Matter can exist in three states Solid fixed



















- Slides: 39


Matter can exist in three states: • Solid: fixed volume, fixed shape • Liquid: fixed volume, no fixed shape • Gas: no fixed volume, no fixed shape

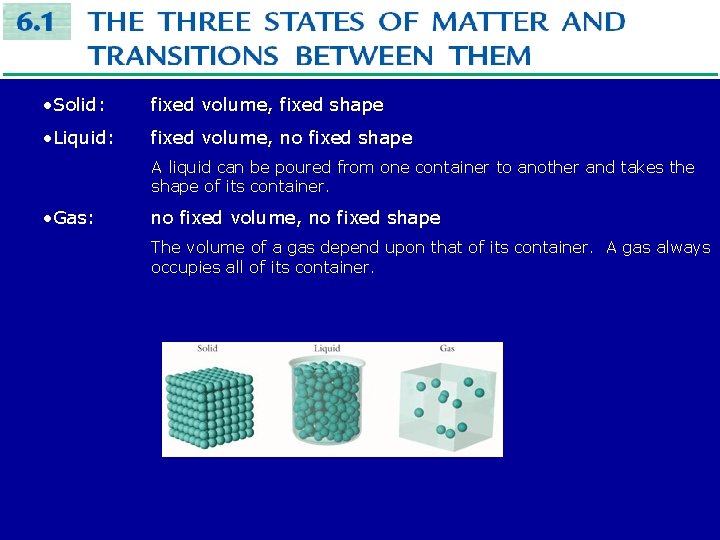
• Solid: fixed volume, fixed shape • Liquid: fixed volume, no fixed shape A liquid can be poured from one container to another and takes the shape of its container. • Gas: no fixed volume, no fixed shape The volume of a gas depend upon that of its container. A gas always occupies all of its container.

• Solids and liquids are more or less incompressible. Their volumes change very little when pressure is applied. • There is little volume change when a solid melts, but there is a large increase in volume when a liquid boils. 1 mol liquid H 2 O at 100 o C and 1 atm 18. 8 m. L 1 mol gaseous H 2 O at 100 o C and 1 atm 3. 1 x 104 m. L • The constituent molecules in a solid are touching and highly ordered. • The constituent molecules in a liquid are touching but not so highly ordered. • The constituent molecules in a gas are not touching and are highly disordered.

• The molecules in a gas move a high speeds and change directions only when they hit the walls of their container (pressure) or each other. • Gaseous molecule do not collide often so attractive forces between molecules have little effect. Because of this all compounds in the gaseous state at 1 atm behave as ideal gases.

• Energy of motion is called kinetic energy (KE). Kinetic energy is the work required to bring a moving object to rest or to impart motion to a stationary body. • At the molecular level kinetic energy is directly related to temperature. The molecules in a hot gas is large while that in a cold gas is small. • Kinetic energy opposes the attractive forces between molecules. When the kinetic energy is small and the attractive forces large, molecules can condense from the gaseous state to the liquid state.

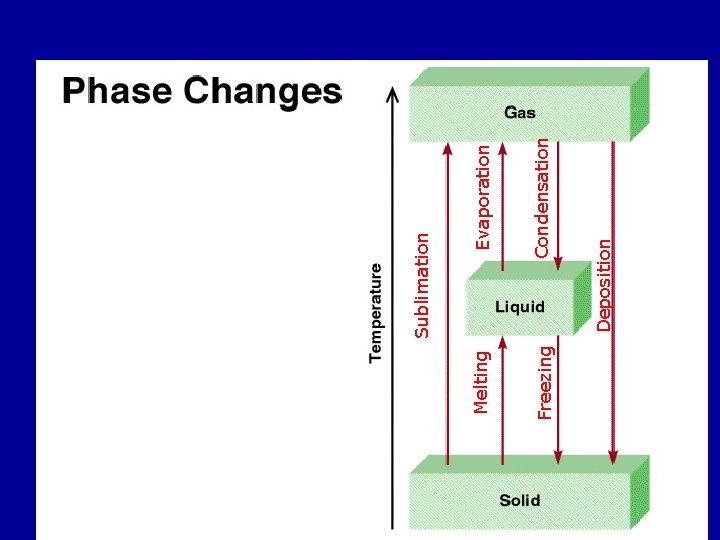
The three states of matter can be interconverted by adding and removing heat: • Melting: A solid may melt to the corresponding liquid when heat is added. • Freezing: A liquid may freeze to the corresponding solid when heat is removed • Vaporization: A liquid may boil to the corresponding gas when heat is added. • Condensation: A gas may condense to the corresponding liquid when heat is removed.

• Melting and freezing occur when the solid is at its melting temperature or the liquid is at its freezing temperature. The melting and freezing temperatures are the same for a given substance and are insensitive to any pressure applied to the solid or liquid. • Vaporization and condensation occur when the liquid is at its boiling temperature or the gas is at its condensation temperature. The boiling and condensation temperatures are the same for a given substance and are sensitive to any pressure applied to the gas or liquid. • The boiling point measured when atmospheric pressure is 1 atm is called the normal boiling point.

• Melting/freezing and boiling/condensation are examples of phase transitions. Phase transitions are reversible and can occur in either direction. • During a phase transition, heat is continuously added or removed until one phase is completely transformed into the other. During this process the temperature of the system remains constant. • The total amount of heat required to melt one mole of a substance is known as the molar heat of fusion, Hf, and is unique for each substance. • The total amount of heat required to boil one mole of a substance is known as the molar heat of vaporization, Hv, and is also unique for each substance. The molar heat of vaporization is always much greater than the molar heat of fusion.

Deposition Condensation Freezing ( Hess’s Law) Evaporation H 2 O (g) Melting Sublimation H 2 O (s) 11. 8

Energy is needed to • heat a solid • heat a liquid • heat a gas • convert a solid to a liquid • convert a liquid to a gas • convert a solid to a gas

Forces between molecules at the molecular level are called secondary forces. Secondary forces can be divided into two types: • Intermolecular forces or van der Waals forces between two molecules. These forces are those responsible for holding solids and liquids together. • Intramolecular forces between different parts of the same molecule. These forces are responsible for the correct folding of protein and other large biomolecules. • Secondary forces are only 10 -20% as strong as covalent or ionic bonds.

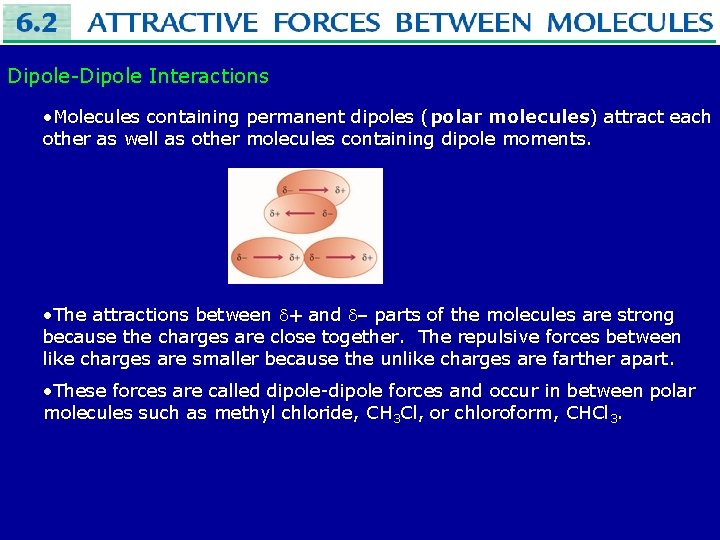
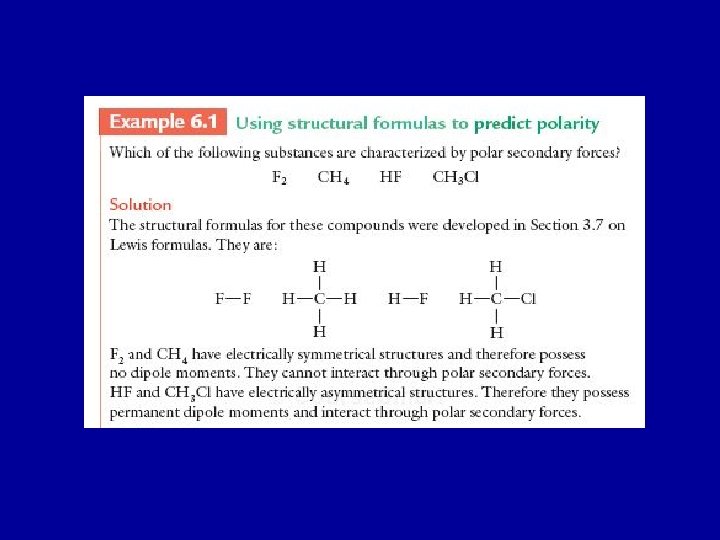
Dipole-Dipole Interactions • Molecules containing permanent dipoles (polar molecules) attract each other as well as other molecules containing dipole moments. • The attractions between + and – parts of the molecules are strong because the charges are close together. The repulsive forces between like charges are smaller because the unlike charges are farther apart. • These forces are called dipole-dipole forces and occur in between polar molecules such as methyl chloride, CH 3 Cl, or chloroform, CHCl 3.

• Water, H 2 O, a polar substance, is a liquid at room temperature while methane, a non-polar substance, CH 4, is a gas. Water molecules are bent and have a large dipole moment while methane molecules are symmetrical and have no net dipole moment.

London Forces of Interaction • Non-polar molecules, such as methane, will condense into liquids at low temperatures, so some type of force must exist between individual nonpolar molecules. • At any instant in time, the electrons moving about the atoms in a molecule may be unequally distributed with respect to the protons in the molecule. This results in a temporary, small dipole moment. (Think of a helium atom with both electrons on one side of the molecule). • This brief, small dipole moment affects the electron distribution in neighboring molecules in such a way to create an attractive force.

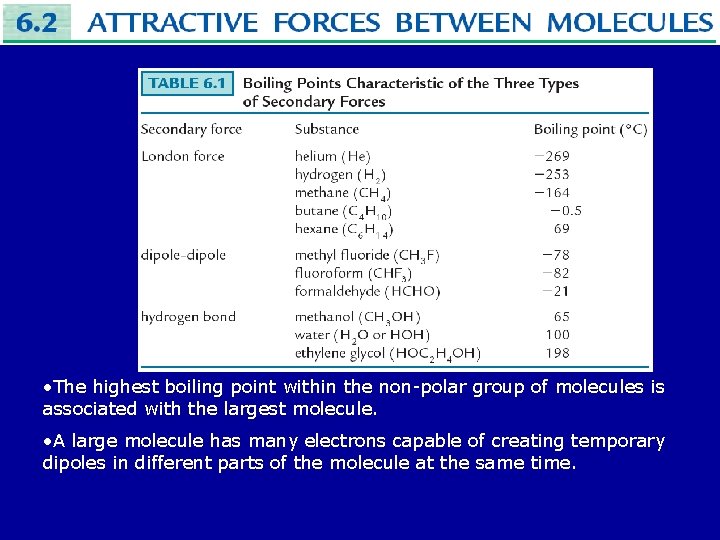
• The attractive force due to these temporary dipoles is called a London force and occurs in all molecules, polar or not. • The strength of the London force can range from very small to about that of a regular dipole-dipole interaction. • The magnitude of London forces in non-polar molecules is reflected in their boiling points.

• The highest boiling point within the non-polar group of molecules is associated with the largest molecule. • A large molecule has many electrons capable of creating temporary dipoles in different parts of the molecule at the same time.


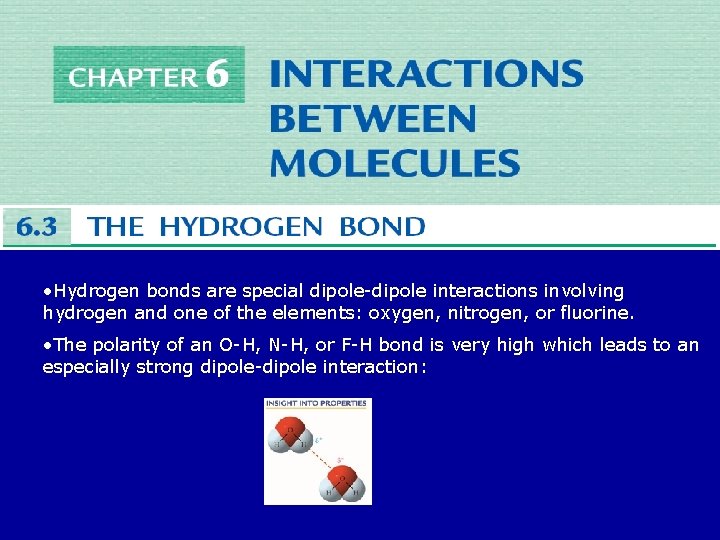
• Hydrogen bonds are special dipole-dipole interactions involving hydrogen and one of the elements: oxygen, nitrogen, or fluorine. • The polarity of an O-H, N-H, or F-H bond is very high which leads to an especially strong dipole-dipole interaction:

• Hydrogen bonds form between identical molecules, as in liquid water, or between different molecules in mixtures, such as ammonia, NH 3, dissolved in water. • Hydrogen bonds are weaker than covalent bonds and are often denoted by dotted lines connecting one molecule to the other: O–H···N • The hydrogen atom acts as a “glue” bonding the oxygen and nitrogen atoms together.

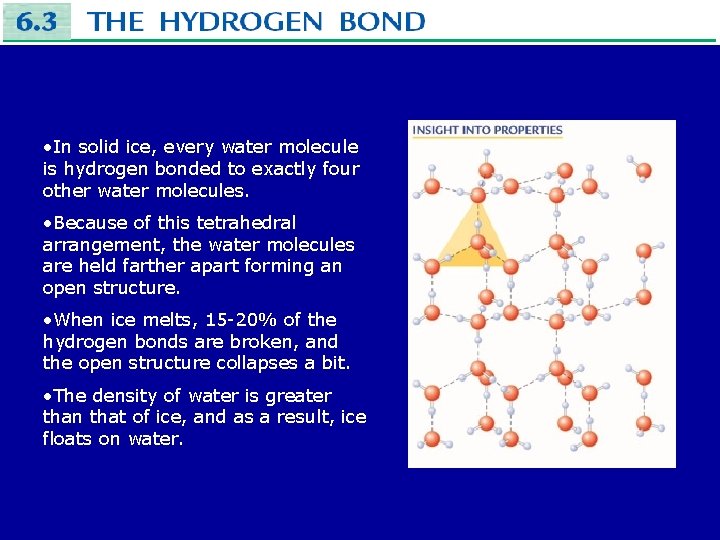
• In solid ice, every water molecule is hydrogen bonded to exactly four other water molecules. • Because of this tetrahedral arrangement, the water molecules are held farther apart forming an open structure. • When ice melts, 15 -20% of the hydrogen bonds are broken, and the open structure collapses a bit. • The density of water is greater than that of ice, and as a result, ice floats on water.

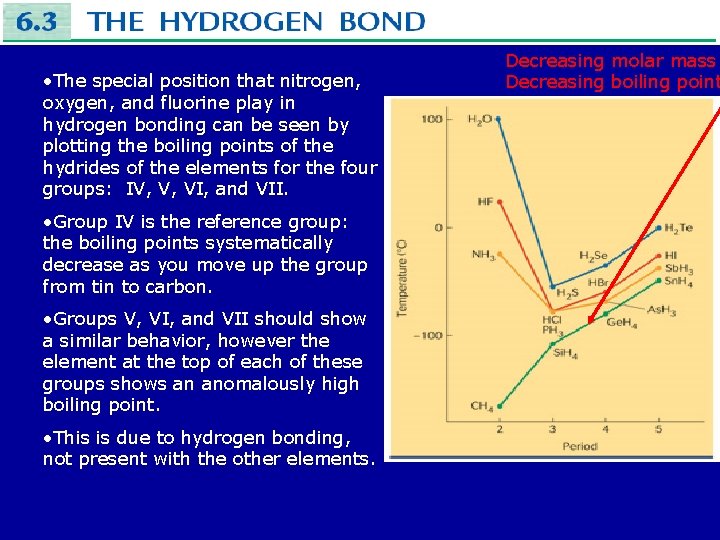
• The special position that nitrogen, oxygen, and fluorine play in hydrogen bonding can be seen by plotting the boiling points of the hydrides of the elements for the four groups: IV, V, VI, and VII. • Group IV is the reference group: the boiling points systematically decrease as you move up the group from tin to carbon. • Groups V, VI, and VII should show a similar behavior, however the element at the top of each of these groups shows an anomalously high boiling point. • This is due to hydrogen bonding, not present with the other elements. Decreasing molar mass Decreasing boiling point

The ability of water to hydrogen bond is of crucial importance for living systems. • Ice floats on water providing an insulating layer which allows fish and other aquatic organisms to exist under the ice during the winter. • The characteristics of proteins, nucleic acids, and other biological molecules depends upon their abilities to hydrogen bond to themselves, to each other, and to water. • The high heat of vaporization allows organism to cool themselves by the evaporation of perspiration.


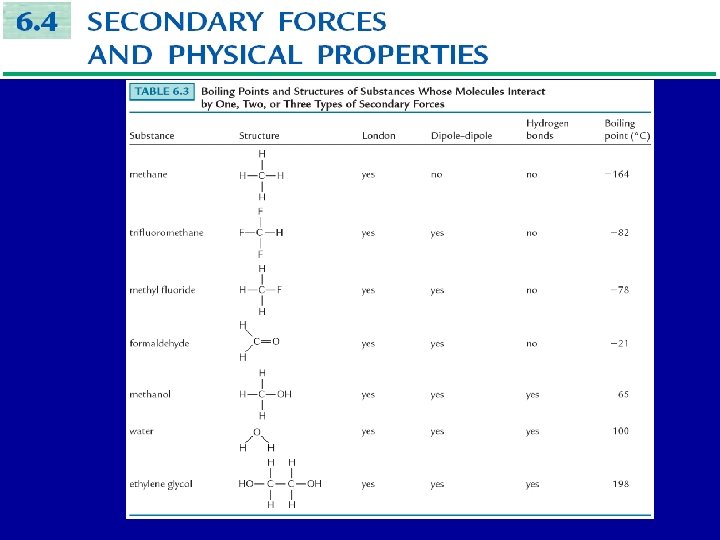
The value normal boiling point of a substance is largely determined by the types of secondary forces between molecules in the liquid state: • London forces • Dipole-dipole forces • Hydrogen bonds

• Hydrogen bonding substances interact by all three types of forces. • Polar substances interact by dipole-dipole and London forces. • Non-polar substances interact by London forces only.


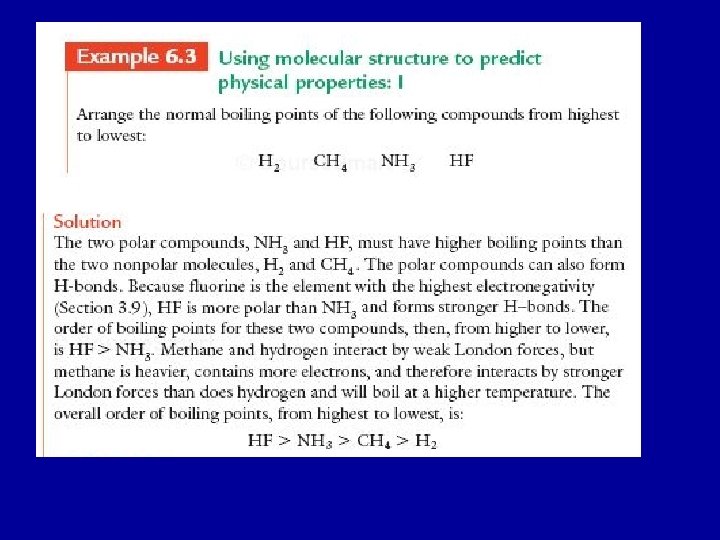
Trends in the physical properties (melting points and boiling points) of compounds can be predicted by answering two questions : • Are the compounds polar or non-polar? • Can the compounds form hydrogen bonds?


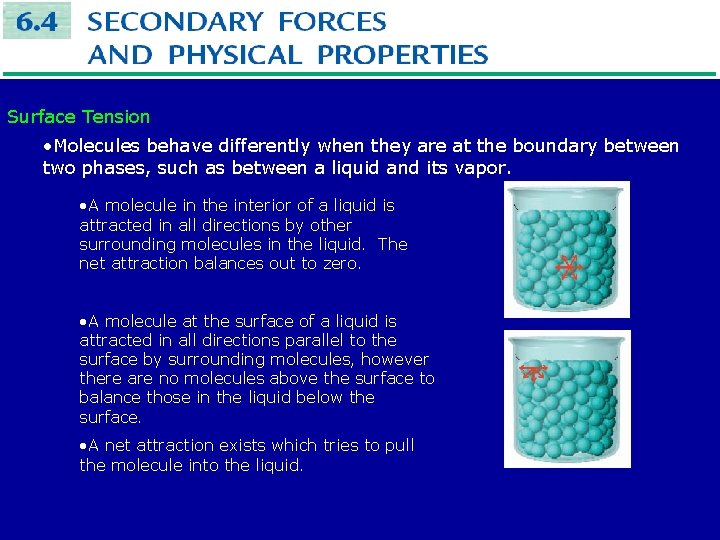
Surface Tension • Molecules behave differently when they are at the boundary between two phases, such as between a liquid and its vapor. • A molecule in the interior of a liquid is attracted in all directions by other surrounding molecules in the liquid. The net attraction balances out to zero. • A molecule at the surface of a liquid is attracted in all directions parallel to the surface by surrounding molecules, however there are no molecules above the surface to balance those in the liquid below the surface. • A net attraction exists which tries to pull the molecule into the liquid.

• The net force pulling molecules into the surface of a liquid is called the surface tension. • This force resists the expansion of a liquid’s surface area and behaves like an “elastic skin”. • Paper clips and insects can float on water due to this effect. As long as the weight of the object does not exceed the force exerted by the surface tension, the object floats.

• The surface tension of water can be reduced by dissolving substances called surface-active agents or surfactants, such as soap or detergent, to the water. • The paper clip and water strider would sink in soapy water. • Other examples of surfactants are the bile salts (dietary fat digestion and absorption) and certain molecules such as lecithin (decrease the surface tension of the water layer inside our lungs, making it easier to breath).

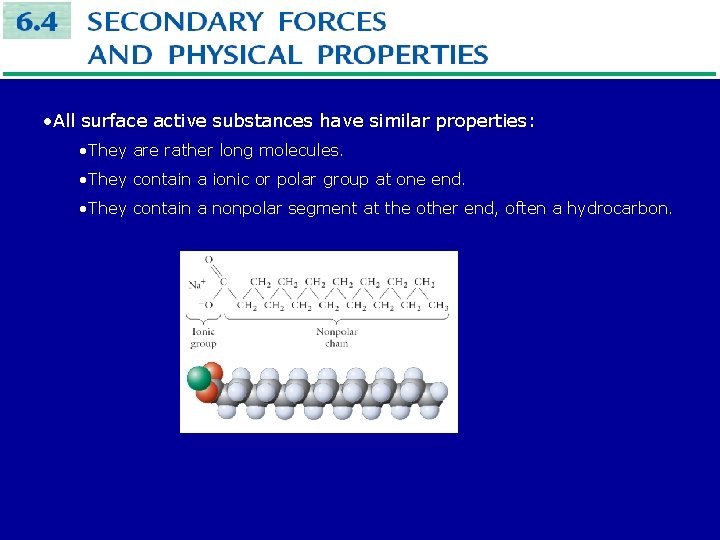
• All surface active substances have similar properties: • They are rather long molecules. • They contain a ionic or polar group at one end. • They contain a nonpolar segment at the other end, often a hydrocarbon.

The non-polar or hydrocarbon part of the molecule is called hydrophobic, or water hating. The ionic or polar part of the molecule is called hydrophilic, or water loving. Molecules containing both hydrophobic and hydrophilic parts are called amphipathic. Amphipathic molecules tend to stay at interfaces (air-water or oil-water) rather than in the interior of either liquid. This decreases the attractive forces between the liquid molecules at the surface, and the surface tension is decreased.

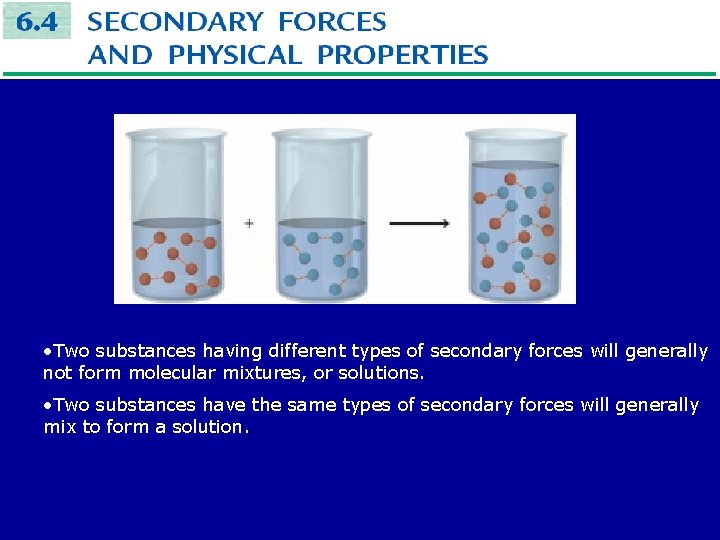
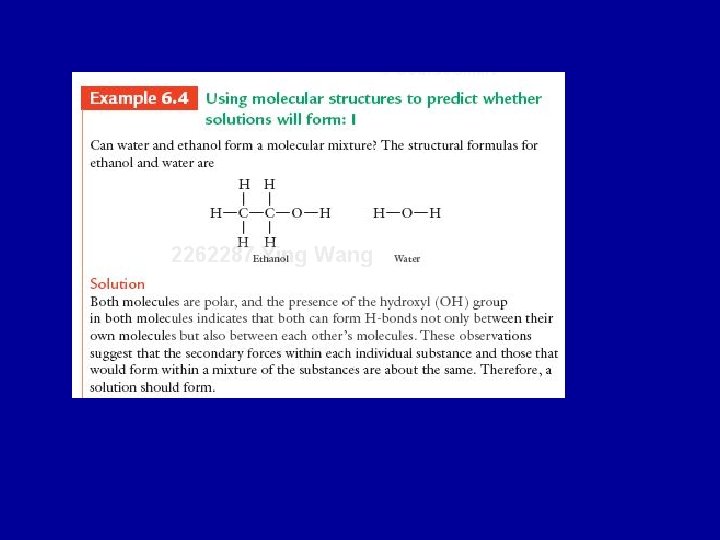
Molecular Mixtures • A mixture of substances with different kinds of molecules or ions uniformly distributed throughout is called a solution. • Solutions form when the intermolecular secondary forces of each substance are similar: like dissolves like. • Consider two substances, A and B. • Molecules of pure A are held together by polar forces: A····A • Molecules of pure B are also held together by polar forces: B····B • The process of forming a solution can be though of as exchanging an A and a B in the above relationships to form: A····B and B····A. • The number of polar interactions in the solution is the same as in the two pure liquids, so A and B will form a solution.

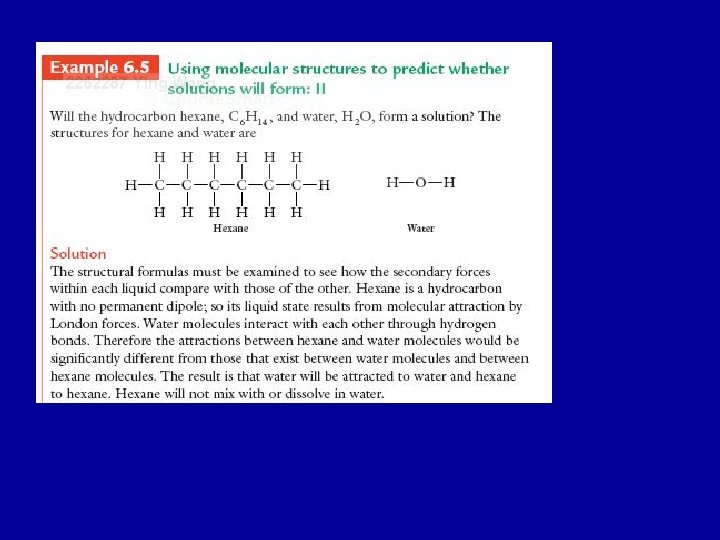
• Two substances having different types of secondary forces will generally not form molecular mixtures, or solutions. • Two substances have the same types of secondary forces will generally mix to form a solution.


