Physical Chemical Properties Changes 1 A physical property




- Slides: 12

Physical & Chemical Properties & Changes

1. A physical property can be observed or measured ________ without changing the matter’s identity. • Examples of physical properties: - color - odor -magnetism

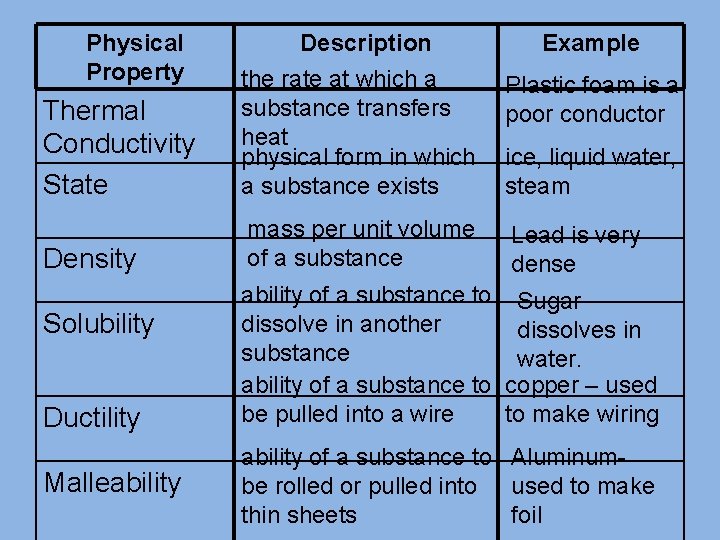
Physical Property Thermal Conductivity State Description the rate at which a substance transfers heat physical form in which a substance exists Density mass per unit volume of a substance Solubility Ductility Malleability Example Plastic foam is a poor conductor ice, liquid water, steam Lead is very dense ability of a substance to Sugar dissolve in another dissolves in substance water. ability of a substance to copper – used be pulled into a wire to make wiring ability of a substance to Aluminumbe rolled or pulled into used to make thin sheets foil

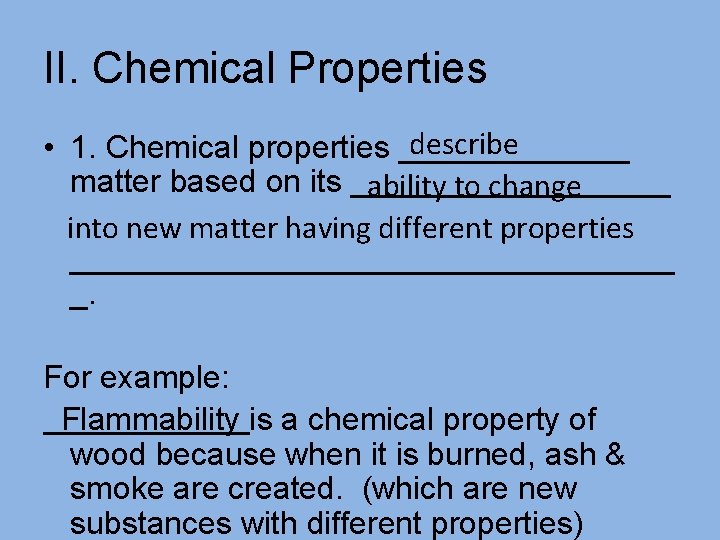
II. Chemical Properties describe • 1. Chemical properties _______ matter based on its _________ ability to change into new matter having different properties _________________ _. For example: Flammability is a chemical property of wood because when it is burned, ash & smoke are created. (which are new substances with different properties)

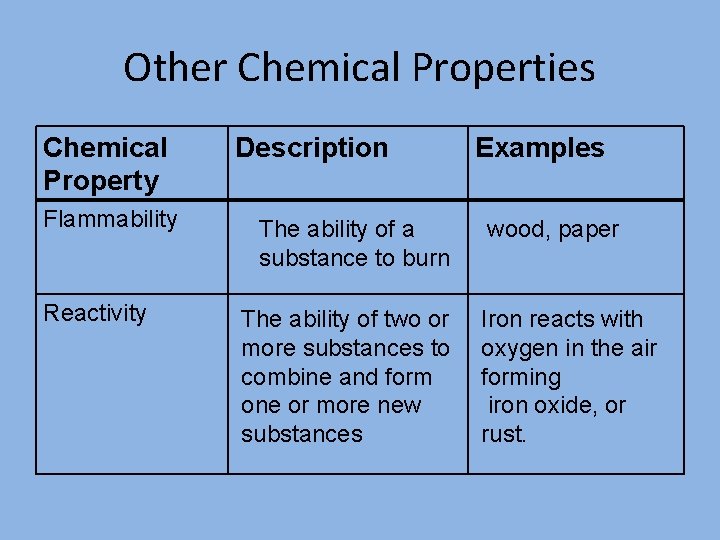
Other Chemical Properties Chemical Property Flammability Reactivity Description The ability of a substance to burn The ability of two or more substances to combine and form one or more new substances Examples wood, paper Iron reacts with oxygen in the air forming iron oxide, or rust.

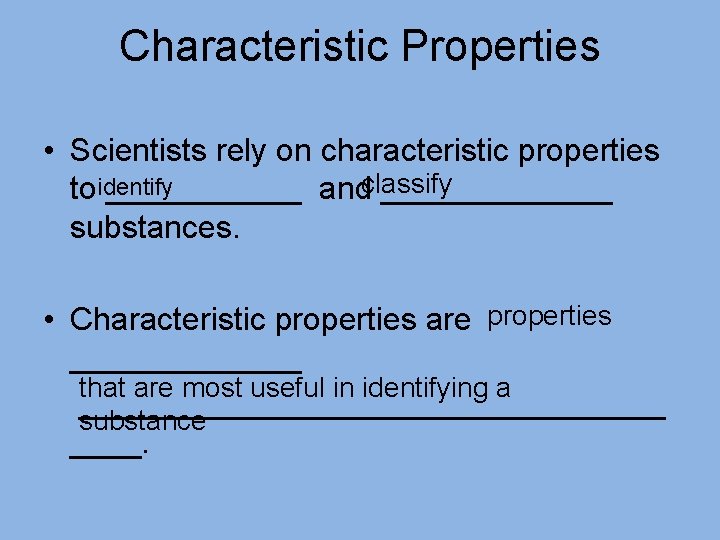
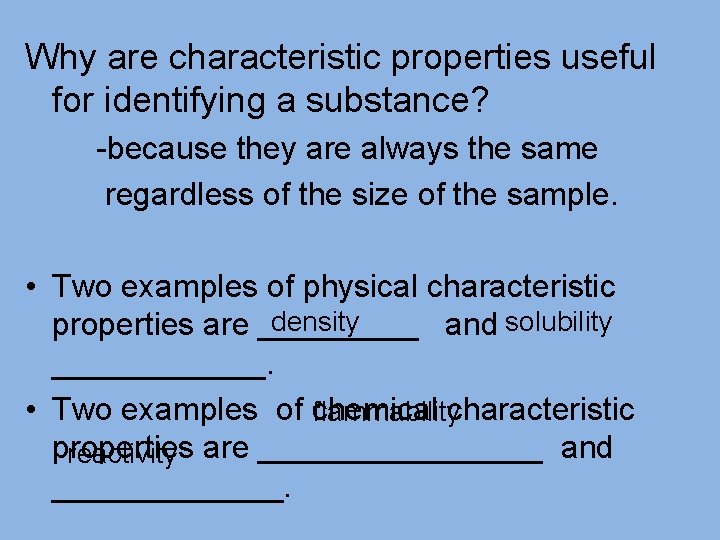
Characteristic Properties • Scientists rely on characteristic properties toidentify ______ andclassify _______ substances. • Characteristic properties are properties _______ that are most useful in identifying a _________________ substance ____.

Why are characteristic properties useful for identifying a substance? -because they are always the same regardless of the size of the sample. • Two examples of physical characteristic density properties are _____ and solubility ______. • Two examples of chemical characteristic flammability properties reactivity are ________ and _______.

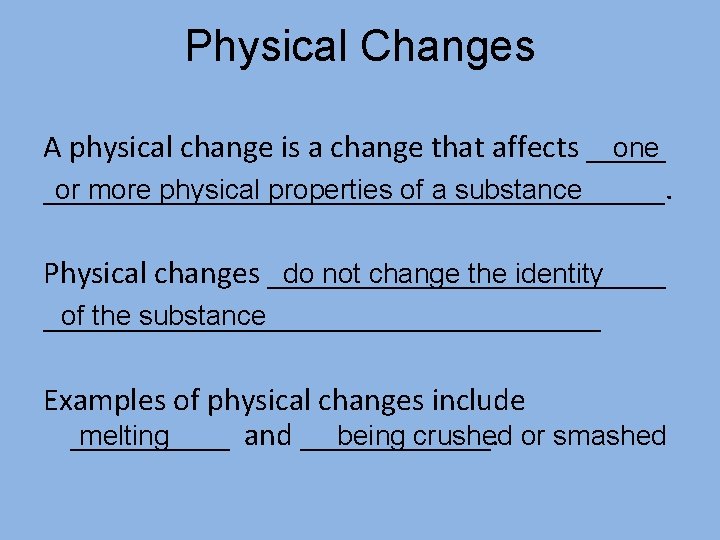
Physical Changes A physical change is a change that affects _____ one ____________________. or more physical properties of a substance do not change the identity Physical changes _____________ of the substance __________________ Examples of physical changes include _____ and ______. melting being crushed or smashed

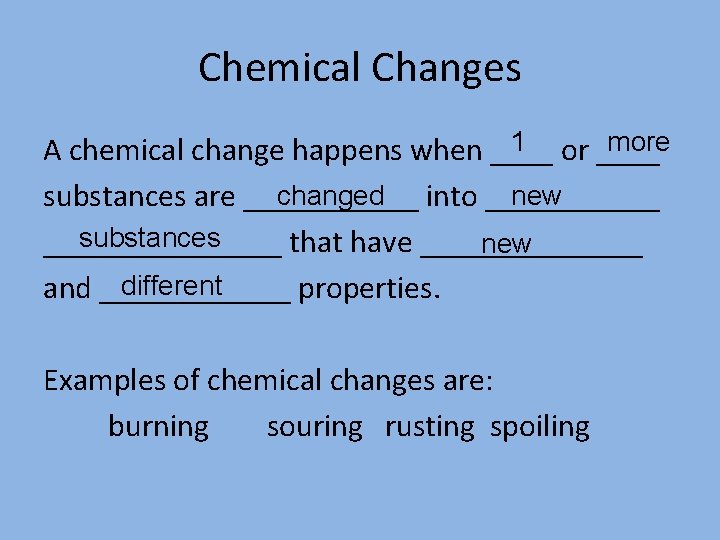
Chemical Changes 1 or ____ more A chemical change happens when ____ changed into ______ new substances are ______ substances ________ that have _______ new different and ______ properties. Examples of chemical changes are: burning souring rusting spoiling

Signs that a chemical change has occurred: –change in color or odor – production of heat – fizzing or foaming –sound or light being released cannot Most chemical changes ________be reversed

The most important question to ask yourself to determine what kind of change has occurred: Did the composition of the substance change? What do we mean by composition? the type of The composition an object particles (matter)ofthat makes is up______ the object ___________________ and the way that the particles are arranged ___________________.

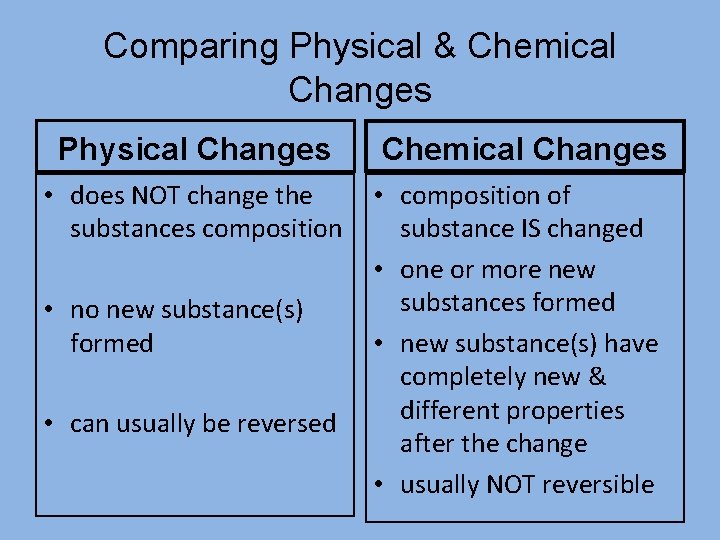
Comparing Physical & Chemical Changes Physical Changes • does NOT change the substances composition • no new substance(s) formed • can usually be reversed Chemical Changes • composition of substance IS changed • one or more new substances formed • new substance(s) have completely new & different properties after the change • usually NOT reversible