2 Physical Properties vs Physical Changes Physical property









- Slides: 18


2 Physical Properties vs Physical Changes • Physical property of a substance is a trait of the substance that can be observed OR can be measured by tools without changing the identity • Physical Change is a change in matter from one form to another but does not change identity

3 Chemical Properties vs Chemical Changes • Chemical property is a description of the ability of a substance to undergo, or not undergo, a change that will alter the composition of the original substance • Chemical change- occurs when there is a change in the arrangement of the atoms involved so a different substance with different properties is produced

The Phases of Matter

As a review • Matter is anything that has mass and volume. ▫ How do we measure them? ▫ Tools? ▫ Units? • The 2 types of matter that we have already talked about are: ▫ Pure substances ▫ Mixtures


Phases of Matter • There are four different states or phases of matter that we as humans associate with. ▫ ▫ Solid Liquid Gas Plasma • Each of the states have characteristics that define them as that particular state.

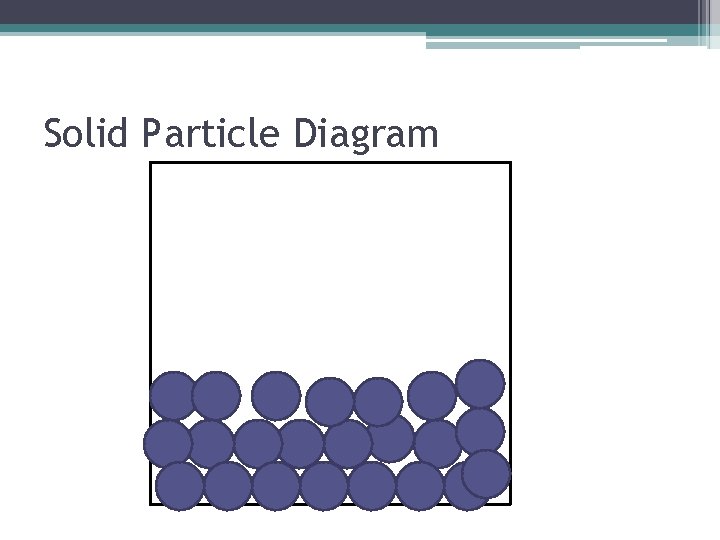
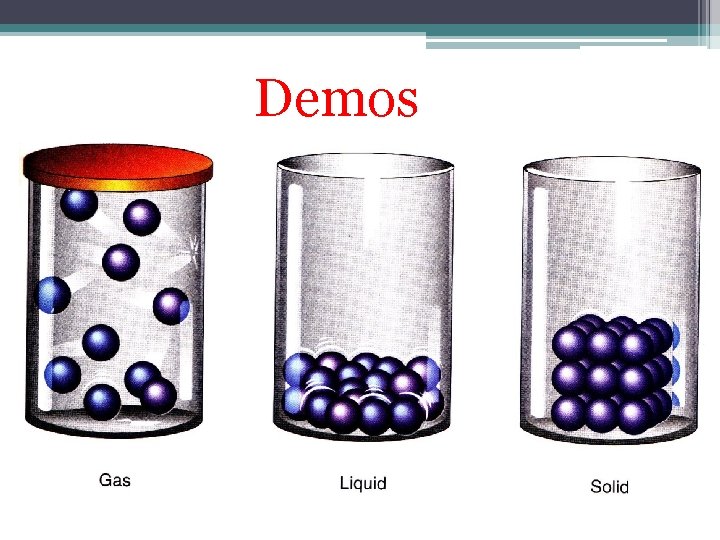
Characteristics of Solids • The particles are constantly vibrating, but they do not slip past one another • Because the particles do not slip past one another, a solid has a DEFINITE volume and shape. • Particles are closely packed together because there is an attractive force holding them together.

Solid Particle Diagram

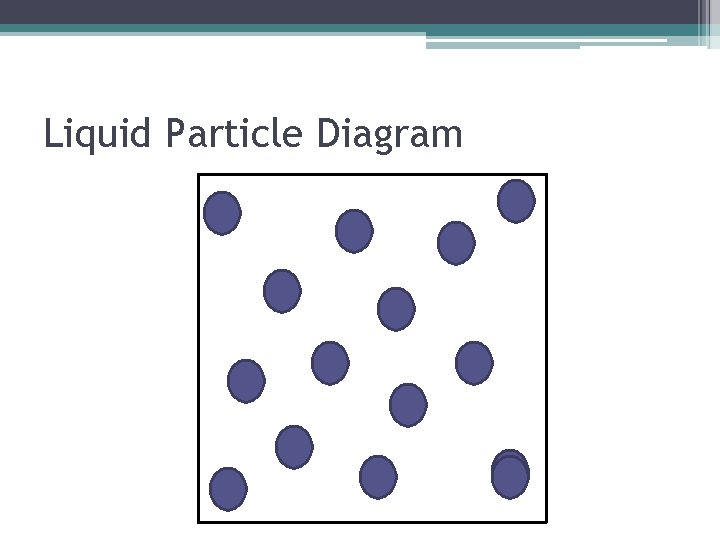
Characteristics of Liquids • The particles can slip and slide past one another. • Because the particles can slip past one another, liquids DO NOT have a definite shape by themselves, but they do take the shape and volume of their container. • The particles are touching one another due to an attractive force holding them together. • The slipping allows the liquids to be poured.

Liquid Particle Diagram

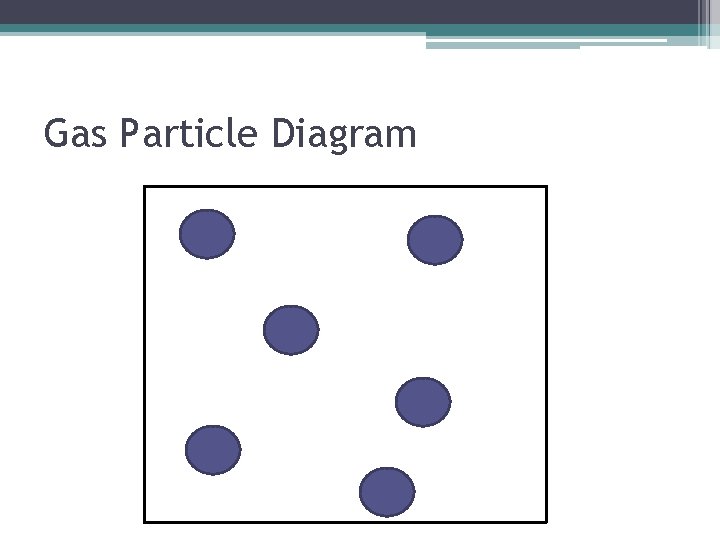
Characteristics of Gases • The particles are moving in random directions. When particles collide, they bounce off one another and continue moving. • Gases do not have a definite shape or volume and fill the entire area of the container • The particles of gasses are not in contact with one another, because they can overcome the attractive forces.

Gas Particle Diagram

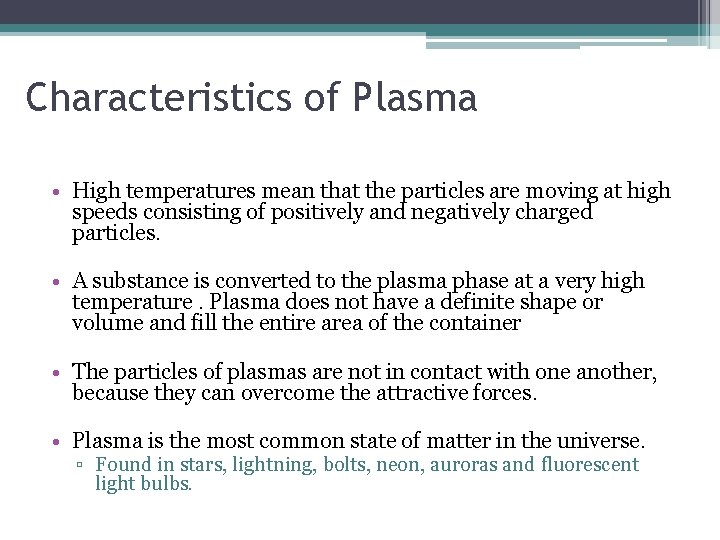
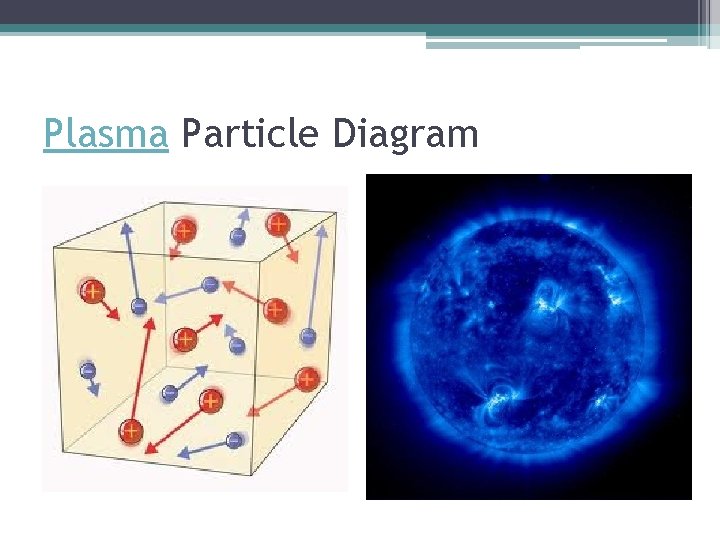
Characteristics of Plasma • High temperatures mean that the particles are moving at high speeds consisting of positively and negatively charged particles. • A substance is converted to the plasma phase at a very high temperature. Plasma does not have a definite shape or volume and fill the entire area of the container • The particles of plasmas are not in contact with one another, because they can overcome the attractive forces. • Plasma is the most common state of matter in the universe. ▫ Found in stars, lightning, bolts, neon, auroras and fluorescent light bulbs.

Plasma Particle Diagram

Demos


“Molecules on the move” activity • Partner up! • 1. What Phase is the matter starting with and ending with. ▫ Ex : Solid to liquid, Gas to Liquid • 2. Is heat being added or removed • 3. Tell me are the Molecules…. ▫ Speeding up (increasing energy) ▫ Slowing down (decreasing energy)