Mass Spectrometry in MCAL Two systems GCMS LCMS






- Slides: 39

Mass Spectrometry in MCAL • Two systems: GC-MS, LC-MS – GC separates small, volatile, non-polar material – MS is detection devise (Agilent 320 -MS TQ Mass Spectrometer) • Full scan monitoring • SIM single ion monitoring • MSMS monitoring • Sample can be injected as a liquid, a gas or even a solid

Different Types of MS • GC-MS - Gas Chromatography MS – separates volatile compounds in gas column and ID’s by mass • LC-MS - Liquid Chromatography MS – separates compounds in HPLC column and ID’s by mass • MS-MS - Tandem Mass Spectrometry – separates compound fragments

GC-MS Injection port GC 3800 MS 320 Probe

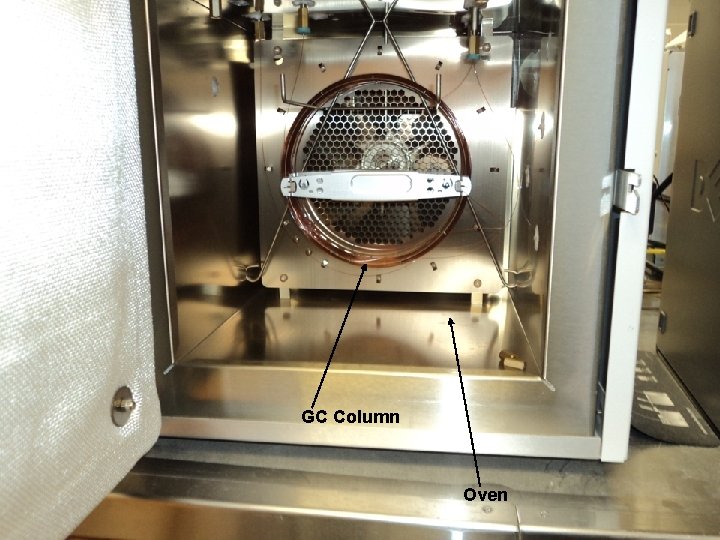
GC Column Oven

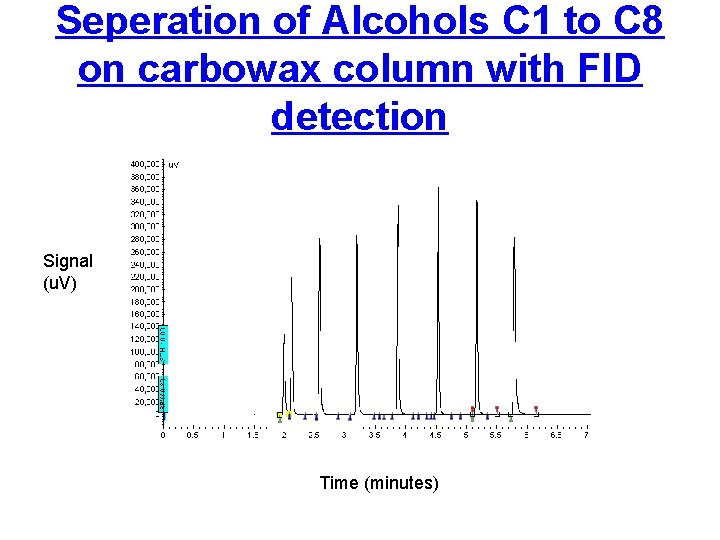
How Does Gas Chromatography Separate compounds • Chromatographic technique that separates volatile organic compounds. • A gas chromatograph consists of a 1) flowing mobile phase, 2) injection port, 3) a separation column containing the stationary phase, 4) controller (computer) detector and a (integrator) and data collection and storage (computer). • Organic compounds are separated due to differences in their partitioning behavior between the mobile gas phase and the stationary phase in the column.

Seperation of Alcohols C 1 to C 8 on carbowax column with FID detection Signal (u. V) Time (minutes)

How Does MS Measure Mass • Deflection of ions in electric or magnetic field which is related to mass of ion. • Ions need to be charged. Can be either positive or negative.

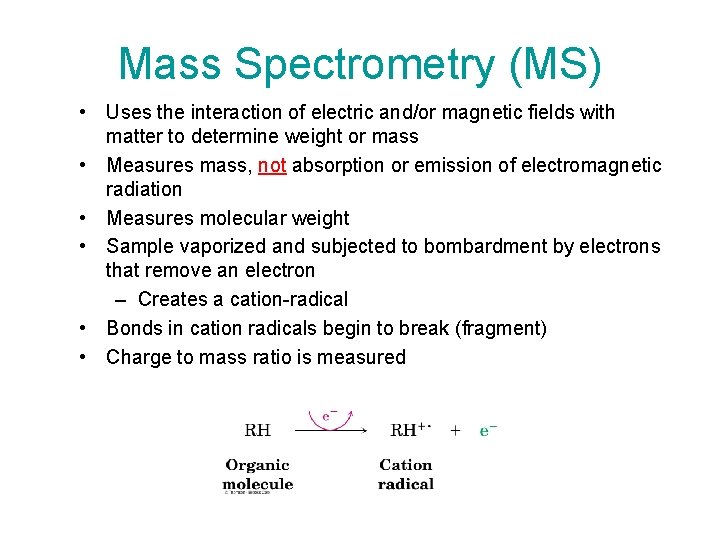
Mass Spectrometry (MS) • Uses the interaction of electric and/or magnetic fields with matter to determine weight or mass • Measures mass, not absorption or emission of electromagnetic radiation • Measures molecular weight • Sample vaporized and subjected to bombardment by electrons that remove an electron – Creates a cation-radical • Bonds in cation radicals begin to break (fragment) • Charge to mass ratio is measured

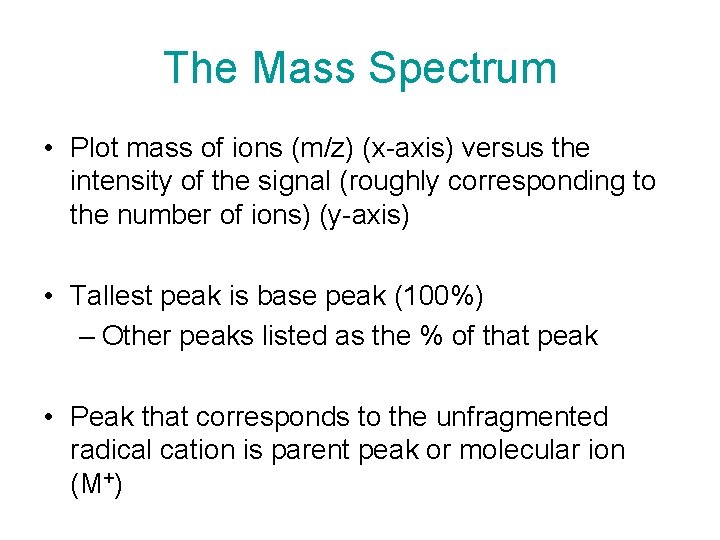
The Mass Spectrum • Plot mass of ions (m/z) (x-axis) versus the intensity of the signal (roughly corresponding to the number of ions) (y-axis) • Tallest peak is base peak (100%) – Other peaks listed as the % of that peak • Peak that corresponds to the unfragmented radical cation is parent peak or molecular ion (M+)

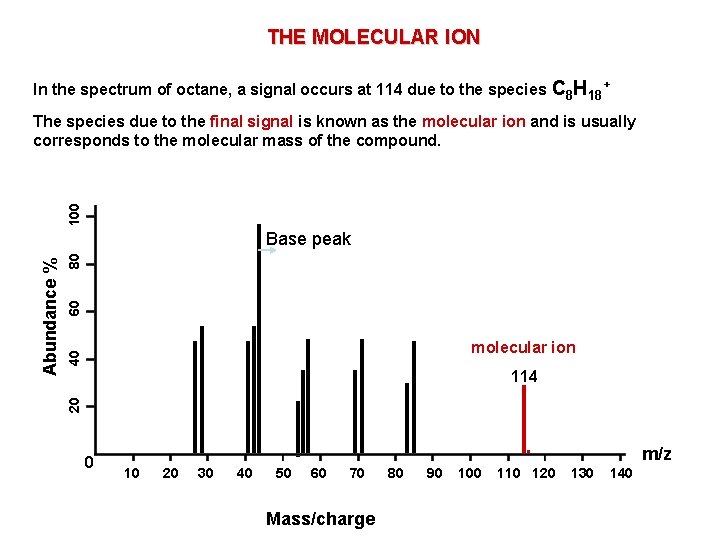
THE MOLECULAR ION In the spectrum of octane, a signal occurs at 114 due to the species C 8 H 18+ 100 The species due to the final signal is known as the molecular ion and is usually corresponds to the molecular mass of the compound. 80 60 40 molecular ion 114 20 Abundance % Base peak 0 . 10 20 30 40 50 60 70 Mass/charge 80 90 100 110 120 m/z 130 140

THE MASS SPECTRUM 1) Spectra (full scan) obtained for organic molecules have many peaks. 2) Each peak is due to a particular fragment with a certain m/z value. 3) Highest m/z value usually corresponds to the molecular ion 4) The tallest peaks come from the most stable species

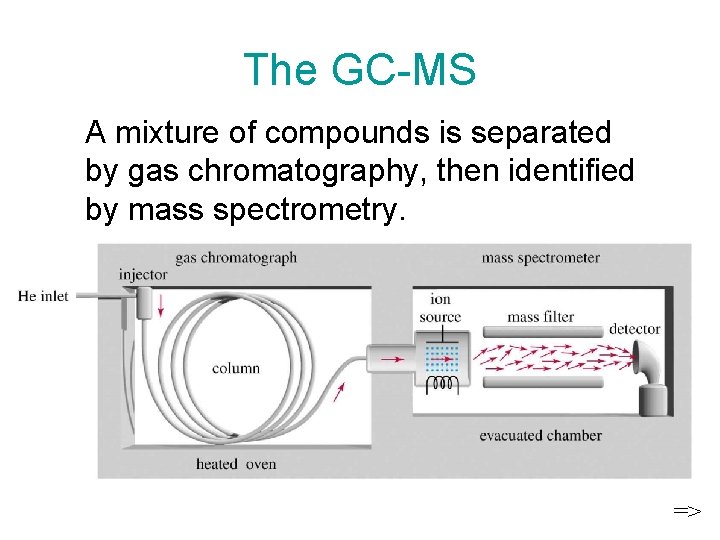
The GC-MS A mixture of compounds is separated by gas chromatography, then identified by mass spectrometry. =>

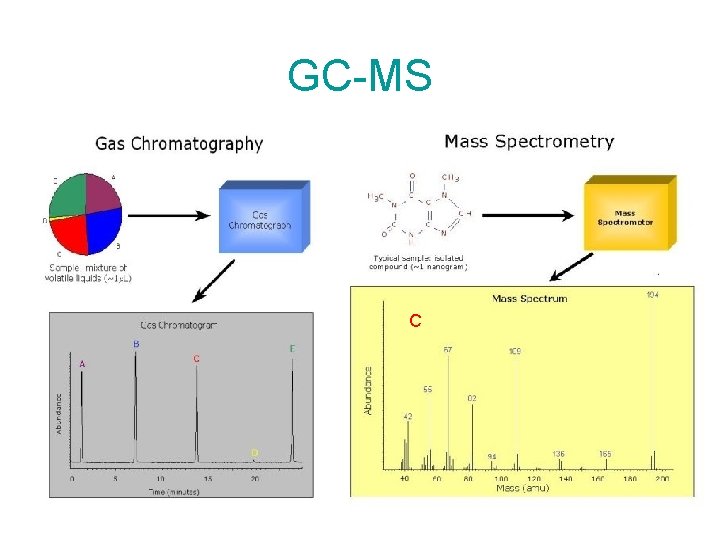
GC-MS C

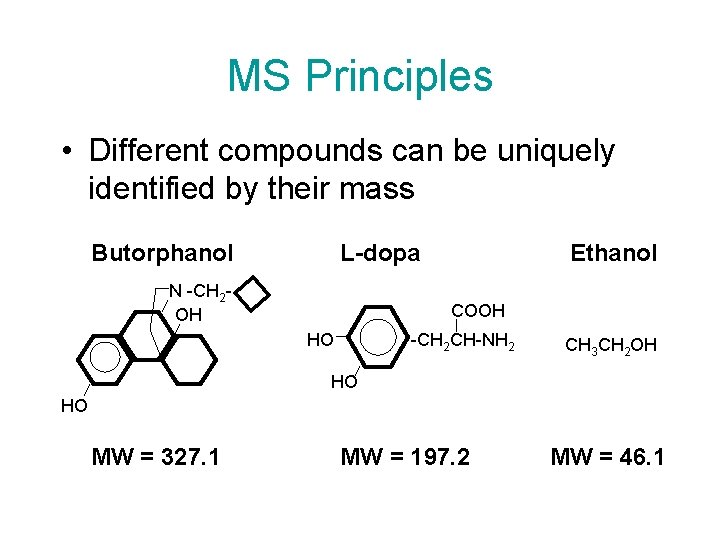
MS Principles • Different compounds can be uniquely identified by their mass Butorphanol L-dopa N -CH 2 OH Ethanol COOH HO -CH 2 CH-NH 2 CH 3 CH 2 OH HO HO MW = 327. 1 MW = 197. 2 MW = 46. 1

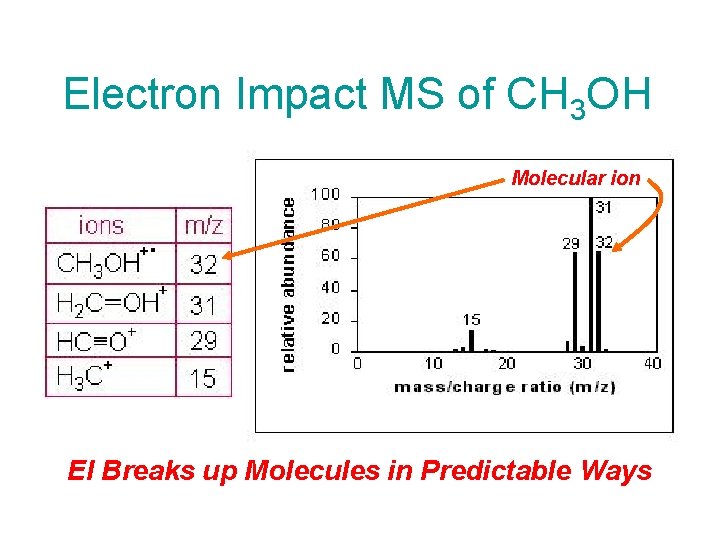
Electron Impact MS of CH 3 OH Molecular ion EI Breaks up Molecules in Predictable Ways

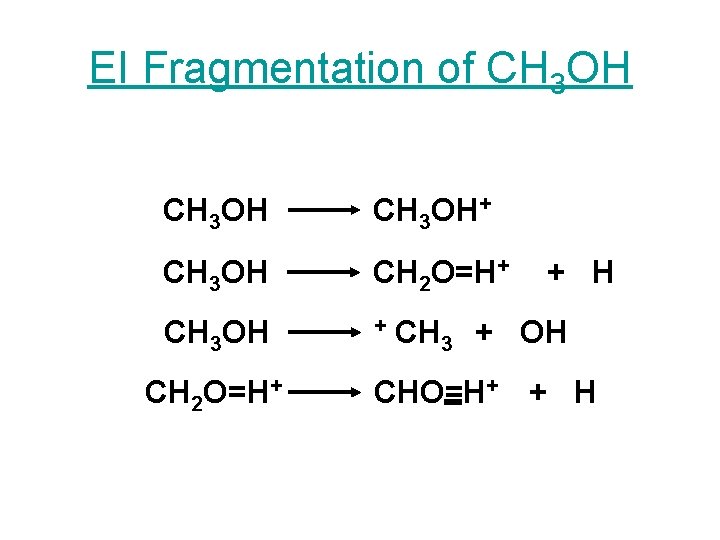
EI Fragmentation of CH 3 OH+ CH 3 OH CH 2 O=H+ CH 3 OH + CH 2 O=H+ + H CH 3 + OH CHO=H+ + H

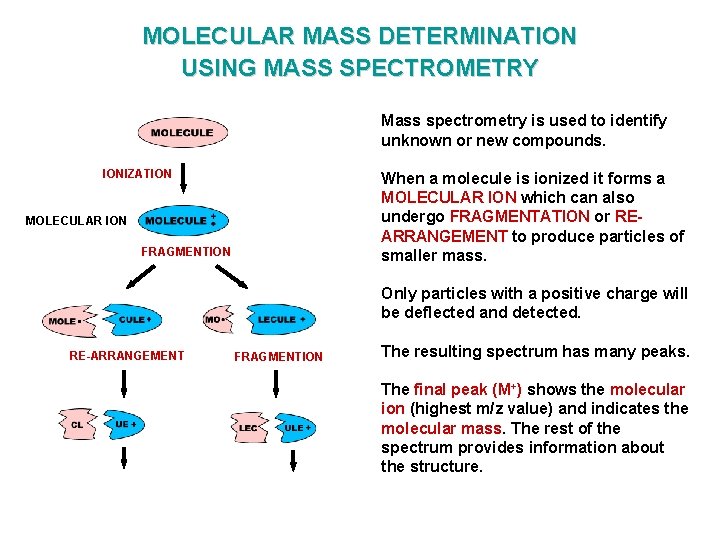
MOLECULAR MASS DETERMINATION USING MASS SPECTROMETRY Mass spectrometry is used to identify unknown or new compounds. IONIZATION When a molecule is ionized it forms a MOLECULAR ION which can also undergo FRAGMENTATION or REARRANGEMENT to produce particles of smaller mass. MOLECULAR ION FRAGMENTION Only particles with a positive charge will be deflected and detected. RE-ARRANGEMENT FRAGMENTION The resulting spectrum has many peaks. The final peak (M+) shows the molecular ion (highest m/z value) and indicates the molecular mass. The rest of the spectrum provides information about the structure.

ce vials Liquid sampling syringe Heating block with stirrer

GC-MS in MCAL Peppermint and Lavendar Essential Oils Experiment

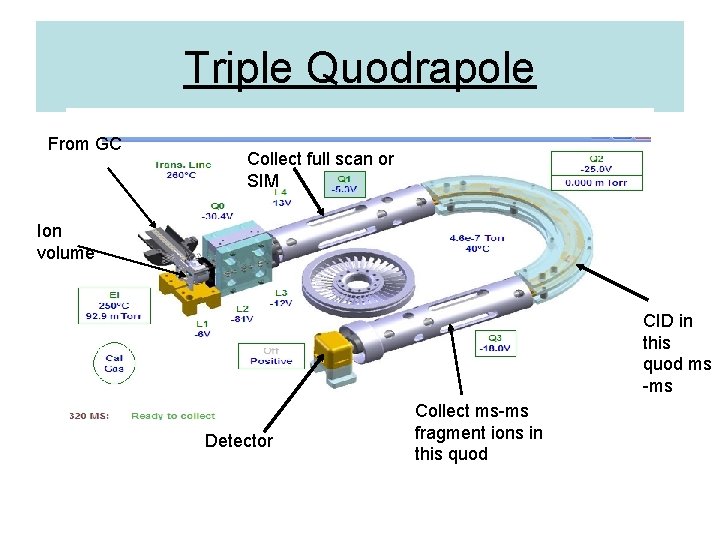
Triple Quodrapole From GC Collect full scan or SIM Ion volume CID in this quod ms -ms Detector Collect ms-ms fragment ions in this quod

MS The gas containing the separated analytes from the GC are ionized (as they elute from the GC) in the ion volume and the ions focused into the first quadrapole (Q 1). The first quadrapole can measure the ions (full scan or selected ions [SIM]) or select certain ions to be transported to the collission quad (Q 2). In Q 2 the ions can be broken into smaller fragments by interaction with energized argon collission gas (CID). These ions are transported to the Q 3 where they are analysed usually as single or small groups of ions (MSMS). These ions are directed to the detector

GC-MS • The MS is equipped with a triple quadrupole analyzer and allows several types of mass detection to be performed. • Full scan (direct MS) mode is used. • SIM can be used • MS-MS can be used

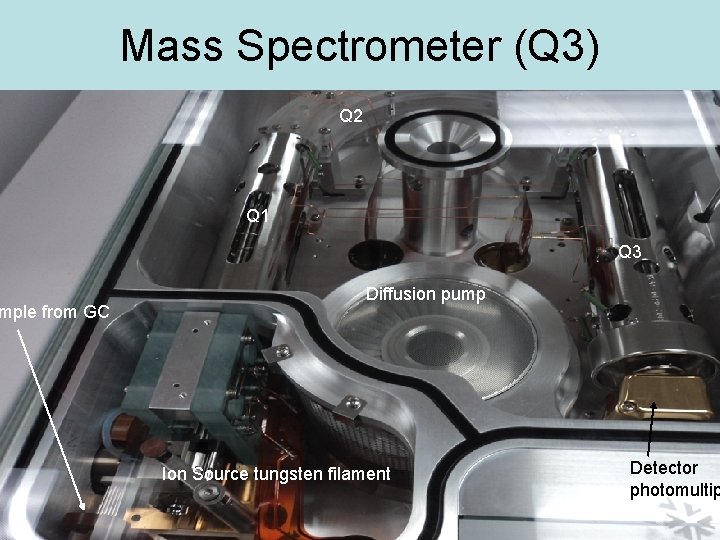
mple from GC Mass Spectrometer (Q 3) Q 2 Q 1 Q 3 Diffusion pump Ion Source tungsten filament Detector photomultip

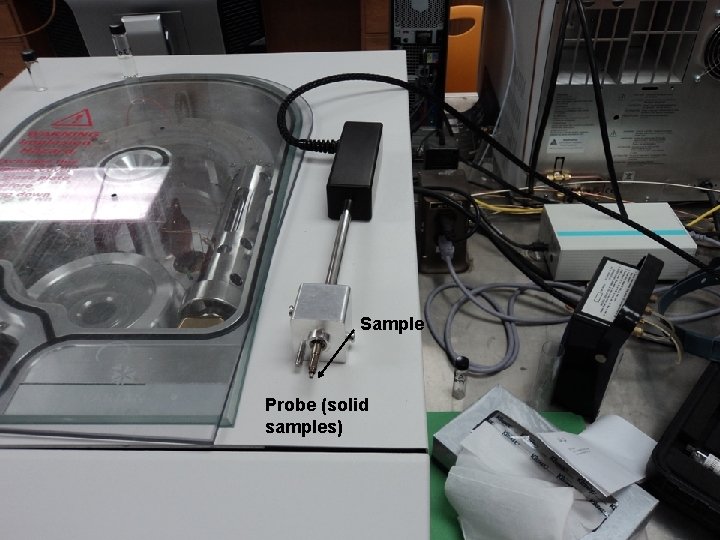
Sample Probe (solid samples)

Probe

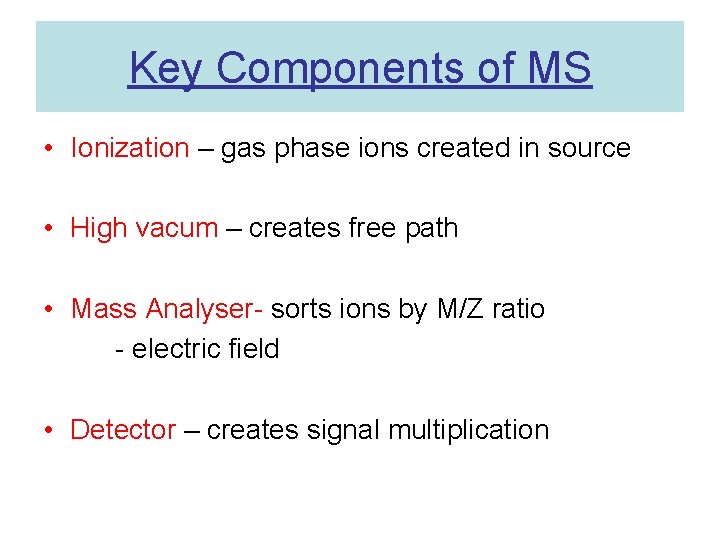
Key Components of MS • Ionization – gas phase ions created in source • High vacum – creates free path • Mass Analyser- sorts ions by M/Z ratio - electric field • Detector – creates signal multiplication

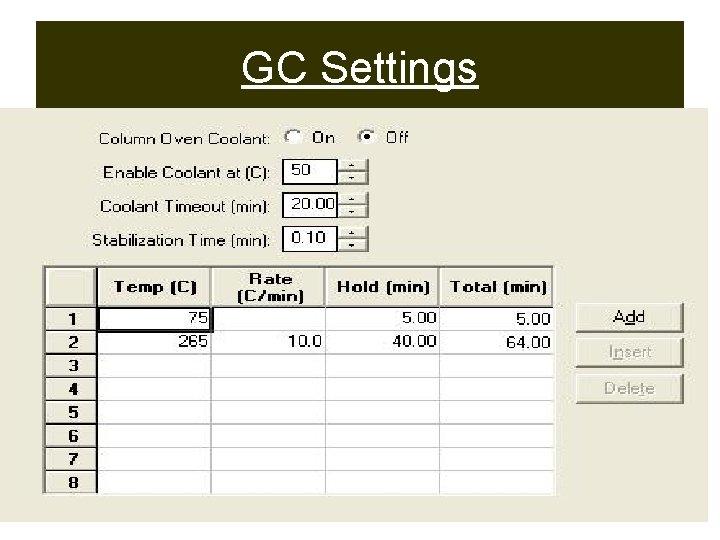
GC Settings

Varian (Agilent) Factor. Four Capillary GC Columns. • Capillary column: thin fusedsilica capillary. • 50 m in length and 250 µm inner diameter. • The stationary phase is CPsil 8, with 5% phenyl 95% dimethyl polysiloxane coated on the inner surface.

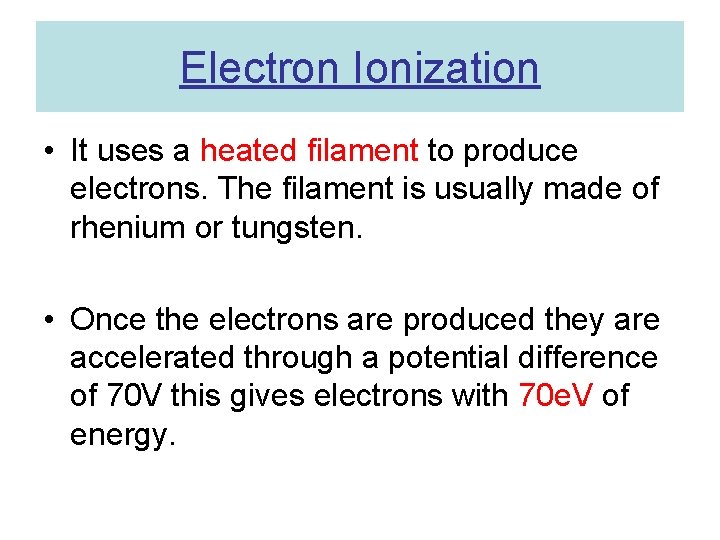
Electron Ionization • It uses a heated filament to produce electrons. The filament is usually made of rhenium or tungsten. • Once the electrons are produced they are accelerated through a potential difference of 70 V this gives electrons with 70 e. V of energy.

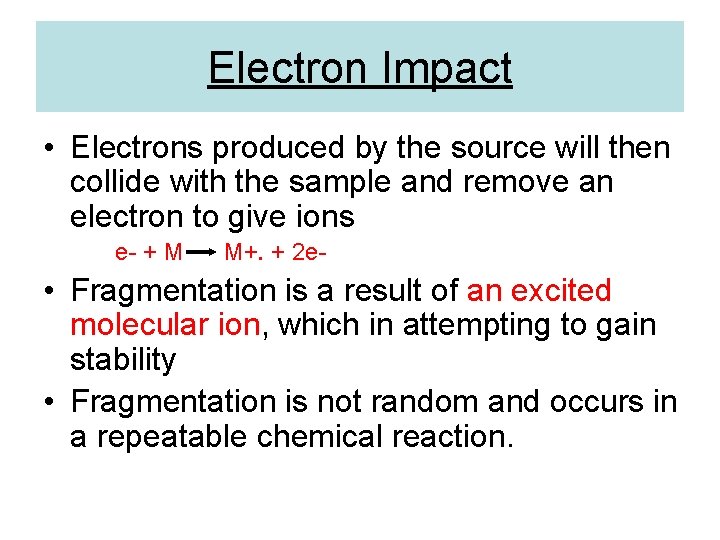
Electron Impact • Electrons produced by the source will then collide with the sample and remove an electron to give ions e- + M M+. + 2 e- • Fragmentation is a result of an excited molecular ion, which in attempting to gain stability • Fragmentation is not random and occurs in a repeatable chemical reaction.

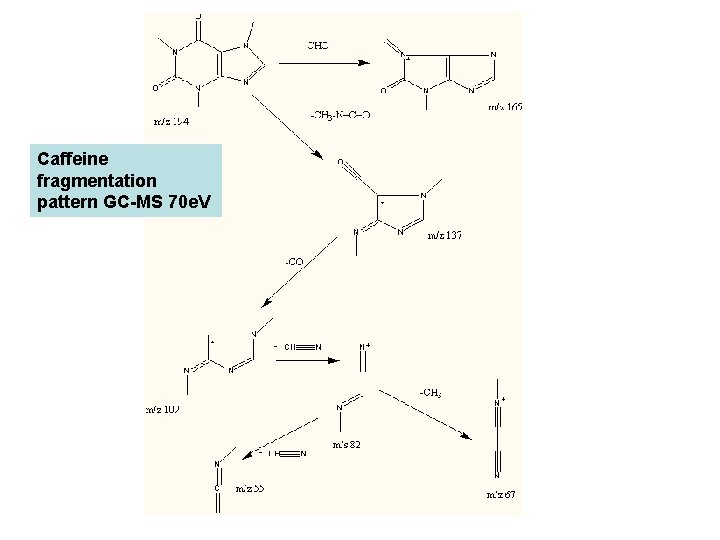
Caffeine fragmentation pattern GC-MS 70 e. V

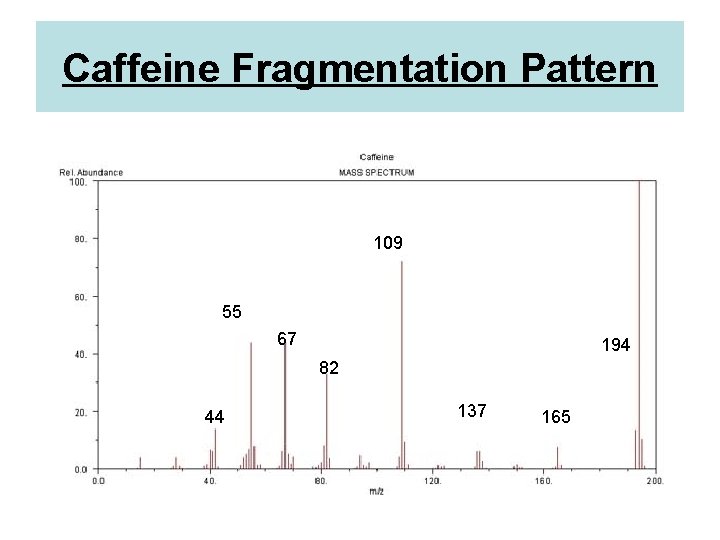
Caffeine Fragmentation Pattern 109 55 67 194 82 44 137 165

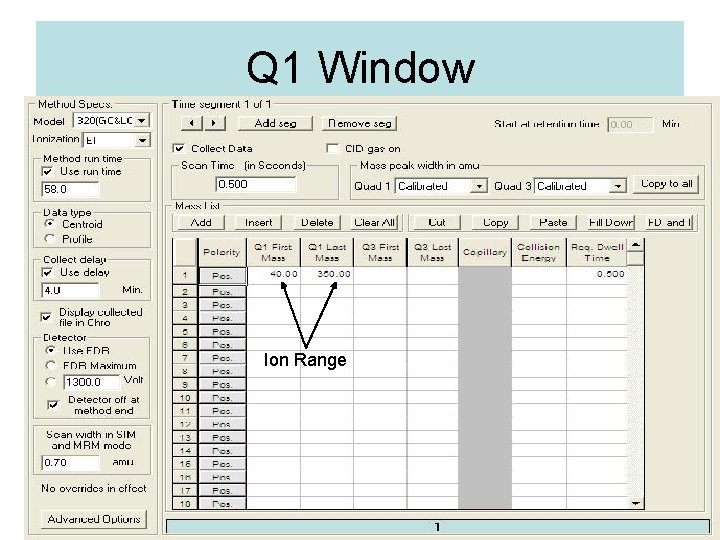
Q 1 Window Ion Range

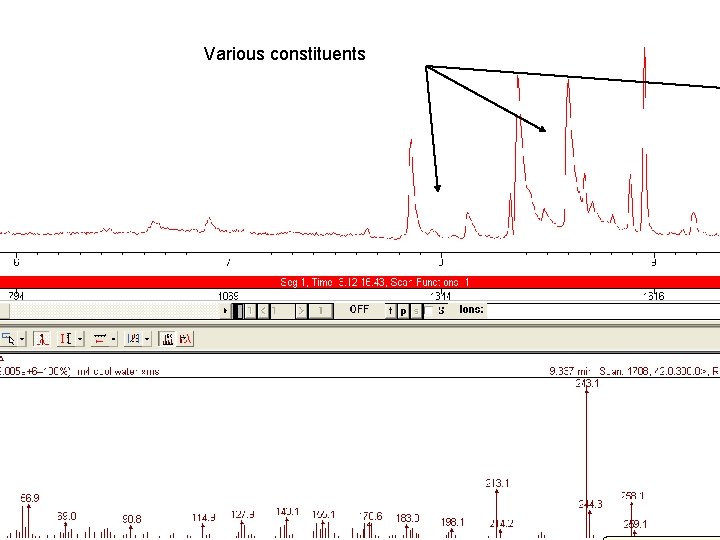
Various constituents


Quantitation of Ingredients • You usually quantitate the ingredient that you are interested in by: – SIM which measures one of the ions in your sample or – MSMS which measures one of the ions produced in the collission cell (Quad 2)

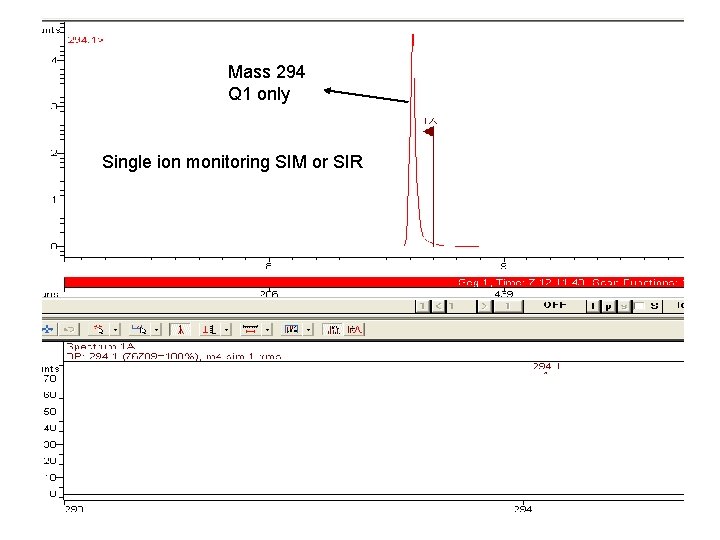
Mass 294 Q 1 only Single ion monitoring SIM or SIR

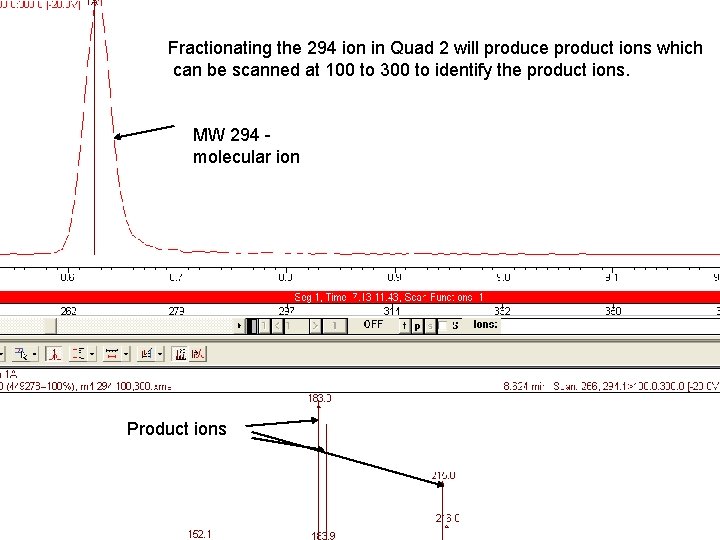
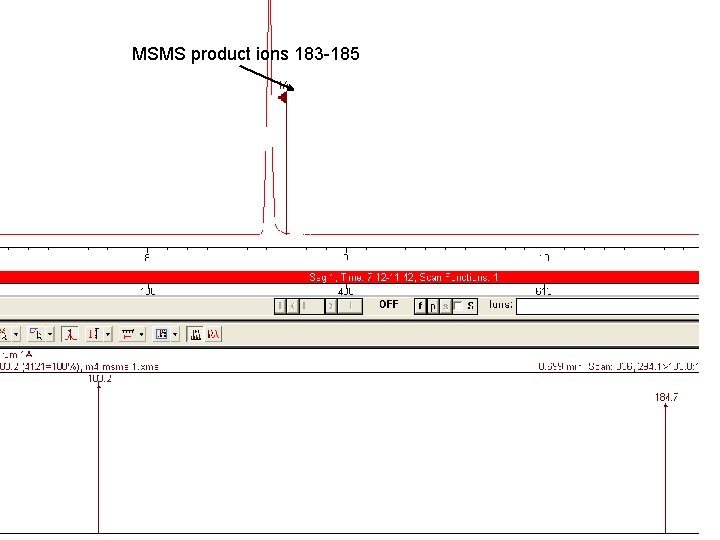
Fractionating the 294 ion in Quad 2 will produce product ions which can be scanned at 100 to 300 to identify the product ions. MW 294 molecular ion Product ions

MSMS product ions 183 -185