PHYSICAL vs CHEMICAL CHANGE PHYSICAL PROPERTY A PROPERTY













- Slides: 15

PHYSICAL vs CHEMICAL CHANGE

PHYSICAL PROPERTY - A PROPERTY is the same as a characteristic. - Anything that you can see from direct observation. - The Physical Property of a substance includes: - Shape - Size - Color - Texture - Density



DESCRIBE THE PHYSICAL PROPERTIES OF A BASKETBALL: - Spherical - Orange - Bounces - Small bumps on surface - Black Lines & Writing

PHYSICAL CHANGE - Does not change the type of matter. - Nothing new or different is formed. - Could be a change in: - Mass - Volume - Density - Different state of matter

EXAMPLES OF PHYSICAL CHANGE: - Crushing an aluminum can. - Tearing a sheet of paper. - Painting a wall a different color. - Cutting a steak.

MAKING A MIXTURE: -2 or more types of matter (substances) mixed together. - Not in specific amounts. - Can be separated physically.

SEPARATING A MIXTURE

CHEMICAL PROPERTY - How a substance reacts with another substance. - Examples: - When water (oxygen) is added to a nail, it rusts. - When baking soda & vinegar are mixed, it forms bubbles.

CHEMICAL CHANGE -When a substance (chemical) changes into a new substance, having different physical & chemical properties.


- During a chemical change, atoms are re-arranged; atoms are not created or destroyed. - LAW OF CONSERVATION OF MATTER: - Matter is conserved; the type & amount of matter cannot change.

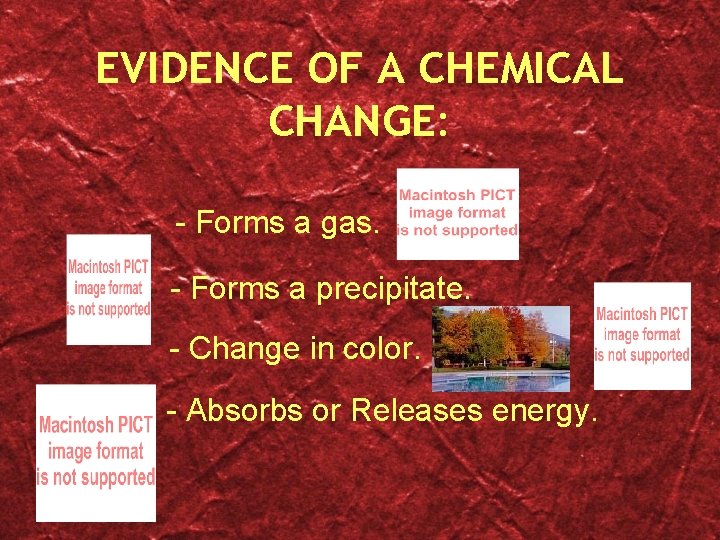
EVIDENCE OF A CHEMICAL CHANGE: - Forms a gas. - Forms a precipitate. - Change in color. - Absorbs or Releases energy.

QUIZ: Physical or Chemical change? 1. A piece of paper gets wet? physical 2. Two chemicals are mixed & a purple gas appears? chemical 3. You add green food coloring to a pancake batter? physical 4. Water freezes and becomes ice? physical 5. Batter placed in oven makes a cake? chemical
 Chemical property definition
Chemical property definition Examples of physical changes
Examples of physical changes Chemical change definition
Chemical change definition Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change Physical change and chemical change
Physical change and chemical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats physical change
Whats physical change Physical change
Physical change When does a physical change occur study jams
When does a physical change occur study jams Chopping wood physical or chemical
Chopping wood physical or chemical Blue color physical or chemical property
Blue color physical or chemical property Qualitative and quantitative physical properties
Qualitative and quantitative physical properties Is compressibility a physical or chemical property
Is compressibility a physical or chemical property Undesirable change
Undesirable change Is the halloween clock reaction a chemical change
Is the halloween clock reaction a chemical change Is aluminum foil cut in half a physical or chemical change
Is aluminum foil cut in half a physical or chemical change