MATERIALKONTAK PANGAN dan KEAMANAN KEMASAN PANGAN UU No























- Slides: 46

MATERIALKONTAK PANGAN dan KEAMANAN KEMASAN PANGAN UU No 7. Tahun 1998 ttg Pangan Bahan yang digunakan untuk mewadahi dan/atau membungkus pangan baik yang bersentuhan langsung dengan pangan maupun tidak 1

INTRODUCTION Definisi FCM oleh EU Any MATERIAL or ARTICLE intended to come directly or indirectly with food must be sufficiently inert to proclude subtances from being transferred to food in quantities large enough to endanger human kealth or to bring about anacceptable change in the composition of the food or deterioration in its organoleptic properties 2

FUNGSI KEMASAN Sebagai WADAH (penyimpanan, penataan, transportasi) Memberi PROTEKSI dan memperpanjang daya simpan agar terhindar dari kerusakan secara : wadah proteksi promosi transportasi fisik (mekanik, cahaya) kimiawi (permeasi gas, kelembaban) Sebagai PROMOSI dan informasi Memudahkan TRANSPORTASI 3

PERSYARATAN Persyaratan kemasan makanan dan minuman tara pangan (food grade) kemasan yang dapat digunakan tanpa adanya pengaruh atau kontaminasi kemasan terhadap produk yang dikemas, sehingga aman bagi kesehatan manusia. 4

JENIS BAHAN KEMASAN (FOOD CONTACT MATERIALS) (Peraturan Ka Badan POM No: HK. 00. 05. 55. 6497 Thn 2007) 1. Plastik (termasuk varnishes dan coating) 2. Selulosa teregenerasikan (Regenerated cellulose) 3. Elastomer dan karet 4. Kertas dan karton 5. Keramik 6. Kaca/ gelas 7. Logam dan paduan logam (alloy) 8. Kayu/ gabus 9. Produk tekstil 10. Lilin parafin dan mikrokristal 5

REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on materials and articles intended to come into contact with food Ada 17 item bahan kemasan pangan : 1. Active and intelligent materials and articles 2. Adhesives 3. Ceramics 4. Cork 5. Rubbers 6. Glass 7. Ion-exchange resins 8. Metals and alloys 9. 10. 11. 12. 13. 14. 15. 16. 17. Paper and board Plastics Printing inks Regenerated cellulose Silicones Textiles Varnishes and coatings Waxes Wood 6

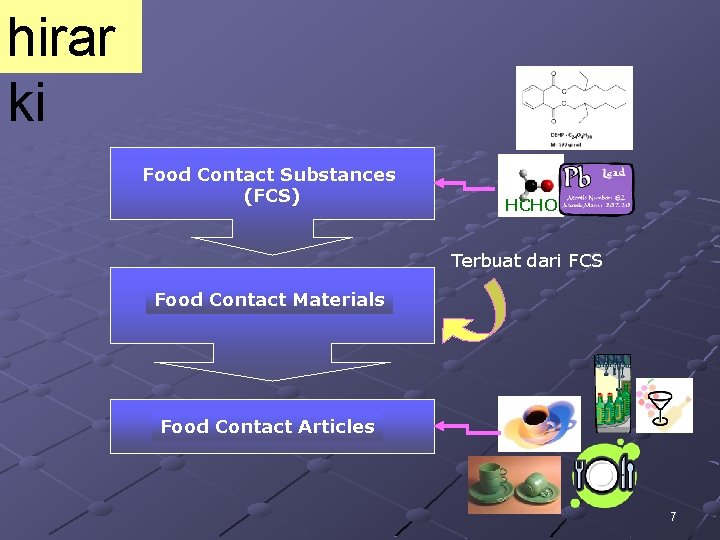
hirar ki Food Contact Substances (FCS) HCHO Terbuat dari FCS Food Contact Materials Food Contact Articles 7

FOOD CONTACT ARTICLES Interaksi antara bahan kemasan dan pangan : Merupakan reaksi kimia dan/atau fisika antara makanan, kemasannya dan lingkungan yang dapat mengubah komposisi, kuallitas atau sifat fisik makanan dan/atau bahan kemasan Interaksi dapat bersifat merugikan atau mungkin dapat meningkatkan kualitas pangan Konsekuensinya akan terjadi migrasi, sorpsi atau permeasi 8

Migrasi : Perpindahan komponen kemasan kedalam makanan Sorpsi : Perpindahan komponen makanan ke dalam kemasan Permeasi : Perpindahan komponen melalui kemasan ke lingkungan dan sebaliknya 9

fokus perhatian utama Klasifikasi kemasan 10

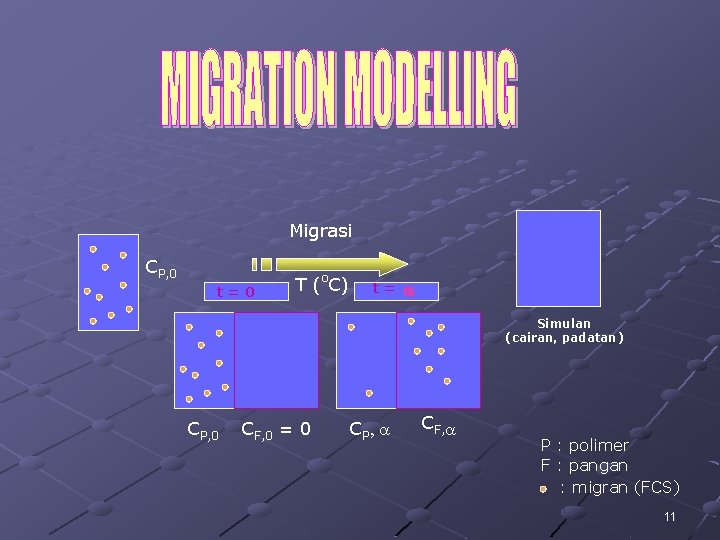
Migrasi CP, 0 t=0 T (o. C) t= a Simulan (cairan, padatan) CP, 0 CF, 0 = 0 CP , a CF, a P : polimer F : pangan : migran (FCS) 11

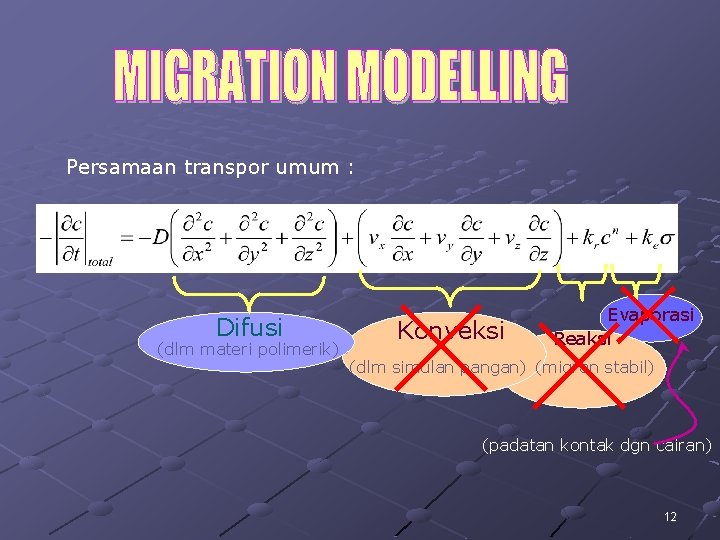
Persamaan transpor umum : Difusi (dlm materi polimerik) Konveksi Evaporasi Reaksi (dlm simulan pangan) (migran stabil) (padatan kontak dgn cairan) 12

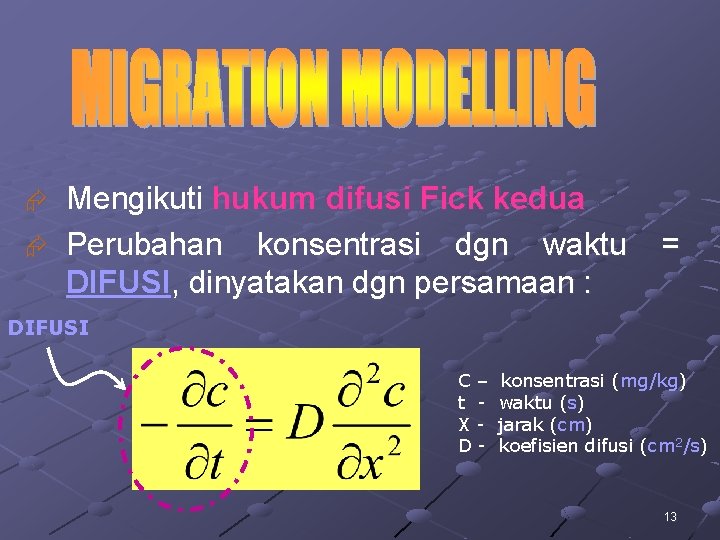
Mengikuti hukum difusi Fick kedua Æ Perubahan konsentrasi dgn waktu DIFUSI, dinyatakan dgn persamaan : Æ = DIFUSI C– t XD- konsentrasi (mg/kg) waktu (s) jarak (cm) koefisien difusi (cm 2/s) 13

Migrasi : Perpindahan zat/komponen dari bahan kemasan ke produk pangan yang dikemas 1. 2. 3. 4. 5. 6. 7. Sumber bahaya Bahan kimia polimernya Bahan asal proses produksinya Bahan fasilitator : katalis dan aditif Sisa bahan pelarut Bahan pemgisi : plasticizer Bahan perekat Tinta 14

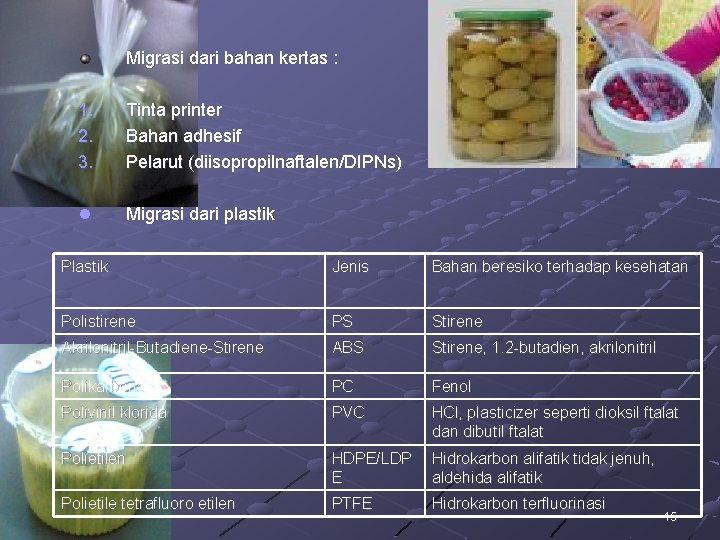
Migrasi dari bahan kertas : 1. 2. 3. Tinta printer Bahan adhesif Pelarut (diisopropilnaftalen/DIPNs) l Migrasi dari plastik Plastik Jenis Bahan beresiko terhadap kesehatan Polistirene PS Stirene Akrilonitril-Butadiene-Stirene ABS Stirene, 1. 2 -butadien, akrilonitril Polikarbonat PC Fenol Polivinil klorida PVC HCl, plasticizer seperti dioksil ftalat dan dibutil ftalat Polietilen HDPE/LDP E Hidrokarbon alifatik tidak jenuh, aldehida alifatik Polietile tetrafluoro etilen PTFE Hidrokarbon terfluorinasi 15

Migrasi dari bahan kaleng : 1. Resiko terhadap kesehatan dapat timbul dari kandungan logam sebagai komponen utama penyusun kaleng 2. Dapat terkandung bahan kimia lainnya dalam bahan pelapis kaleng seberti aluminium, seng atau bahan organik seperti epoksi-fenol dan organosol Migrasi dari Gelas : Gelas telah digunakan bertahun-tahun dan beberapa problem khusus pada terlarutnya timbal dari gelas kristal yang kemungkinkan mengandung peroksida Migrasi dari Keramik : Logam atau enamel atau gelas tembikar 16

• Bahan berbahaya dan resiko terhadap kesehatan Komponen Resiko Polivinil klorida Dapat menyebabkan kanker, cacat lahir, perubahan genetik, bronkitis, penyakit kulit, penurunan kemampuan pendengaran, kegagalan fungsi penglihatan disfungsi hati Ftalat (DEHP, DEHA) Disrupsi endokrin, terkait dengan asma, berpengaruh terhadap pertumbuhan dan reproduksi PET Kanker Polyester Iritasi pada mata Stirene Iritasi saluran pernafasan, kulit, mata, depresi sistem saraf pusat, kanker Timbal (Pb) Menghambat pertumbuhan, anemia, kerusakan sistem syaraf pusat, esteoporosis Kromium Kanker dan alergi kulit Merkuri Kerusakan sistem syaraf pusat, ginjal 17

When you eat or drink things stored in plastic, wear plastic, sit on plastic, taste it, smell it, and so on, plastic is incorporated into you. There is a bi-directional communication between plastic and things that contact it, meaning that plastic gets into the food, and food gets into the plastic, as well as you. So, when you eat the things that plastic contacts, quite literally, it becomes you. In other words, you are what you eat . . drink. . . and breathe. . . Plastic! 18

First, plastic is made by combining many toxic synthetic man-made chemicals by a process called polymerization. The plastics industry tells us that this process binds the toxic chemicals together so tightly that they are no longer toxic to us. But they don't tell us that the polymerization process is never 100% perfect. It always leaves some of those toxic chemicals available to migrate out of the plastic product and into whatever contacts it— your food, you, air, water, and so on. Secondly, many of these chemicals not only cause cancer, but also disrupt the normal functioning of the endocrine system of most animals, including humans. They have been given the name endocrine disruptors. These toxic man-made chemicals have been shown to be accumulating in the bodies of both humans and the animals we eat. Hormones act in single digit part/per/trillion (PPT) concentrations, and have an effect on virtually every bodily function. The effects of disrupting the normal activities of hormones can be devastating and permanent. 19

PVC: A Truly “Poison” Plastic Unlike the many plastics made without chlorine, PVC poses serious environmental health threats from the start. The production of PVC requires the manufacture of raw chemicals, including highly polluting chlorine, and cancer-causing vinyl chloride monomer (VCM) and ethylene dichloride (EDC). Communities surrounding U. S. vinyl chloride chemical facilities, half of which are in Louisiana, suffer from serious toxic chemical pollution of their groundwater supplies, surface waters and air. Residents of the town of Mossville, Louisiana had dioxin levels in their blood that were three times higher than normal. PVC plastic also requires large amounts of toxic additives to make it stable and usable. These additives are released during the use (and disposal) of PVC products, resulting in elevated human exposures to phthalates, lead, cadmium, tin and other toxic chemicals. Dioxin emissions from PVC combustion occur regularly due to the 1 million annual fires that burn buildings and vehicles, two sectors that 20 use substantial amounts of PVC.

Studies have found that all the plastics commonly used for foodpackaging can leach, so the safest bet is to store foods in Pyrex-likeglass or Corningware-type ceramic when possible, and to avoid heating ormicrowaving foods in plastic. Also, since most grocery stores use PVC for their cling-wrapped cheeses and meats, you may want to trim off the outer layer of these pre-packaged foods to reduce ingestion of DEHA, which has caused reproductive effects and liver tumors in test animals. Canned foods, which can contain traces of bisphenol-A from the plastic inner lining of the can, may also be a source of concern. 21

#1: Polyethylene Terephthalate (PETE or PET), used to bottle soda pop, most bottled water and cooking oils, juice, salad dressings, peanutbutter, etc. #2: High Density Polyethylene (HDPE), a cloudy or opaque plastic used for milk jugs, most one-gallon water bottles, and to bottle some foods. #3: Low Density Polyethylene (LDPE) is used for MOST (for other brands, see PVC) cling wraps and food storage bags, such as Glad wrap, Handi-Wrap, and Ziploc and Glad bags. #4: Polypropylene (PP) is a cloudy or opaque plastic used to make many deli soup containers, most Rubbermaid containers, cloudy plastic baby bottles, ketchup bottles, and other clouded plastic food bottles. 22

#5. Polyvinyl Chloride (PVC or vinyl) is used to make Reynolds Wrap and Polyvinyl Films (Stretchtite and Freezetite brands) cling wraps and the cling wrap of choice for most grocery stores. PVC can also turn up in the plastic trays in boxed cookies or chocolates, in candy bar wrappers, and occasionally as bottles for grocery items, including large Bertolli Lucca olive oils, 64 -ounce Wesson Cooking Oil, and Appalachian Mountain spring water, among others. Some plastic squeeze bottles are made of PVC as well. #6. Polystyrene (PS) is most familiar to consumers in its inflated formas Styrofoam, but non-inflated plastic PS can be found in some brands of disposable plastic cups and bowls and in most opaque plastic cutlery. #7: "Other" resins, most typically polycarbonate, from which bisphenol-A can migrate. Most plastic baby bottles are made of polycarbonate, as are 5 -gallon water bottles, clear plastic "sippy" cups for kids, and some brands o fclear plastic cutlery. Other possible sources of bisphenol-A exposure 23

• Tujuan test migrasi adalah untuk menyakinkan keamanan makanan dan melindungi konsumen dari bahan-bahan berbahaya yang mungkin berpindah dari kemasan ke dalam makanan yang dikemas • Ada 2 macam migrasi : • Migrasi total/overall/global : Total massa yang bermigrasi dari kemasan kedalam makanan atau simulan pada kondisi tertentu 24

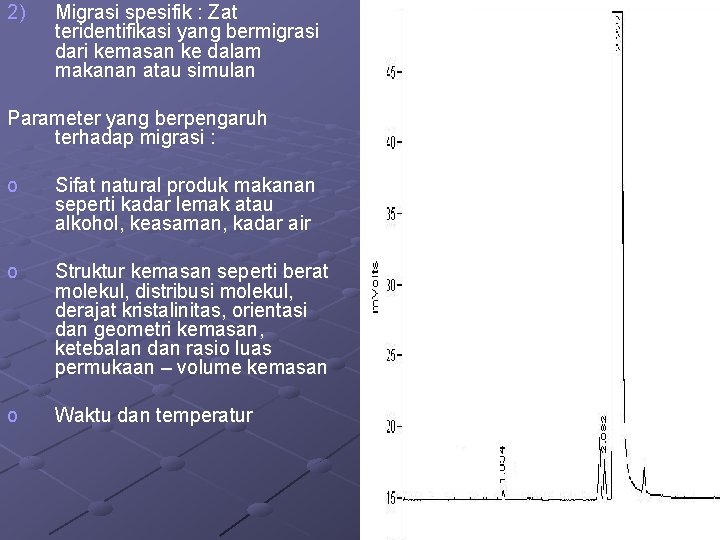
2) Migrasi spesifik : Zat teridentifikasi yang bermigrasi dari kemasan ke dalam makanan atau simulan Parameter yang berpengaruh terhadap migrasi : o Sifat natural produk makanan seperti kadar lemak atau alkohol, keasaman, kadar air o Struktur kemasan seperti berat molekul, distribusi molekul, derajat kristalinitas, orientasi dan geometri kemasan, ketebalan dan rasio luas permukaan – volume kemasan o Waktu dan temperatur 25

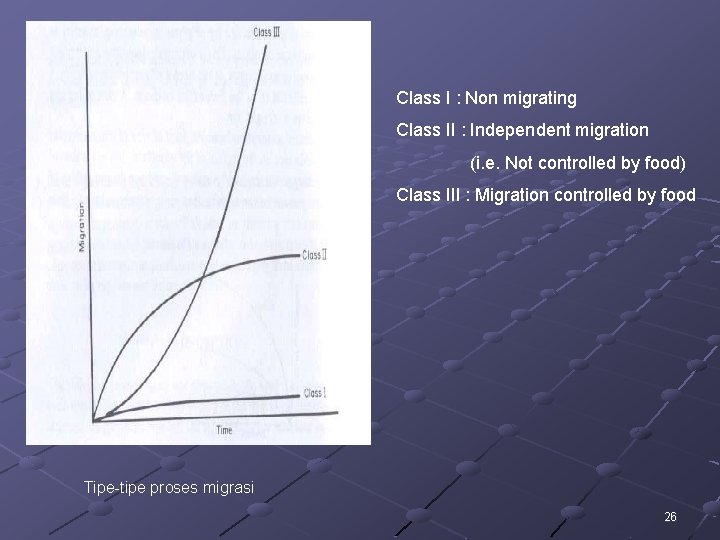
Class I : Non migrating Class II : Independent migration (i. e. Not controlled by food) Class III : Migration controlled by food Tipe-tipe proses migrasi 26

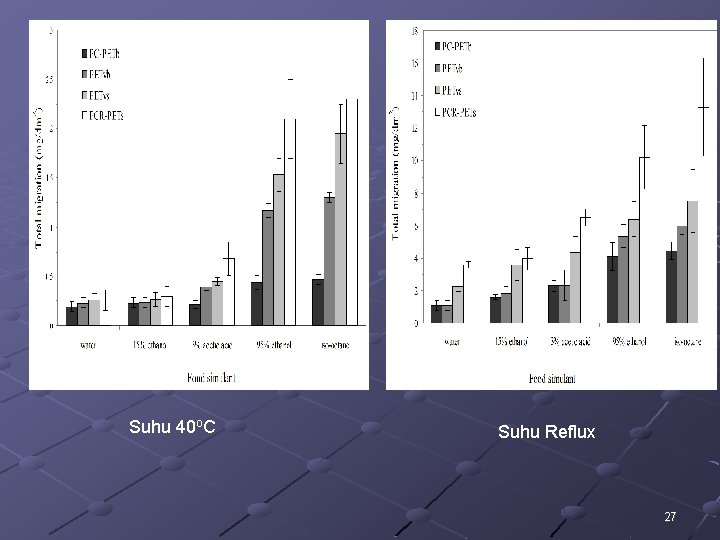
Suhu 40 o. C Suhu Reflux 27

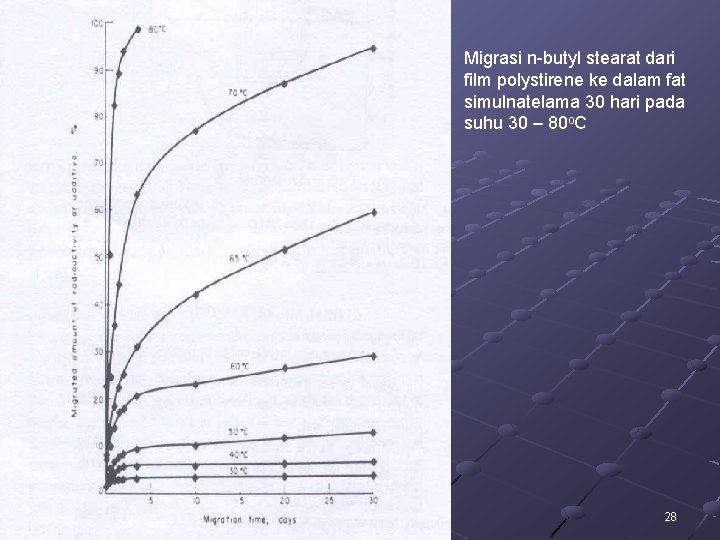
Migrasi n-butyl stearat dari film polystirene ke dalam fat simulnatelama 30 hari pada suhu 30 – 80 o. C 28

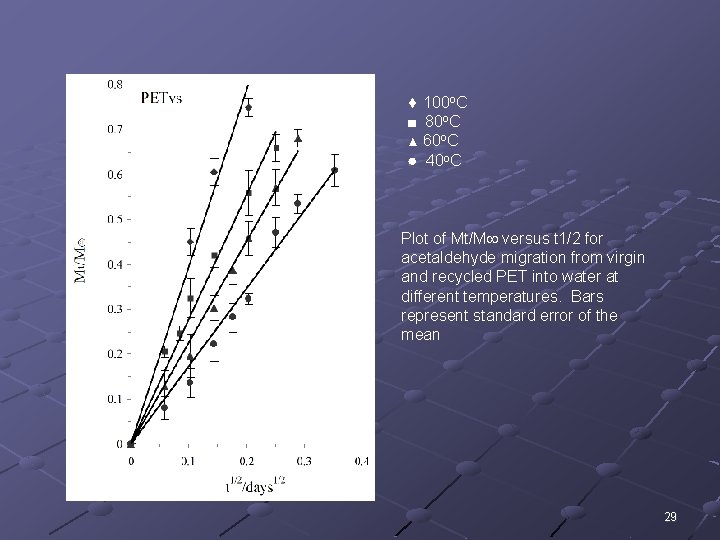
100 o. C ■ 80 o. C ▲ 60 o. C ● 40 o. C Plot of Mt/M versus t 1/2 for acetaldehyde migration from virgin and recycled PET into water at different temperatures. Bars represent standard error of the mean 29

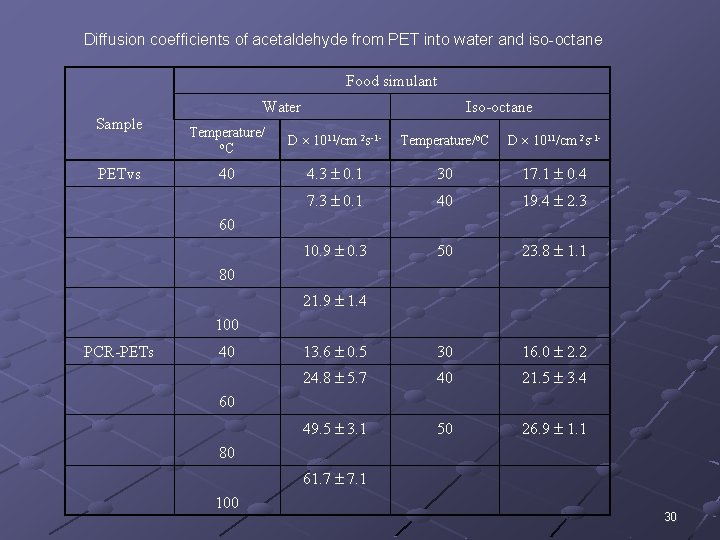
Diffusion coefficients of acetaldehyde from PET into water and iso-octane Food simulant Sample PETvs Water Iso-octane Temperature/ o. C D 1011/cm 2 s-1 - Temperature/o. C D 1011/cm 2 s-1 - 40 4. 3 0. 1 30 17. 1 0. 4 7. 3 0. 1 40 19. 4 2. 3 10. 9 0. 3 50 23. 8 1. 1 13. 6 0. 5 30 16. 0 2. 2 24. 8 5. 7 40 21. 5 3. 4 49. 5 3. 1 50 26. 9 1. 1 60 80 21. 9 1. 4 100 PCR-PETs 40 60 80 61. 7 7. 1 100 30

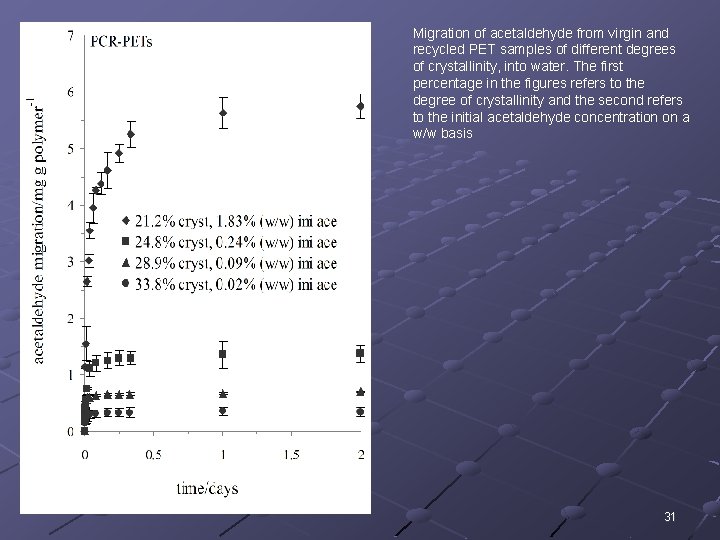
Migration of acetaldehyde from virgin and recycled PET samples of different degrees of crystallinity, into water. The first percentage in the figures refers to the degree of crystallinity and the second refers to the initial acetaldehyde concentration on a w/w basis 31

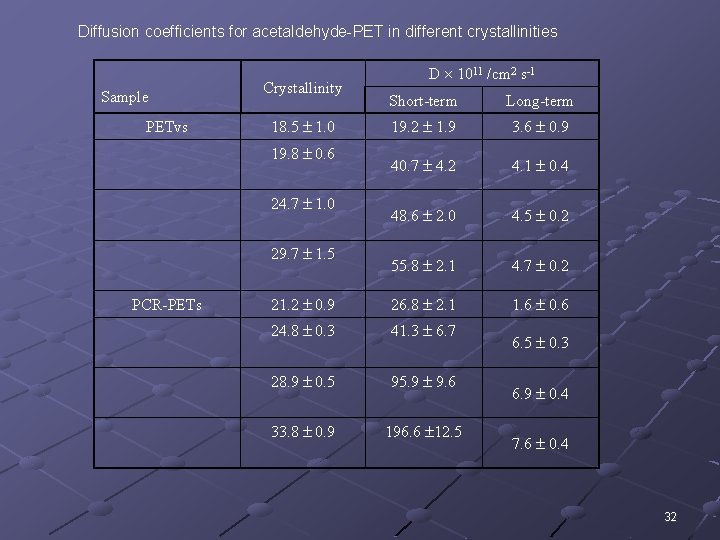
Diffusion coefficients for acetaldehyde-PET in different crystallinities Sample PETvs Crystallinity Short-term Long-term 19. 2 1. 9 3. 6 0. 9 40. 7 4. 2 4. 1 0. 4 48. 6 2. 0 4. 5 0. 2 55. 8 2. 1 4. 7 0. 2 21. 2 0. 9 26. 8 2. 1 1. 6 0. 6 24. 8 0. 3 41. 3 6. 7 28. 9 0. 5 95. 9 9. 6 33. 8 0. 9 196. 6 12. 5 18. 5 1. 0 19. 8 0. 6 24. 7 1. 0 29. 7 1. 5 PCR-PETs D 1011 /cm 2 s-1 6. 5 0. 3 6. 9 0. 4 7. 6 0. 4 32

FOOD SIMULANTS • Makanan : berkomposisi sangat kompleks sehingga sangat sulit mengukur zat atau komponen yang bermigrasi dari bahan kemasan kedalam makanan tersebut • Oleh karena itu digunakan SIMULANT sebagai pengganti Simulan Diskripsi A Air B 3 % asam asetat C 15 % alkohol dalam air D Minyak zaitun/lemak sintetik HB 307 A, B dan C disebut “ Aqueous food simulant” D disebut “Fatty food simulant” 33

Dalam analisis, penggunaan minyak zaitun tidaklah mudah karena : 1) Beberapa bahan kemasan menyerap minyak sehingga memerlukan pengkoreksian dalam penghitungan akhir migrasi 2) Minyak sering komplek jika dianalisa menggunakan kromatografi Oleh karena digunakan “ALTERNATIVE FATTY FOOD SIMULANT” yaitu iso-heptane, iso-oktan, iso-propanol atau 95% alkohol dalam air 34

35

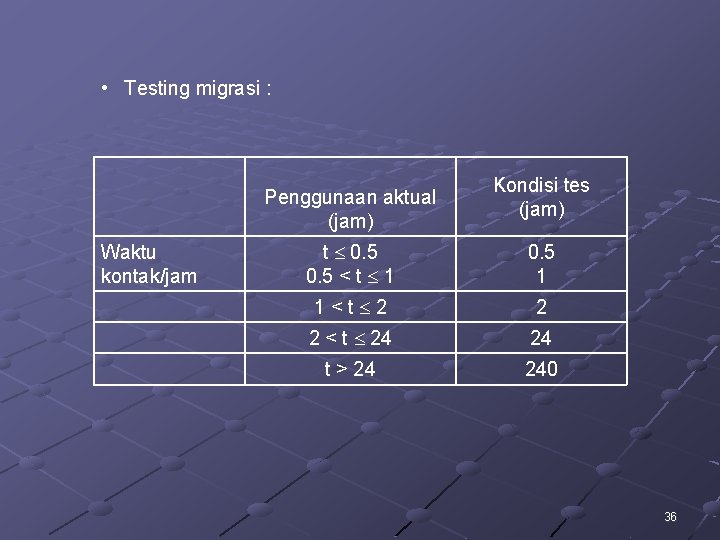
• Testing migrasi : Penggunaan aktual (jam) Waktu kontak/jam Kondisi tes (jam) t 0. 5 < t 1 0. 5 1 1<t 2 2 2 < t 24 24 t > 24 240 36

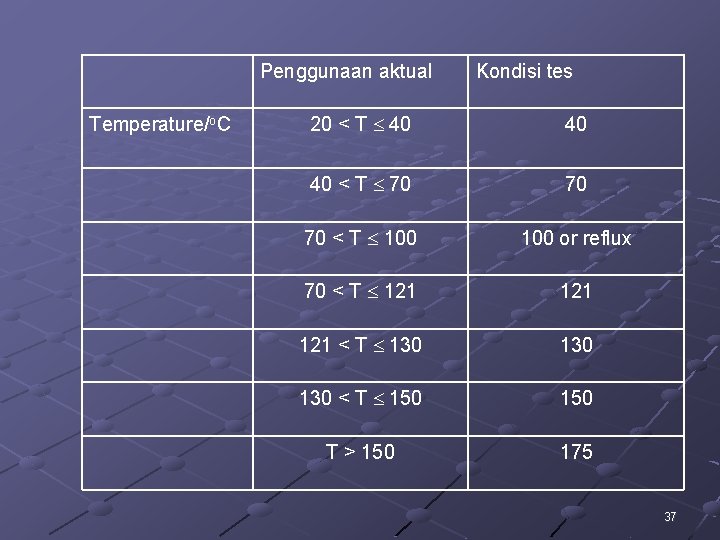
Penggunaan aktual Temperature/o. C Kondisi tes 20 < T 40 40 40 < T 70 70 70 < T 100 or reflux 70 < T 121 121 < T 130 130 < T 150 T > 150 175 37

PRACTICAL PROBLEMS Plastik sangat bervariasi dari fleksibel fil tipis sampai specimen rigid dan cukup tebal. Specimen mungkin terapung dan sulit dipastikan semua permukaan bersentuhan dengan food simulants 38

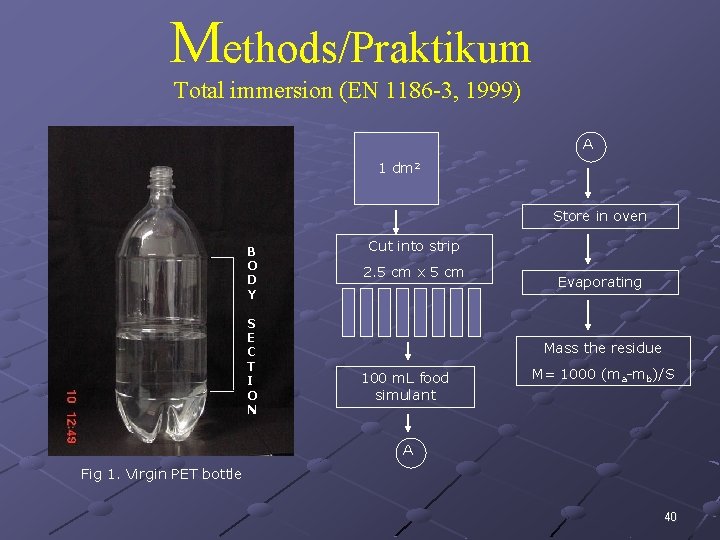
Tes migrasi total : The tests will be carried out by conventional total immersion in 100 m. L of the simulant per dm 2 polymer area (the results of the immersion experiments will be calculated for the area of sheet used. The tube containing the polymer and food simulant will be stored for 10 days in a thermostatically controlled oven at 40 o. C. At the end of the contact time an aliquot of each exposed solution of food simulant will be transferred to a pre-weighed quartz dish and placed on a hot plate. The simulant will be carefully evaporated on the hot plate to leave behind the solid residue. During evaporation, the temperature will be set to 80 o. C in order to avoid simulant losses by splashing. Once dried, the samples will be put into an oven at 105 o. C for 4 h, cooled to room temperature in a desiccator containing silica gel and held for 2 h at that temperature. The quantity of non-volatile residue from the test will be used to determine the overall migration value. TM = 1000 * (ma-mb)/S 39

Methods/Praktikum Total immersion (EN 1186 -3, 1999) A 1 dm 2 Store in oven B O D Y S E C T I O N Cut into strip B O D Y S E C T I O N 2. 5 cm x 5 cm Evaporating Mass the residue 100 m. L food simulant M= 1000 (ma-mb)/S A Fig 1. Virgin PET bottle 40

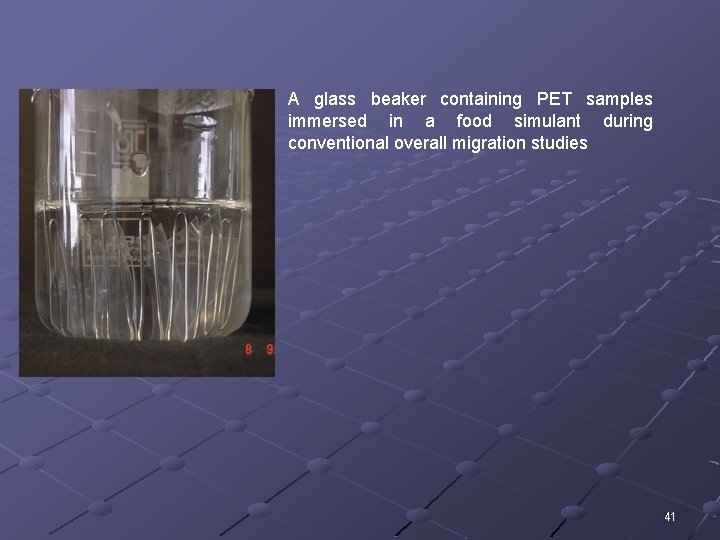
A glass beaker containing PET samples immersed in a food simulant during conventional overall migration studies 41

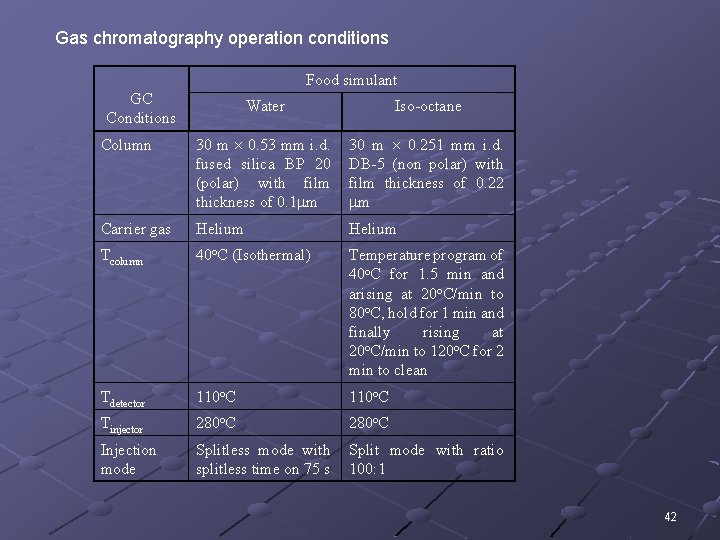
Gas chromatography operation conditions Food simulant GC Conditions Water Iso-octane Column 30 m 0. 53 mm i. d. fused silica BP 20 (polar) with film thickness of 0. 1 m 30 m 0. 251 mm i. d. DB-5 (non polar) with film thickness of 0. 22 m Carrier gas Helium Tcolumn 40 o. C (Isothermal) Temperature program of 40 o. C for 1. 5 min and arising at 20 o. C/min to 80 o. C, hold for 1 min and finally rising at o o 20 C/min to 120 C for 2 min to clean Tdetector 110 o. C Tinjector 280 o. C Injection mode Splitless mode with splitless time on 75 s Split mode with ratio 100: 1 42

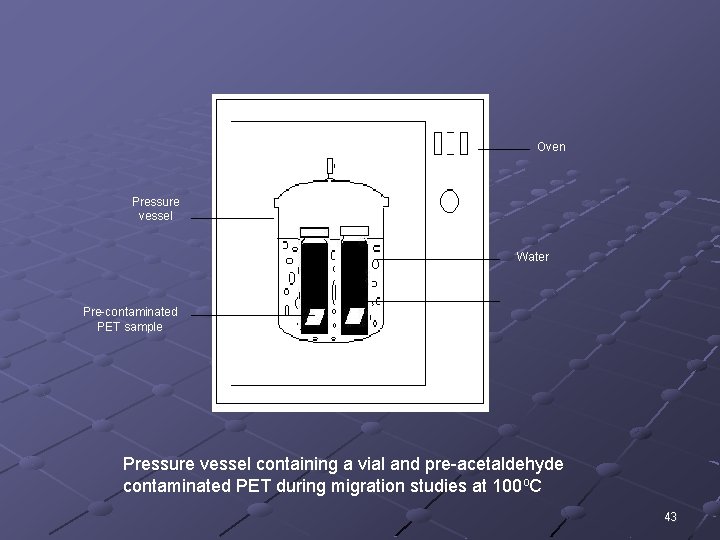
Oven Pressure vessel Water Pre-contaminated PET sample Pressure vessel containing a vial and pre-acetaldehyde contaminated PET during migration studies at 100 o. C 43

Tes spesifik migrasi : KASUS : ACETALDEHYDE MIGRATION FROM PET BOTTLE • It is quite difficult to detect acetaldehyde due to its high volatility. The boiling point of this compound is 20. 8 o. C. • Different methods of analysis have been used for the determination of acetaldehyde from PET. These include simultaneous distillation extraction, reaction with chemicals, dynamic headspace sampling, a static headspace method, membrane dialysis, conventional solid phase extraction, purge-and-trap sampling and high performance liquid chromatography (HPLC) with fluorescent detection. • In principle, there is no different between the two methods of dynamic head-space and purge-trap techniques. • Among these methods, the static headspace gas chromatography appears to be the simplest and least costly. • The other methods are generally very time consuming and require special solvents and/or equipment. • The detection limits, however, depend on the type of detectors being used in the various chromatographic techniques. 44

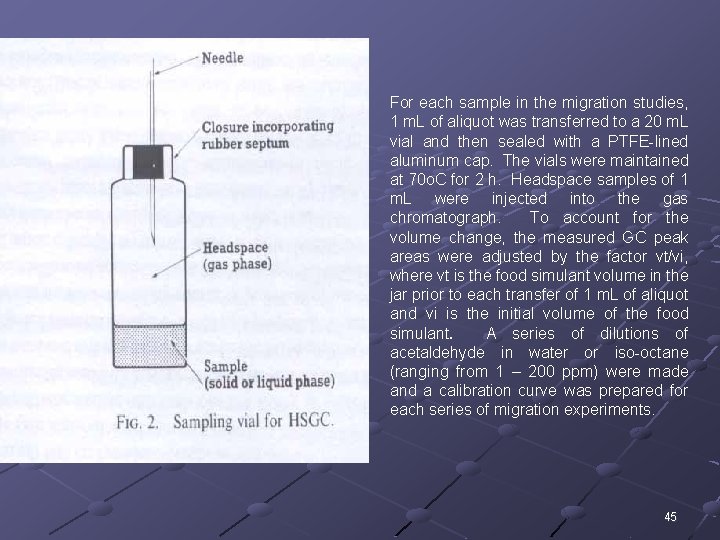
For each sample in the migration studies, 1 m. L of aliquot was transferred to a 20 m. L vial and then sealed with a PTFE-lined aluminum cap. The vials were maintained at 70 o. C for 2 h. Headspace samples of 1 m. L were injected into the gas chromatograph. To account for the volume change, the measured GC peak areas were adjusted by the factor vt/vi, where vt is the food simulant volume in the jar prior to each transfer of 1 m. L of aliquot and vi is the initial volume of the food simulant. A series of dilutions of acetaldehyde in water or iso-octane (ranging from 1 – 200 ppm) were made and a calibration curve was prepared for each series of migration experiments. 45

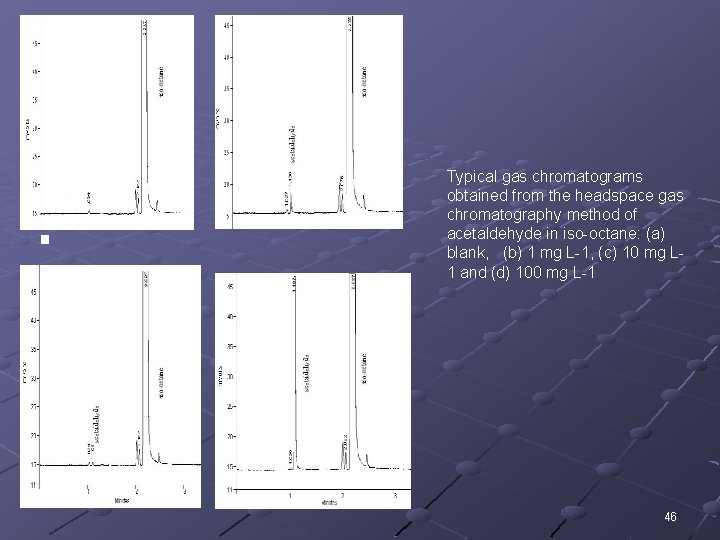
(a) (b) (c) (d) Typical gas chromatograms obtained from the headspace gas chromatography method of acetaldehyde in iso-octane: (a) blank, (b) 1 mg L-1, (c) 10 mg L 1 and (d) 100 mg L-1 46
 Permentan 53 tahun 2018
Permentan 53 tahun 2018 Keamanan pangan menurut who
Keamanan pangan menurut who Jelaskan alat penyajian modern dan contohnya
Jelaskan alat penyajian modern dan contohnya Balai besar kimia dan kemasan
Balai besar kimia dan kemasan Persyaratan mutu obat tradisional
Persyaratan mutu obat tradisional Tata ruang kantor semi tertutup
Tata ruang kantor semi tertutup Kebutuhan keselamatan dan keamanan
Kebutuhan keselamatan dan keamanan Pancasila sebagai pertahanan dan keamanan
Pancasila sebagai pertahanan dan keamanan Andrias darmayadi
Andrias darmayadi Organisasi keamanan
Organisasi keamanan Sifat sifat kemasan
Sifat sifat kemasan Kemasan primer
Kemasan primer Tujuan bahan kemasan bersifat inert adalah
Tujuan bahan kemasan bersifat inert adalah Kemasan dapat didefinisikan sebagai
Kemasan dapat didefinisikan sebagai Apa acuan desain kemasan kayu?
Apa acuan desain kemasan kayu? Indikator pembuatan kemasan
Indikator pembuatan kemasan Belakang kemasan
Belakang kemasan Suhu dingin menurut farmakope
Suhu dingin menurut farmakope Manfaat kemasan
Manfaat kemasan Kemasan primer
Kemasan primer Teknik pembuatan patung loro blonyo
Teknik pembuatan patung loro blonyo Hal-hal yang perlu diperhatikan dalam kemasan produk adalah
Hal-hal yang perlu diperhatikan dalam kemasan produk adalah Tuliskan fungsi kemasan menurut mutu performa
Tuliskan fungsi kemasan menurut mutu performa Susu bebelac kaleng
Susu bebelac kaleng Merubah menggayakan
Merubah menggayakan Kemasan logam
Kemasan logam Kemasan retail
Kemasan retail Pengemasan plastik
Pengemasan plastik Tujuan membuat kemasan model 3d
Tujuan membuat kemasan model 3d Bahan pangan hasil peternakan antara lain adalah .... *
Bahan pangan hasil peternakan antara lain adalah .... * Lapisan keamanan komputer
Lapisan keamanan komputer Kerangka untuk audit keamanan komputer
Kerangka untuk audit keamanan komputer Kebijakan keamanan
Kebijakan keamanan Makalah lapisan keamanan komputer
Makalah lapisan keamanan komputer Ancaman keamanan sistem informasi
Ancaman keamanan sistem informasi Keamanan database
Keamanan database Aspek keamanan informasi
Aspek keamanan informasi Jenis ancaman sistem keamanan komputer
Jenis ancaman sistem keamanan komputer Sistem keamanan pintu menggunakan sidik jari
Sistem keamanan pintu menggunakan sidik jari Definisi keamanan komputer
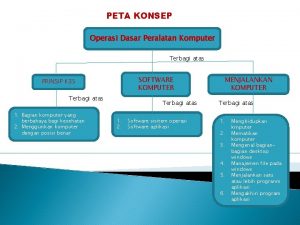
Definisi keamanan komputer Peta konsep sistem keamanan jaringan
Peta konsep sistem keamanan jaringan Mata kuliah keamanan sistem informasi
Mata kuliah keamanan sistem informasi Apa itu physical security
Apa itu physical security Peta minda komponen komputer
Peta minda komponen komputer Mata kuliah keamanan jaringan
Mata kuliah keamanan jaringan Ori keamanan significado
Ori keamanan significado Pengertian patroli keamanan sekolah
Pengertian patroli keamanan sekolah