Matter Matter Physical property is a property that







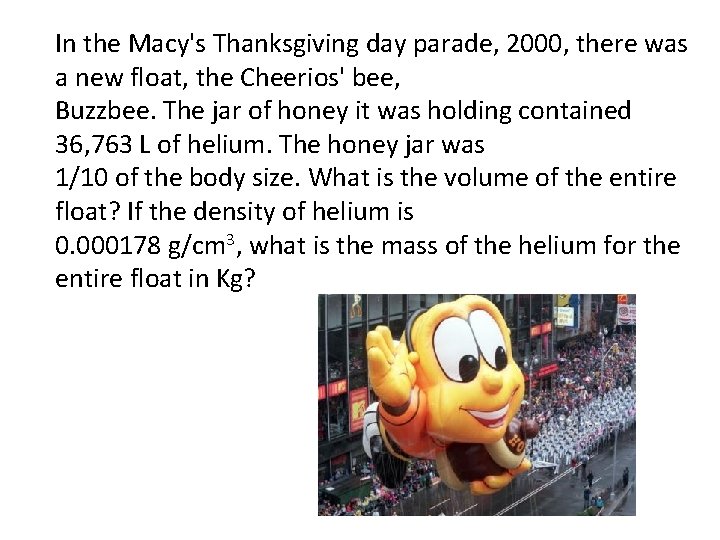
- Slides: 18

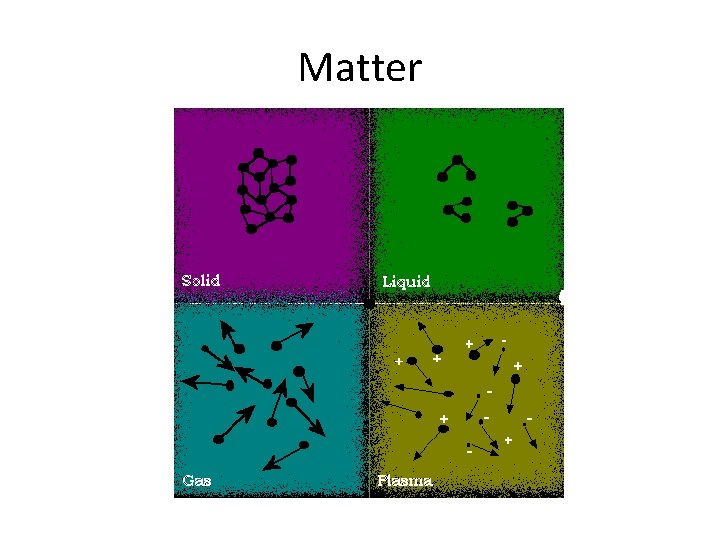
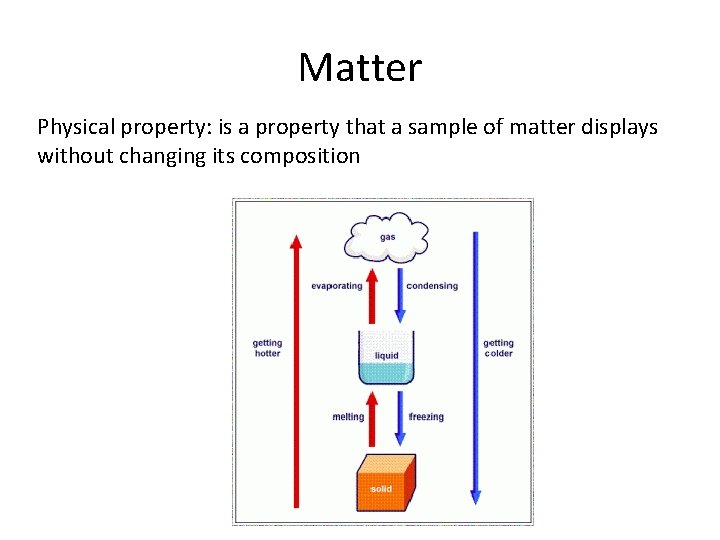
Matter

Matter Physical property: is a property that a sample of matter displays without changing its composition

Matter • Physical change: some of the physical properties of a sample of matter may change but its composition remains unchanged

Physical properties • Qualitative 1. Smell 2. Taste 3. Color 4. Luster(is it shiny? ) 5. Clarity(is it cloudy? ) • Quantitative 1. Boiling point 2. Melting point 3. Vapor pressure 4. Density 5. Specific heat 6. Solubility 7. Elasticity 8. viscosity

Burning steel wool Fe + O 2 Fe 2 O 3 Chemical properties of matter describes its "potential" to undergo some chemical change or reaction by virtue of its composition. What elements, electrons, and bonding are present to give the potential for chemical change. A Chemical change alters the composition of the original matter. Different elements or compounds are present at the end of the chemical change. The atoms in compounds are rearranged to make new and different compounds.

State whether each property is physical or chemical a. Iron nail is attracted to a magnet b. Charcoal lighter fluid is ignited with a match c. A bronze statue develops a green coating (patina) over time d. A block of wood floats on water

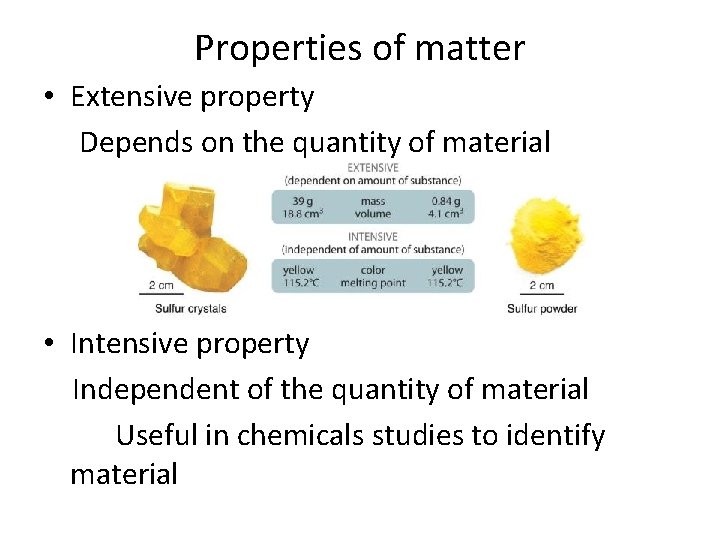
Properties of matter • Extensive property Depends on the quantity of material • Intensive property Independent of the quantity of material Useful in chemicals studies to identify material


Indicate which are extensive and which are intensive quantities a. The mass of air in a balloon b. Temperature of an ice cube c. The time required to boil a sample of water d. The color of light given off by a neon lamp

Intensive vs Extensive • Intensive properties serve as conversions • Can be used to identify a substance Use as a conversion factor from one extensive measurement to another Example mass of substance extensive density of substance intensive Mass/volume=density intensive-physical property

Percent • Per 100 • Seawater 3. 5 % sodium chloride by mass • Write the conversion factors for this %.

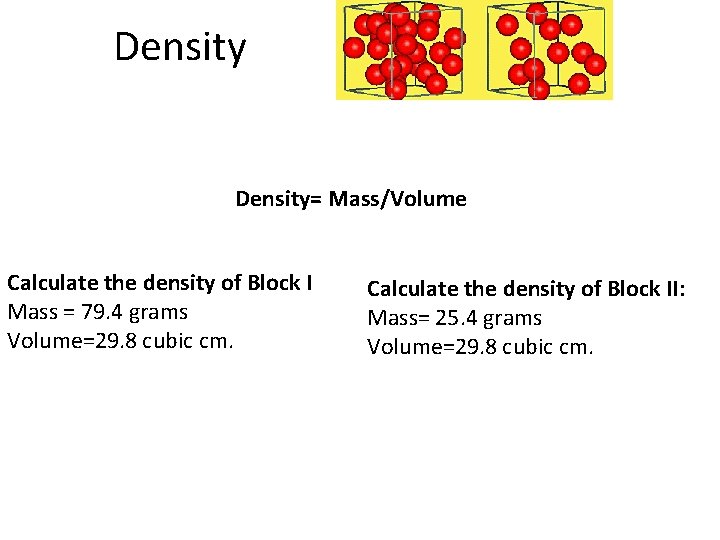
Density= Mass/Volume Calculate the density of Block I Mass = 79. 4 grams Volume=29. 8 cubic cm. Calculate the density of Block II: Mass= 25. 4 grams Volume=29. 8 cubic cm.

Density as a conversion factor • The gasoline in an automobile gas tank has a mass of 60. 0 kg and a density of 0. 752 g/cm 3. What is the volume?

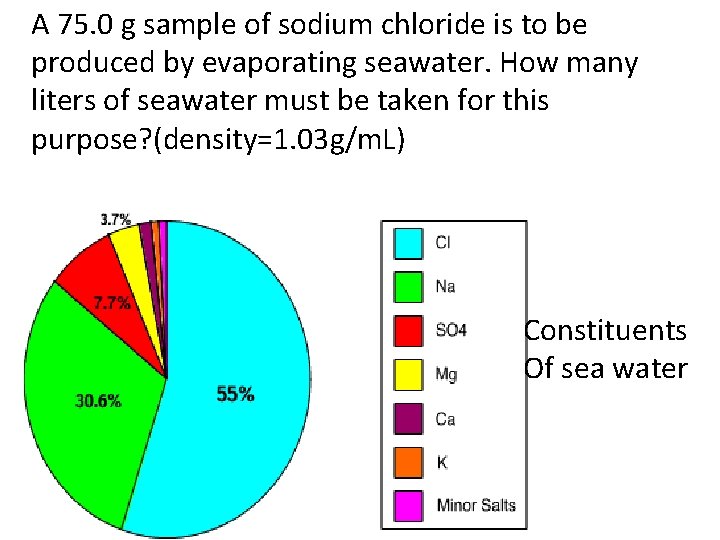
A 75. 0 g sample of sodium chloride is to be produced by evaporating seawater. How many liters of seawater must be taken for this purpose? (density=1. 03 g/m. L) Constituents Of sea water

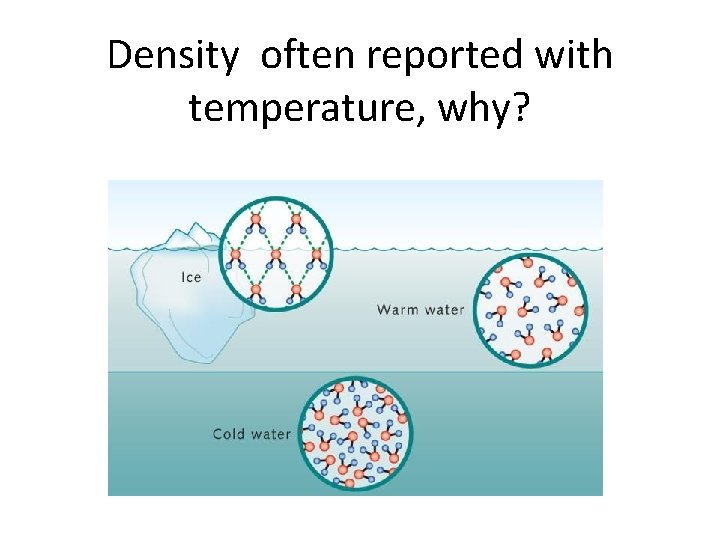
Density often reported with temperature, why?

Ca. Cl 2 is used as a de-icer on roads in the winter. It has a density of 2. 50 g/cm 3. What is the mass of 15. 0 L this substance?
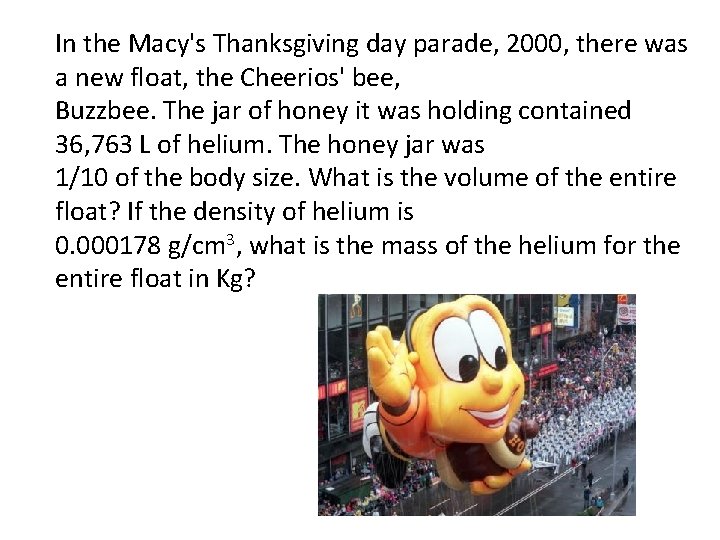
In the Macy's Thanksgiving day parade, 2000, there was a new float, the Cheerios' bee, Buzzbee. The jar of honey it was holding contained 36, 763 L of helium. The honey jar was 1/10 of the body size. What is the volume of the entire float? If the density of helium is 0. 000178 g/cm 3, what is the mass of the helium for the entire float in Kg?

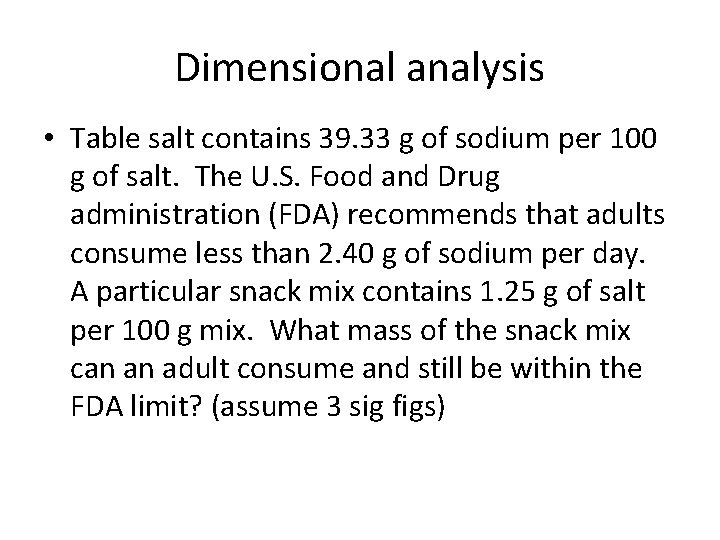
Dimensional analysis • Table salt contains 39. 33 g of sodium per 100 g of salt. The U. S. Food and Drug administration (FDA) recommends that adults consume less than 2. 40 g of sodium per day. A particular snack mix contains 1. 25 g of salt per 100 g mix. What mass of the snack mix can an adult consume and still be within the FDA limit? (assume 3 sig figs)