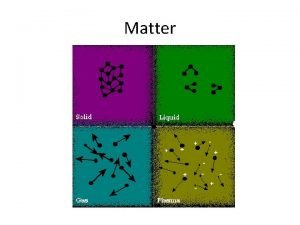
Physical Properties of Matter A physical property is









- Slides: 19


Physical Properties of Matter • A physical property is a characteristic of a pure substance that can be observed without changing it into another substance -Physical state -Temperature for freezing, boiling, or vaporizing -Ability to dissolve in water -Hardness, texture, color, and flexibility • Can be used to classify matter (ex. Metals are flexible and can conduct heat and electricity)

Chemical Properties of Matter • Chemical properties are characteristics of a pure substance that describe its ability to change into different substances • -Properties can be used to classify substances • -Examples: Ability to burn and/or react with other substances § Only observed when the original substance is changed into a different substance • -New substances may have different properties then the original substances

Changes in Matter • Matter can undergo two basic types of changes- physical changes and chemical changes • Physical changes- alters the form or appearance of matter but does not form any new substances • Chemical changes- changes in matter that produce one or more new substances

Physical Changes of Matter • A substance that undergoes a physical change is still the same substance after the change • A change in state, such as from a solid to a liquid or from a liquid to a gas, is an example of a physical change

Chemical Changes in Matter • A substance undergoing a chemical change is transformed into a different substance • In some chemical changes, a single substance changes into one or more other substances (ex. When hydrogen peroxide is poured on a cut it breaks down into water and oxygen gas) • In other chemical changes, two or more substances combine to form different substances (ex. Iron oxide forms when iron combines with oxygen in the air) • Unlike a physical change, a chemical change produces new substances with properties different from those of the original substances

Evidence of Chemical Change • Changed in energy and changes in properties are both signs that a chemical reaction has occurred • Observable changes can indicate a chemical change has occurred • Color Change • May signal that a new substance has formed in an otherwise unchanged object (ex. Bread is browned when toasted) • Gas production • Gases can be produced when solid or liquid reactants chemically combine (ex. Vinegar & baking soda produce carbon dioxide bubbles) • Formation of a precipitate • Mixing two liquids can produce a precipitate, which is a solid that forms from the liquids (ex. Mixing milk & lemon juice forms a chunky precipitate called curds)

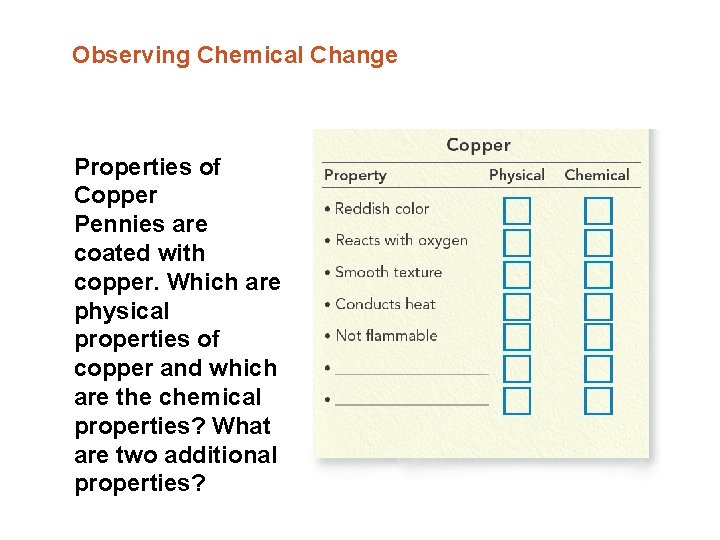
Observing Chemical Change Properties of Copper Pennies are coated with copper. Which are physical properties of copper and which are the chemical properties? What are two additional properties?

Chemical Reactions • A Chemical reaction- (also called a chemical change) is a change that produces one or more new substance • Occur when existing bonds break and new bonds form • Atoms and molecules rearrange to form new substances

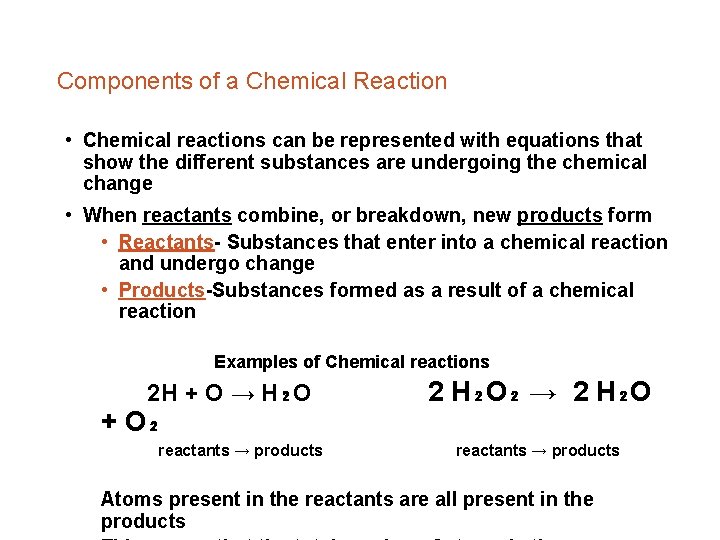
Components of a Chemical Reaction • Chemical reactions can be represented with equations that show the different substances are undergoing the chemical change • When reactants combine, or breakdown, new products form • Reactants- Substances that enter into a chemical reaction and undergo change • Products-Substances formed as a result of a chemical reaction Examples of Chemical reactions 2 H + O → H₂O + O₂ reactants → products 2 H₂O₂ → 2 H₂O reactants → products Atoms present in the reactants are all present in the products

Getting Chemical Reactions Started • Reactants need a initial push to get going • Activation energy – The minimum amount of energy needed to start a chemical reaction • Once the reaction is underway, it will either need more energy to keep going or it will release energy

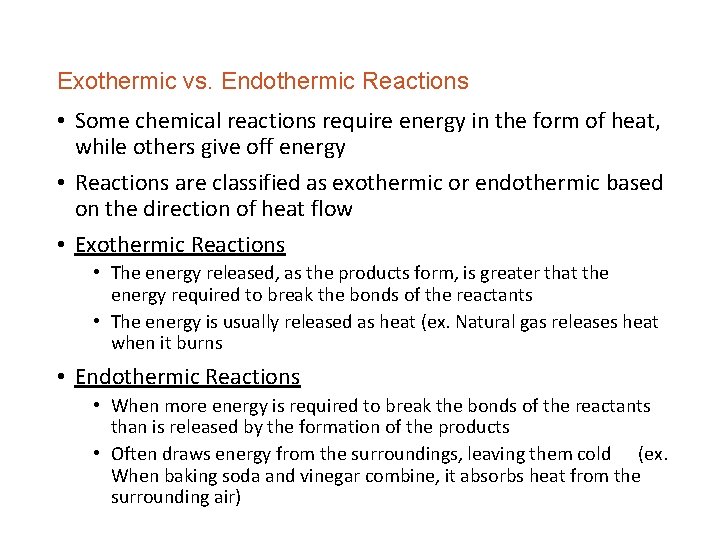
Exothermic vs. Endothermic Reactions • Some chemical reactions require energy in the form of heat, while others give off energy • Reactions are classified as exothermic or endothermic based on the direction of heat flow • Exothermic Reactions • The energy released, as the products form, is greater that the energy required to break the bonds of the reactants • The energy is usually released as heat (ex. Natural gas releases heat when it burns • Endothermic Reactions • When more energy is required to break the bonds of the reactants than is released by the formation of the products • Often draws energy from the surroundings, leaving them cold (ex. When baking soda and vinegar combine, it absorbs heat from the surrounding air)

Rates of Chemical Reactions • Chemical reactions don’t all occur at the same rate • Reactions rates depend of condition such as surface area, temperature, concentration, and the presence of catalysts or inhibitors • Surface Area- Increasing the surface area of a substance will speed up reaction times • Temperature- Warm substances have faster reaction rates due to the particles having more initial energy • Concentration- A measure of the amount of each substance in the reaction • Reaction rates increase when there are higher concentrations, as there are more particles to react

Rates of Chemical Reactions Continued • Catalysts • Substances that cause or accelerate a chemical reaction without being affected themselves • Increase reaction rates by lowering the activation energy needed to start a reaction • Inhibitors • Materials used to decrease the rate of chemical reactions (ex. Preservatives)

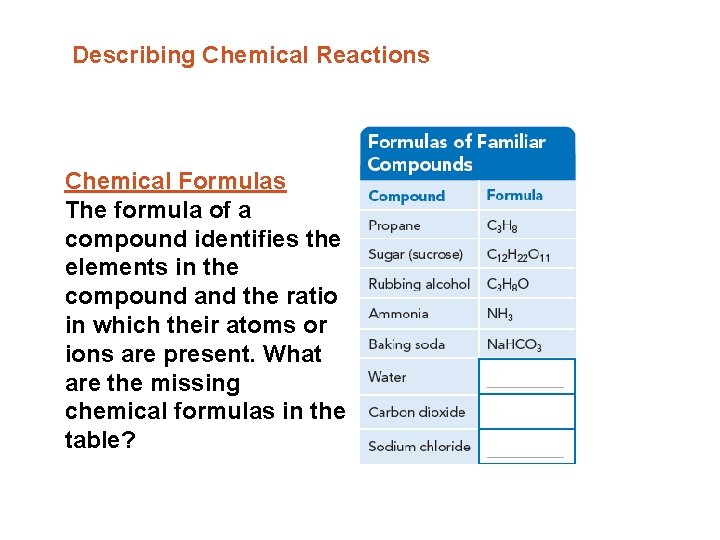
Describing Chemical Reactions Chemical Formulas The formula of a compound identifies the elements in the compound and the ratio in which their atoms or ions are present. What are the missing chemical formulas in the table?

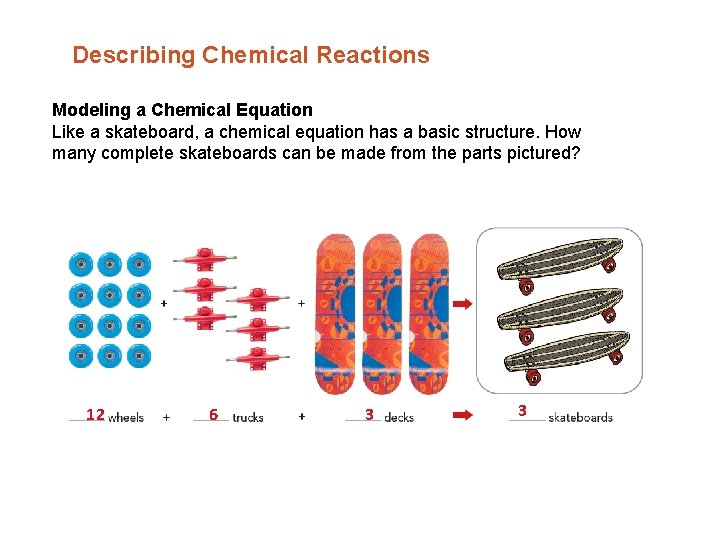
Describing Chemical Reactions Modeling a Chemical Equation Like a skateboard, a chemical equation has a basic structure. How many complete skateboards can be made from the parts pictured? 12 6 3 3

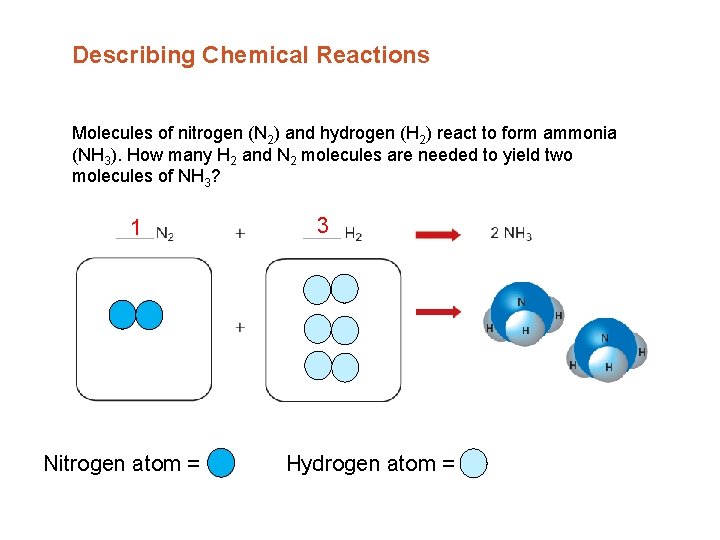
Describing Chemical Reactions Molecules of nitrogen (N 2) and hydrogen (H 2) react to form ammonia (NH 3). How many H 2 and N 2 molecules are needed to yield two molecules of NH 3? 1 Nitrogen atom = 3 Hydrogen atom =

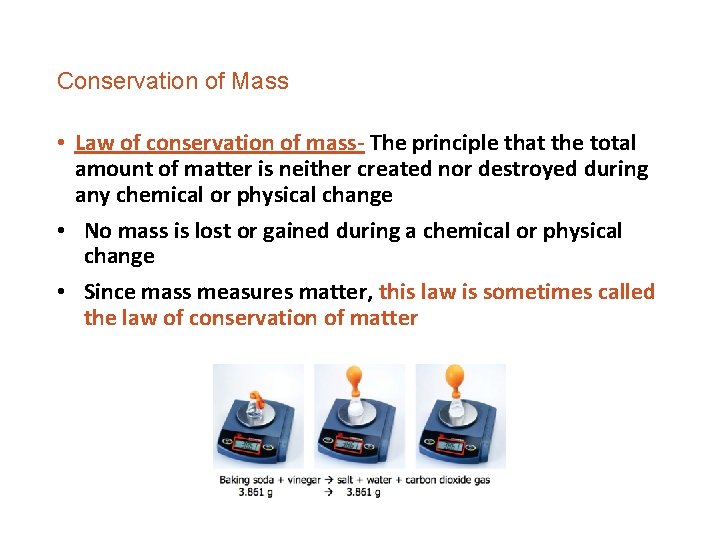
Conservation of Mass • Law of conservation of mass- The principle that the total amount of matter is neither created nor destroyed during any chemical or physical change • No mass is lost or gained during a chemical or physical change • Since mass measures matter, this law is sometimes called the law of conservation of matter

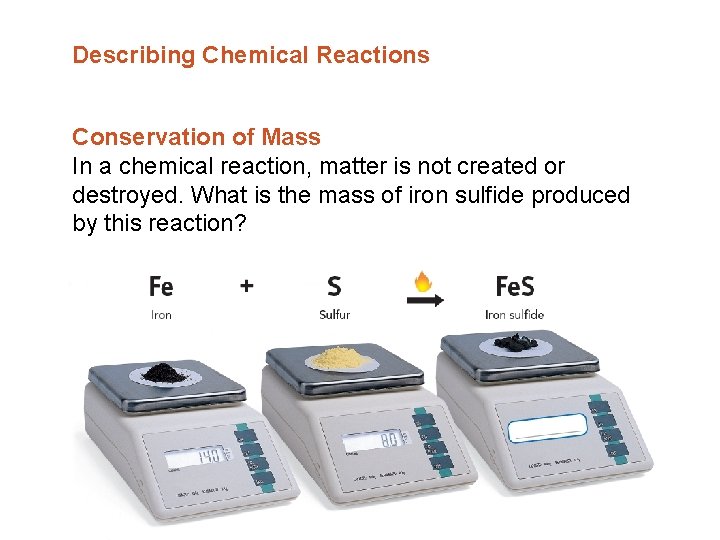
Describing Chemical Reactions Conservation of Mass In a chemical reaction, matter is not created or destroyed. What is the mass of iron sulfide produced by this reaction?
 Physical properties and chemical properties
Physical properties and chemical properties Physical properties of matter jeopardy
Physical properties of matter jeopardy Graphic organizer matter
Graphic organizer matter Chemical properties of notebook paper
Chemical properties of notebook paper Commutative vs associative
Commutative vs associative Obstructed and unobstructed heritage
Obstructed and unobstructed heritage General property of matter
General property of matter General property of matter?
General property of matter? Are thermal and electrical conductivity related
Are thermal and electrical conductivity related What property of matter that describes a rusty anchor.
What property of matter that describes a rusty anchor. F=kx
F=kx Strength property of matter
Strength property of matter Helium physical and chemical properties
Helium physical and chemical properties Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is white matter made of
What is white matter made of Section 1 composition of matter
Section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Cerebral aqueduct
Cerebral aqueduct Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter