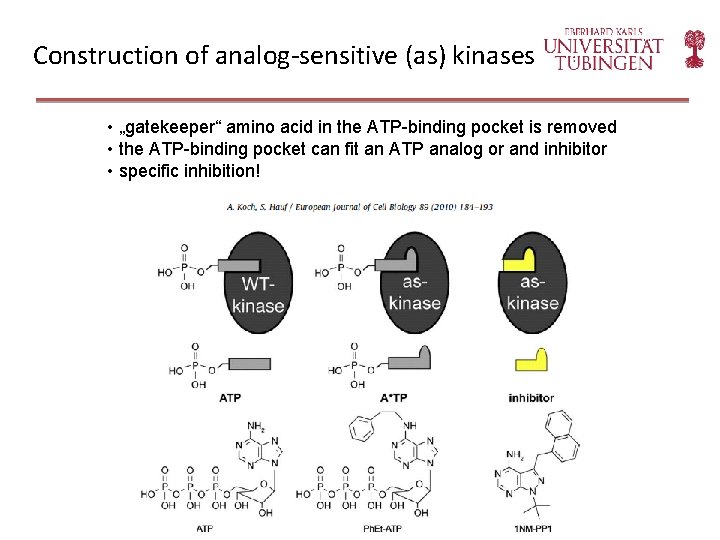
Construction of analog-sensitive (as) kinases • „gatekeeper“ amino acid in the ATP-binding pocket is removed • the ATP-binding pocket can fit an ATP analog or and inhibitor • specific inhibition!

Example 1: Use of analog-sensitive kinases Detection of Aurora kinase targets in S. pombe (with S. Hauf, FMI/MPI)

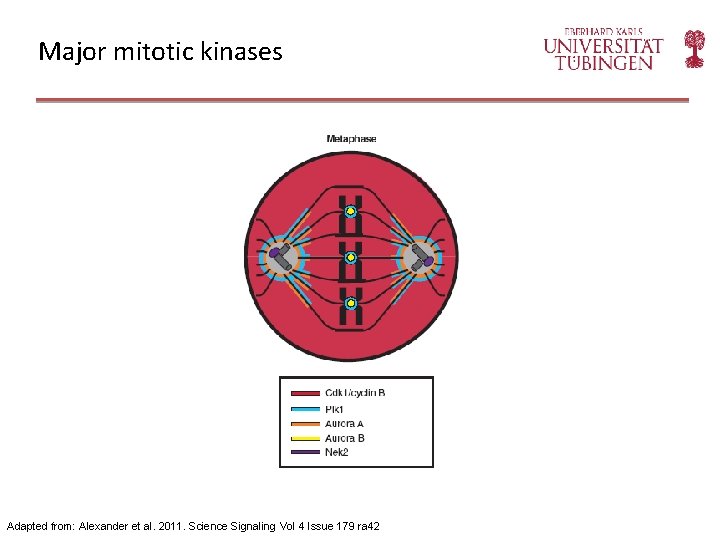
Major mitotic kinases Adapted from: Alexander et al. 2011. Science Signaling Vol 4 Issue 179 ra 42

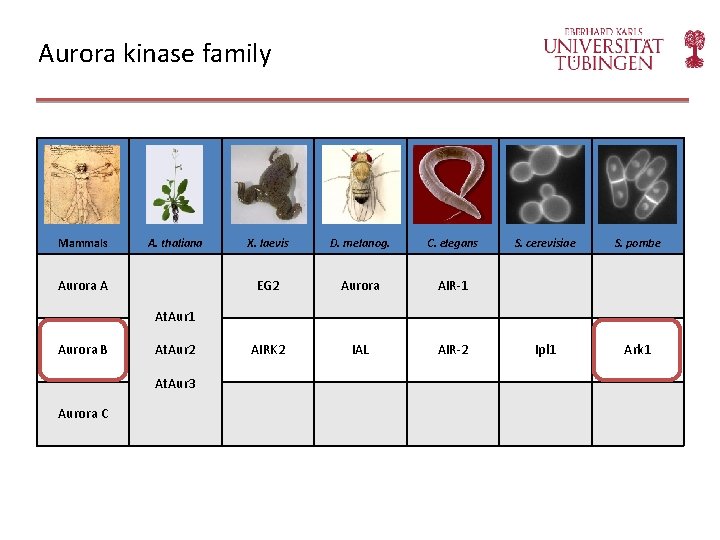
Aurora kinase family Mammals A. thaliana Aurora A X. laevis D. melanog. C. elegans EG 2 Aurora AIR-1 AIRK 2 IAL AIR-2 S. cerevisiae S. pombe Ipl 1 Ark 1 At. Aur 1 Aurora B At. Aur 2 At. Aur 3 Aurora C

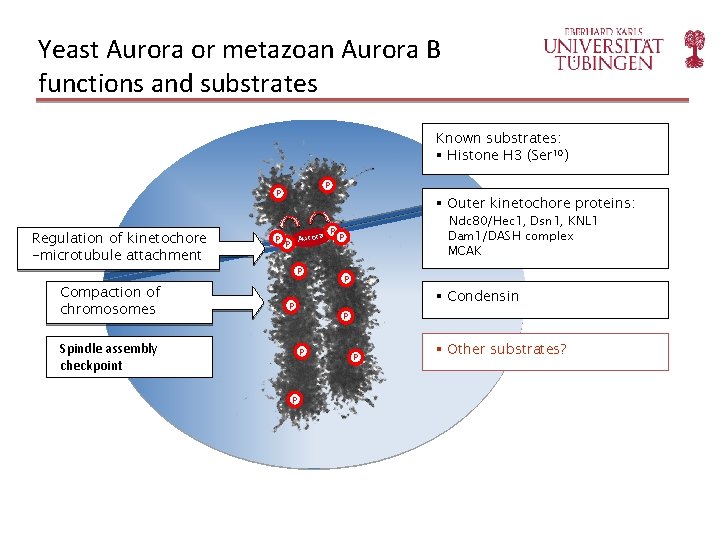
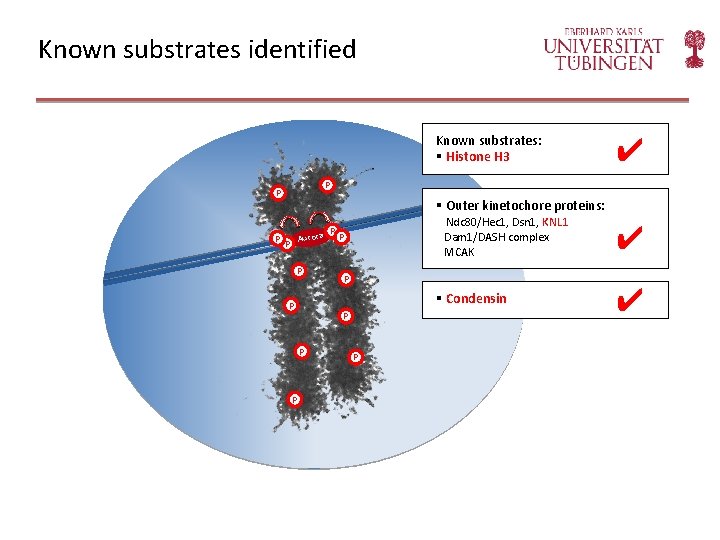
Yeast Aurora or metazoan Aurora B functions and substrates Known substrates: § Histone H 3 (Ser 10) P P Regulation of kinetochore -microtubule attachment Compaction of chromosomes P § Outer kinetochore proteins: Aurora P P P P Ndc 80/Hec 1, Dsn 1, KNL 1 Dam 1/DASH complex MCAK § Condensin P Spindle assembly checkpoint P P § Other substrates?

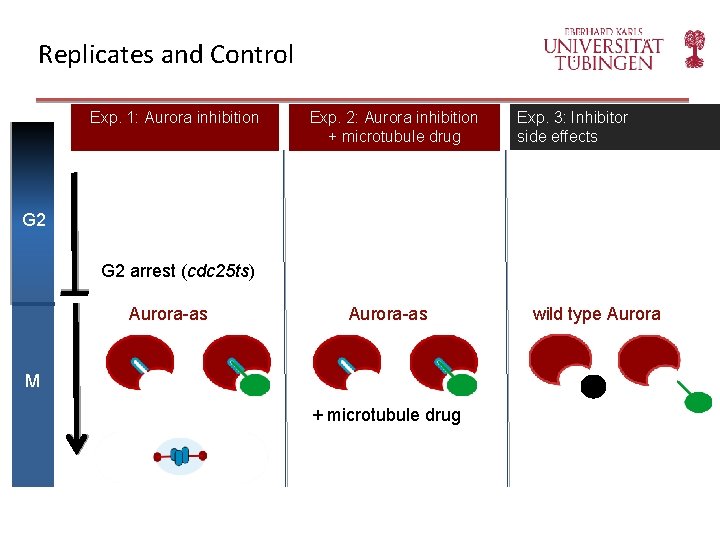
Replicates and Control Exp. 1: Aurora inhibition Exp. 2: Aurora inhibition + microtubule drug Exp. 3: Inhibitor side effects G 2 arrest (cdc 25 ts) Aurora-as M + microtubule drug wild type Aurora

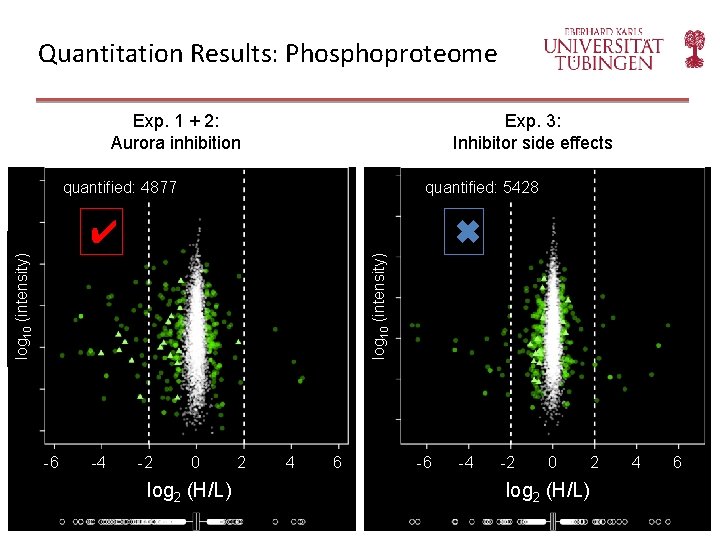
Quantitation Results: Phosphoproteome Exp. 1 + 2: Aurora inhibition Exp. 3: Inhibitor side effects quantified: 4877 quantified: 5428 log 10 (intensity) ✖ log 10 (intensity) ✔ -6 -4 -2 0 log 2 (H/L) 2 4 6 -6 -4 -2 0 2 log 2 (H/L) 4 6

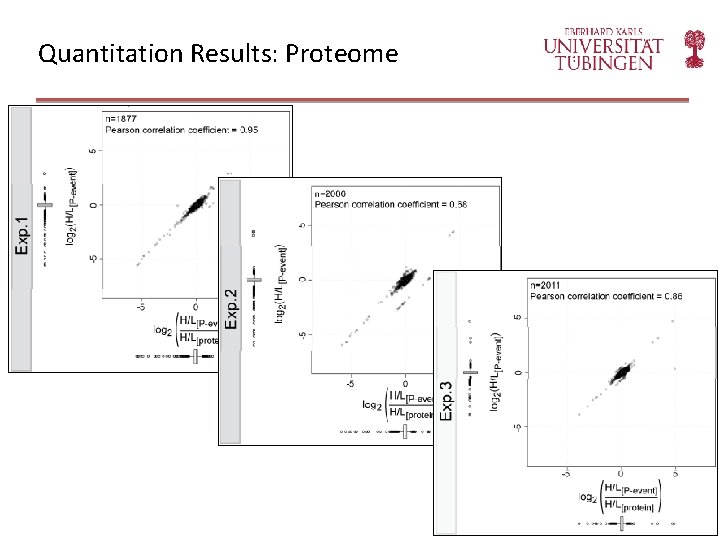
Quantitation Results: Proteome

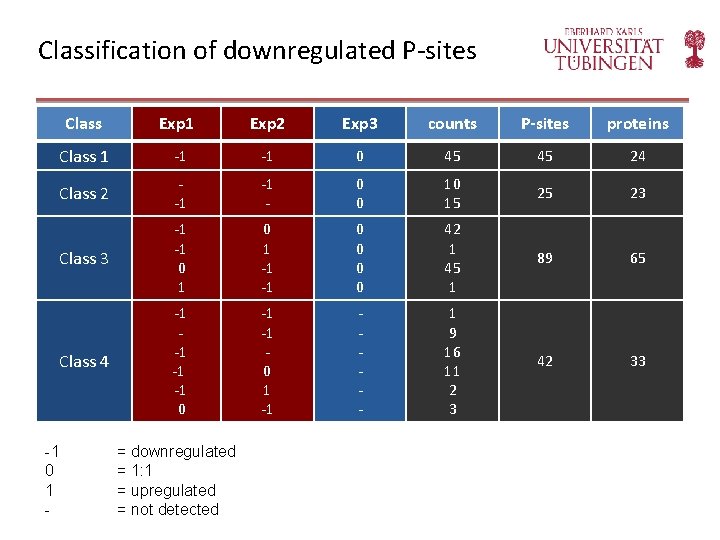
Classification of downregulated P-sites Class Exp 1 Exp 2 Exp 3 counts P-sites proteins Class 1 -1 -1 0 45 45 24 Class 2 -1 -1 - 0 0 10 15 25 23 Class 3 -1 -1 0 1 -1 -1 0 0 42 1 45 1 89 65 Class 4 -1 -1 0 1 -1 - 1 9 16 11 2 3 42 33 -1 0 1 - = downregulated = 1: 1 = upregulated = not detected

Known substrates identified Known substrates: § Histone H 3 P P P ✔ § Outer kinetochore proteins: Aurora P P Ndc 80/Hec 1, Dsn 1, KNL 1 Dam 1/DASH complex MCAK P P § Condensin P P P ✔ ✔
![Refinement of Aurora kinase target sequence Currently accepted target sequences RKXSTILV S serevisiae RKNRXSTILVM Refinement of Aurora kinase target sequence Currently accepted target sequences: [RK]-X-[ST]-[ILV] (S. serevisiae) [RKN]-R-X-[ST]-[ILVM]](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-29.jpg)
Refinement of Aurora kinase target sequence Currently accepted target sequences: [RK]-X-[ST]-[ILV] (S. serevisiae) [RKN]-R-X-[ST]-[ILVM] (human Aurora-A) R-X-[ST] R-K-R-X-[ST]

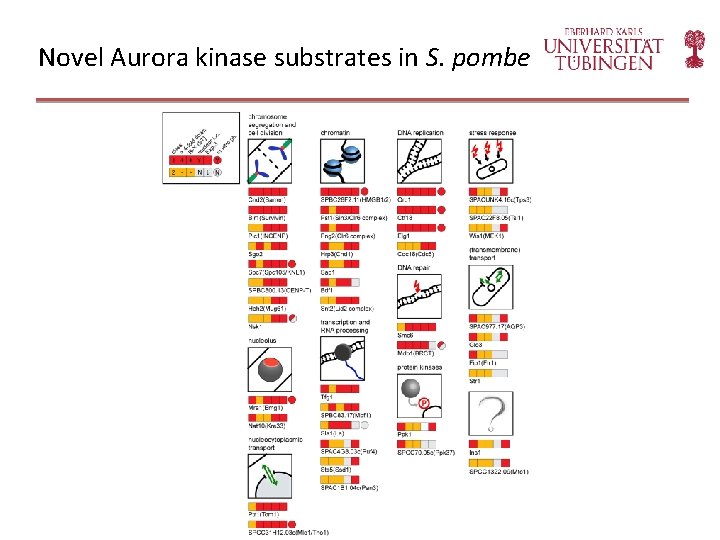
Novel Aurora kinase substrates in S. pombe

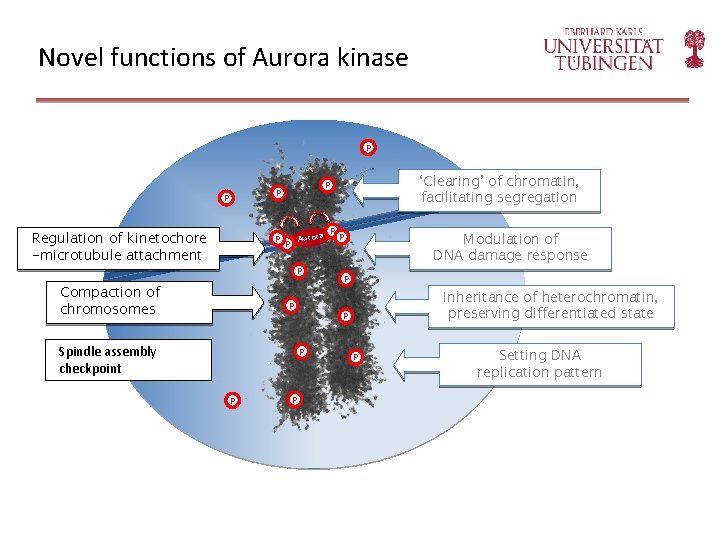
Novel functions of Aurora kinase P P Regulation of kinetochore -microtubule attachment P Aurora P P Compaction of chromosomes P Spindle assembly checkpoint P P Modulation of DNA damage response P P Inheritance of heterochromatin, preserving differentiated state P P P ‘Clearing’ of chromatin, facilitating segregation P P P Setting DNA replication pattern

Example 2: Use of dominant-negative kinase mutants Detection of PKD targets in HEK 293 cells (with A. Hausser, Uni Stuttgart)

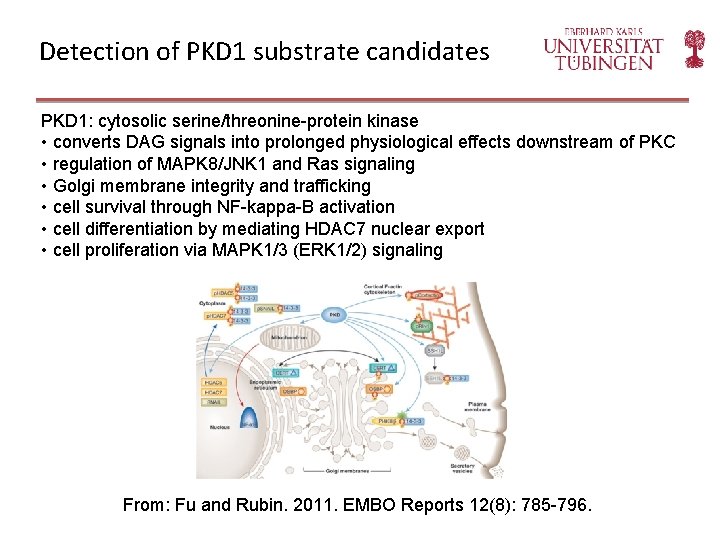
Detection of PKD 1 substrate candidates PKD 1: cytosolic serine/threonine-protein kinase • converts DAG signals into prolonged physiological effects downstream of PKC • regulation of MAPK 8/JNK 1 and Ras signaling • Golgi membrane integrity and trafficking • cell survival through NF-kappa-B activation • cell differentiation by mediating HDAC 7 nuclear export • cell proliferation via MAPK 1/3 (ERK 1/2) signaling From: Fu and Rubin. 2011. EMBO Reports 12(8): 785 -796.

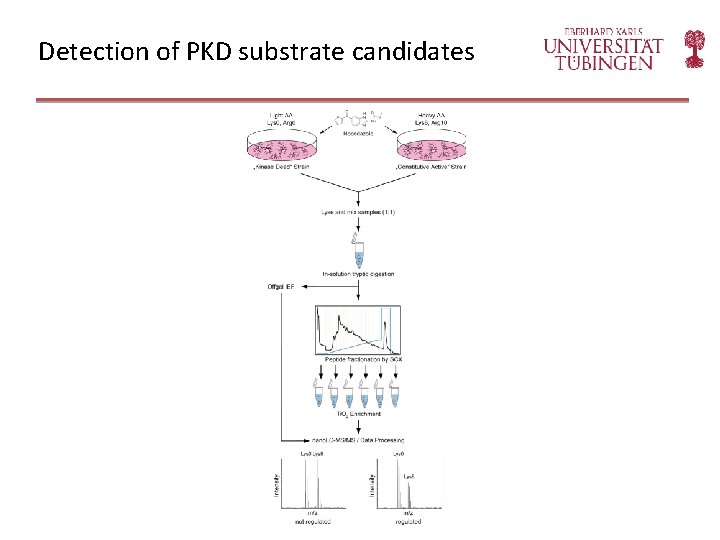
Detection of PKD substrate candidates

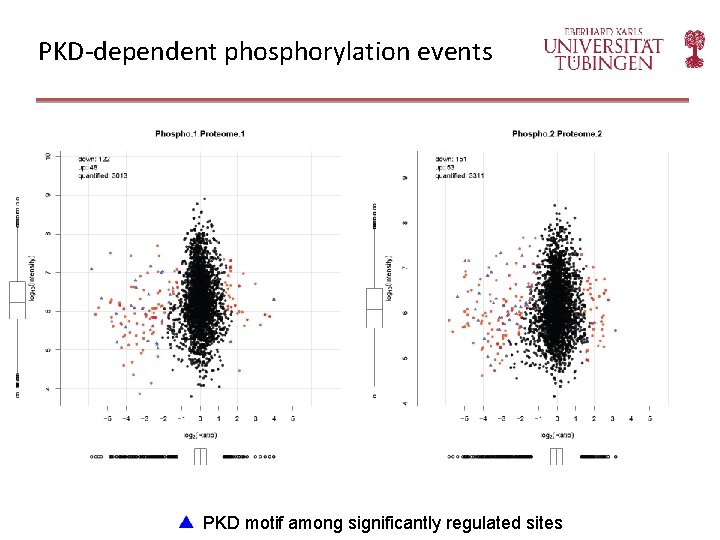
PKD-dependent phosphorylation events PKD motif among significantly regulated sites

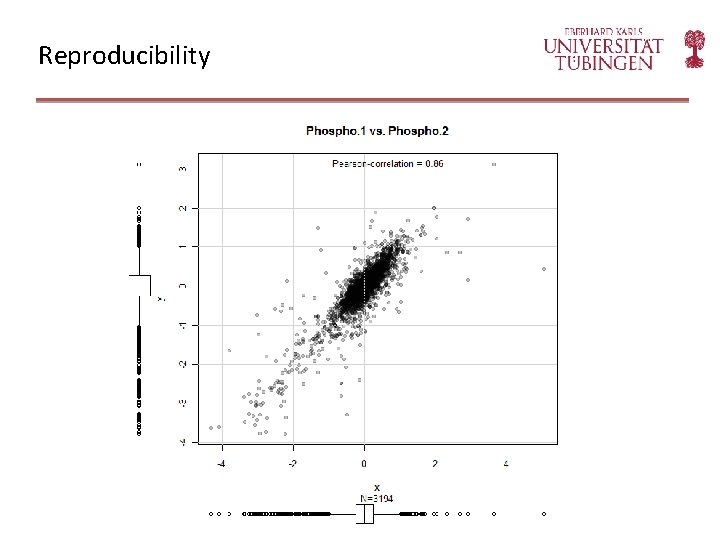
Reproducibility
![Kinase of interest must be active during experiment PKDcaPKDkdNoco parentalPKDkdNoco Kinase of interest must be active during experiment [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-37.jpg)
Kinase of interest must be active during experiment [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+
![Importance of normalization by protein ratio PKDcaPKDkdNoco parentalPKDkdNoco Overexpressed PKDkd Importance of normalization by protein ratio [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+ Overexpressed PKDkd](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-38.jpg)
Importance of normalization by protein ratio [PKDca/PKDkd]Noco+ [parental/PKDkd]Noco+ Overexpressed PKDkd

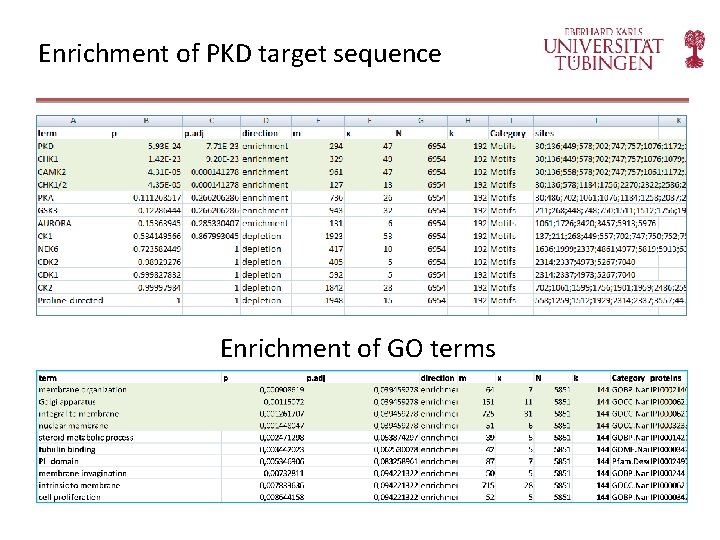
Enrichment of PKD target sequence Enrichment of GO terms
![Refinement of PKD target sequence Currently accepted target sequence LVIXRKXXST LVKKKLST Depletion of Pro Refinement of PKD target sequence Currently accepted target sequence: [LVI]-X-[RK]-X-X-[ST] [LV]-K-K-K-L-[ST] Depletion of Pro!](https://slidetodoc.com/presentation_image_h/10e9a902e7a257d48b6b5688c33db2b1/image-40.jpg)
Refinement of PKD target sequence Currently accepted target sequence: [LVI]-X-[RK]-X-X-[ST] [LV]-K-K-K-L-[ST] Depletion of Pro! X-X-K-X-X-[ST]

Acknowledgements Proteome Center Tuebingen Karsten Krug Mirita Franz-Wachtel Silke Wahl University of Stuttgart Angelika Hausser Stephan Eisler Friedrich Miescher Laboratory Silke Hauf Andre Koch Funding 41