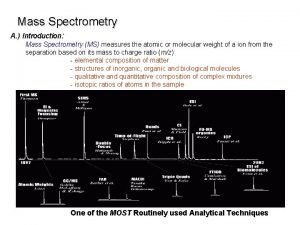
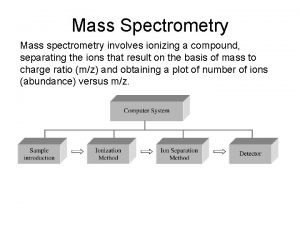
MASS SPECTROMETRY CONTENTS What is mass spectrometry MS


















- Slides: 44

MASS SPECTROMETRY

CONTENTS What is mass spectrometry (MS)? What Information does mass spectrometry provide? Where are mass spectrometers used? How does a mass spectrometer work? Introduction Sample introduction Methods of sample ionization Analysis and separation of sample ions Detection and recording of sample ions Electrospray ionization Electrospray ionisation Nanospray ionisation Data processing 9/9/2021 2

CONTENTS Matrix assisted laser desorption ionization Positive or negative ionization? Tandem mass spectrometry (MS-MS): Structural and sequence information from mass spectrometry Tandem mass spectrometry analyses Peptide sequencing by tandem mass spectrometry Oligonucleotide sequencing by tandem mass spectrometry 9/9/2021 3

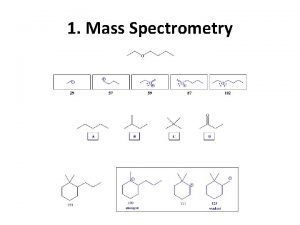
1. What is mass spectrometry (MS)? What information does mass spectrometry provide? Mass spectrometry is an analytical tool used for measuring the molecular mass of a sample. For large samples, molecular masses can be measured to within an accuracy of 0. 01% of the total molecular mass of the sample i. e. within a 4 Daltons (Da) or atomic mass units (amu) error for a sample of 40, 000 Da. This is sufficient to allow minor mass changes to be detected. For small organic molecules the molecular mass can be measured to within an accuracy of 5 ppm or less, which is often sufficient to confirm the molecular formula of a compound, and is also a standard requirement for publication in a journal. Structural information can be generated using certain types of mass spectrometers, usually those with multiple analyzers which are known as tandem mass spectrometers. This is achieved by fragmenting the sample inside the instrument and analyzing the products generated. This procedure is useful for the structural elucidation of organic compounds and for peptide or oligonucleotide sequencing, polymers. 9/9/2021 4

2. Where are mass spectrometers used? Mass spectrometers are used in industry and academia for both routine and research purposes. The following list is just a brief summary of the major mass spectrometric applications: Macromolecules and Oligomers Biotechnology: the analysis of proteins, peptides, oligonucleotides Pharmaceutical: drug discovery, combinatorial chemistry, pharmacokinetics, drug metabolism Clinical: neonatal screening, hemoglobin analysis, drug testing Environmental: PAHs, PCBs, water quality, food contamination Geological: oil composition 9/9/2021 5

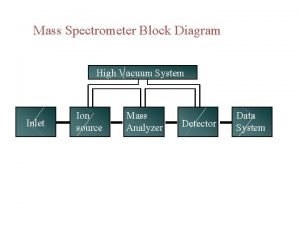
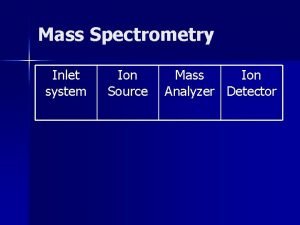
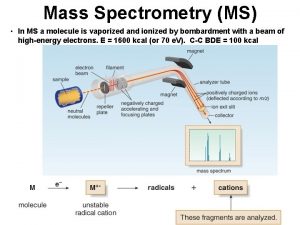
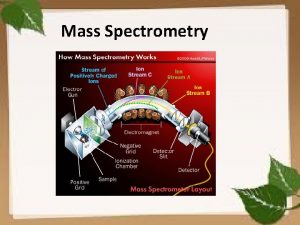
4. How does a mass spectrometer work? Mass spectrometers can be divided into three fundamental parts, namely the ionisation source , the analyzer , and the detector. Ø The sample has to be introduced into the ionization source of the instrument. Once inside the ionization source, the sample molecules are ionized, because ions are easier to manipulate than neutral molecules. These ions are extracted into the analyzer region of the mass spectrometer where they are separated according to their mass (m) -to-charge (z) ratios (m/z). The separated ions are detected and this signal sent to a data system where the m/z ratios are stored together with their relative abundance for presentation in the format of a m/z spectrum. 9/9/2021 6

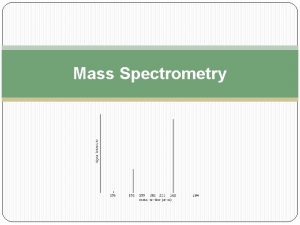
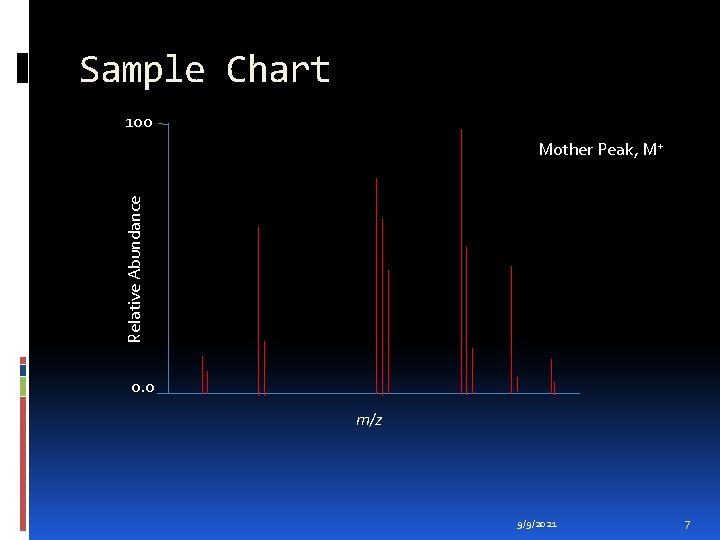
Sample Chart 100 Relative Abundance Mother Peak, M+ 0. 0 m/z 9/9/2021 7

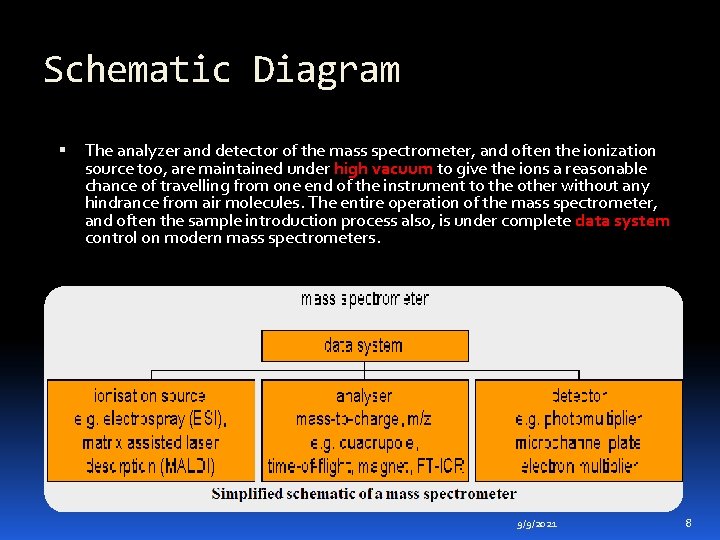
Schematic Diagram The analyzer and detector of the mass spectrometer, and often the ionization source too, are maintained under high vacuum to give the ions a reasonable chance of travelling from one end of the instrument to the other without any hindrance from air molecules. The entire operation of the mass spectrometer, and often the sample introduction process also, is under complete data system control on modern mass spectrometers. 9/9/2021 8

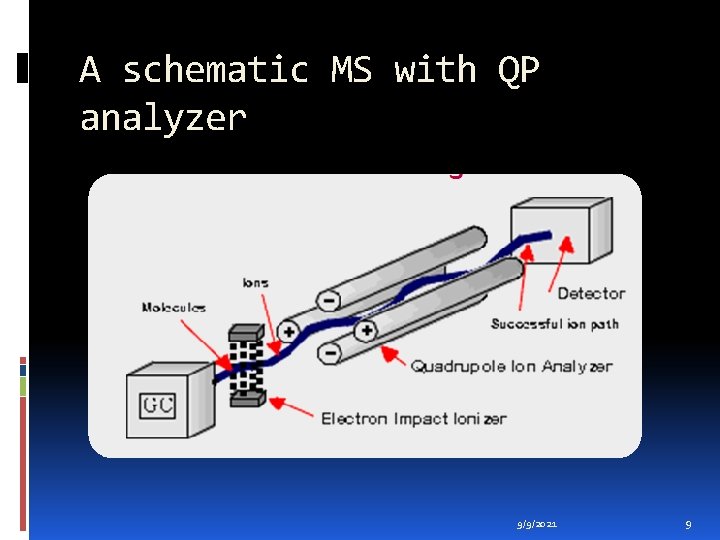
A schematic MS with QP analyzer 9/9/2021 9

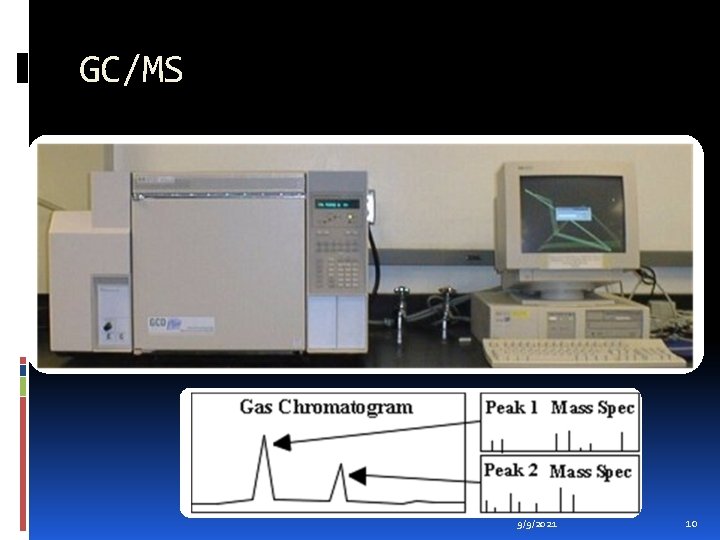
GC/MS 9/9/2021 10

Sample introduction The method of sample introduction to the ionization source often depends on the ionization method being used, as well as the type and complexity of the sample. The sample can be inserted directly into the ionisation source, or can undergo some type of chromatography to the ionization source. This latter method of sample introduction usually involves the mass spectrometer being coupled directly to a high pressure liquid chromatography (HPLC), gas chromatography (GC) or capillary electrophoresis (CE) separation column, and hence the sample is separated into a series of components which then enter the mass spectrometer sequentially for individual analysis. 9/9/2021 11

Methods of sample ionisation The ionisation method to be used should depend on the type of sample under investigation and the mass spectrometer available. Atmospheric Pressure Chemical Ionisation (APCI) Chemical Ionisation (CI) Electron Impact (EI) Electrospray Ionisation (ESI) Fast Atom Bombardment (FAB) Field Desorption / Field Ionisation (FD/FI) Matrix Assisted Laser Desorption Ionisation (MALDI) Thermospray Ionisation (TSP) 9/9/2021 12

Competition of Different Methods 9/9/2021 13

Analysis and Separation of Sample Ions The main function of the mass analyser is to separate , or resolve , the ions formed in the ionisation source of the mass spectrometer according to their mass-to-charge (m/z) ratios. Quadrupoles (QP), Time-of-flight (TOF), Magnetic sectors, Fourier transform Quadrupole ion traps. 9/9/2021 14

Analysis and Separation of Sample Ions (Cont. ) These mass analyzers have different features, including the m/z range that can be covered, the mass accuracy, and the achievable resolution. The compatibility of different analysers with different ionization methods varies. For example, all of the analyzers listed above can be used in conjunction with electrospray ionisation, whereas MALDI is not usually coupled to a quadrupole analyser. 9/9/2021 15

Analysis and Separation of Sample Ions (Cont. ) Tandem (MS-MS) mass spectrometers are instruments that have more than one analyser and so can be used for structural and sequencing studies. Two, three and four analysers have all been incorporated into commercially available tandem instruments, and the analysers do not necessarily have to be of the same type, in which case the instrument is a hybrid one. More popular tandem mass spectrometers include those of the quadrupole-quadrupole, magnetic sector-quadrupole , and more recently, the quadrupole-time-of-flight geometries 9/9/2021 16

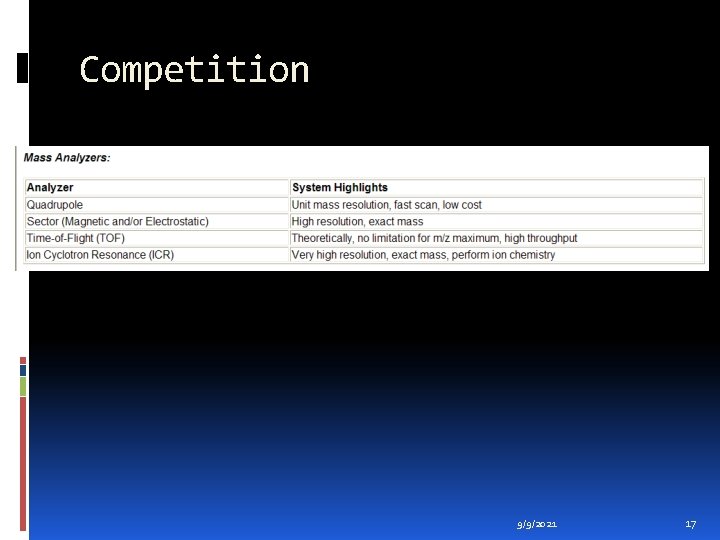
Competition 9/9/2021 17

Detection and recording of sample ions The detector monitors the ion current, amplifies it and the signal is then transmitted to the data system where it is recorded in the form of mass spectra. The m/z values of the ions are plotted against their intensities to show the number of components in the sample, the molecular mass of each component, and the relative abundance of the various components in the sample. Photomultiplier, electron multiplier micro-channel plate detectors. 9/9/2021 18

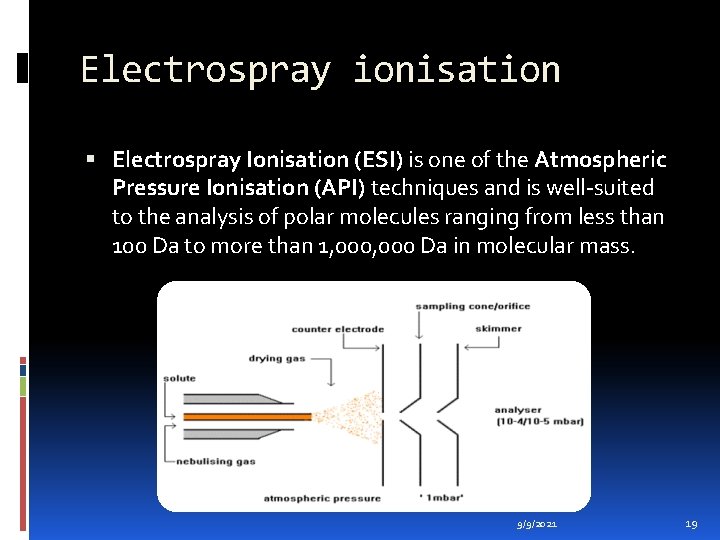
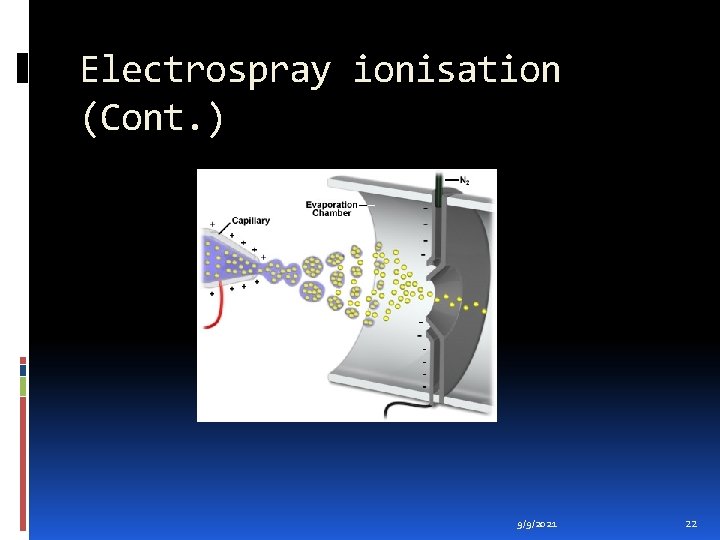
Electrospray ionisation Electrospray Ionisation (ESI) is one of the Atmospheric Pressure Ionisation (API) techniques and is well-suited to the analysis of polar molecules ranging from less than 100 Da to more than 1, 000 Da in molecular mass. 9/9/2021 19

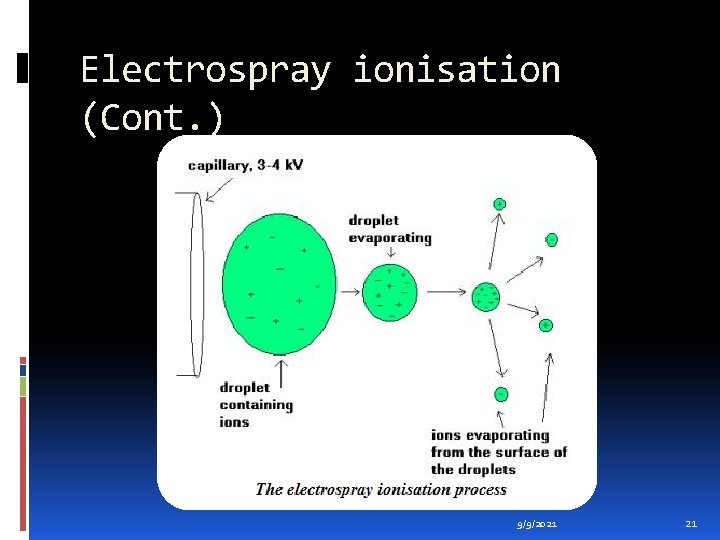
Electrospray ionisation (Cont. ) During standard electrospray ionisation (J. Fenn, J. Phys. Chem. , 1984, 88, 4451), the sample is dissolved in a polar, volatile solvent and pumped through a narrow, stainless steel capillary (75 - 150 micrometers i. d. ) at a flow rate of between 1 µL/min and 1 m. L/min. A high voltage of 3 or 4 k. V is applied to the tip of the capillary, which is situated within the ionisation source of the mass spectrometer, and as a consequence of this strong electric field, the sample emerging from the tip is dispersed into an aerosol of highly charged droplets, a process that is aided by a co-axially introduced nebulising gas flowing around the outside of the capillary. This gas, usually nitrogen, helps to direct the spray emerging from the capillary tip towards the mass spectrometer. The charged droplets diminish in size by solvent evaporation, assisted by a warm flow of nitrogen known as the drying gas which passes across the front of the ionisation source. Eventually charged sample ions, free from solvent, are released from the droplets, some of which pass through a sampling cone or orifice into an intermediate vacuum region, and from there through a small aperture into the analyser of the mass spectrometer, which is held under high vacuum. The lens voltages are optimised individually for each sample. 9/9/2021 20

Electrospray ionisation (Cont. ) 9/9/2021 21

Electrospray ionisation (Cont. ) 9/9/2021 22

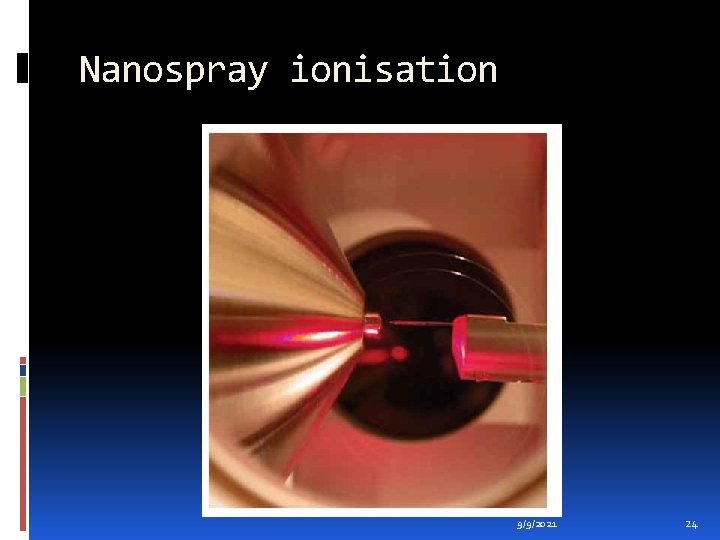
Nanospray ionisation (M. Wilm, M. Mann, Anal. Chem. , 1996, 68, 1) is a low flow rate version of electrospray ionisation. A small volume (1 -4 micro. L) of the sample dissolved in a suitable volatile solvent, at a concentration of ca. 1 - 10 pmol/micro. L, is transferred into a miniature sample vial. A reasonably high voltage (ca. 700 - 2000 V) is applied to the specially manufactured gold-plated vial resulting in sample ionisation and spraying. The flow rate of solute and solvent using this procedure is very low, 30 - 1000 n. L/min, and so not only is far less sample consumed than with the standard electrospray ionisation technique, but also a small volume of sample lasts for several minutes, thus enabling multiple experiments to be performed. A common application of this technique is for a protein digest mixture to be analysed to generate a list of molecular masses for the components present, and then each component to be analysed further by tandem mass spectrometric (MS-MS) techniques. 9/9/2021 23

Nanospray ionisation 9/9/2021 24

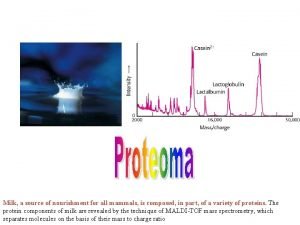
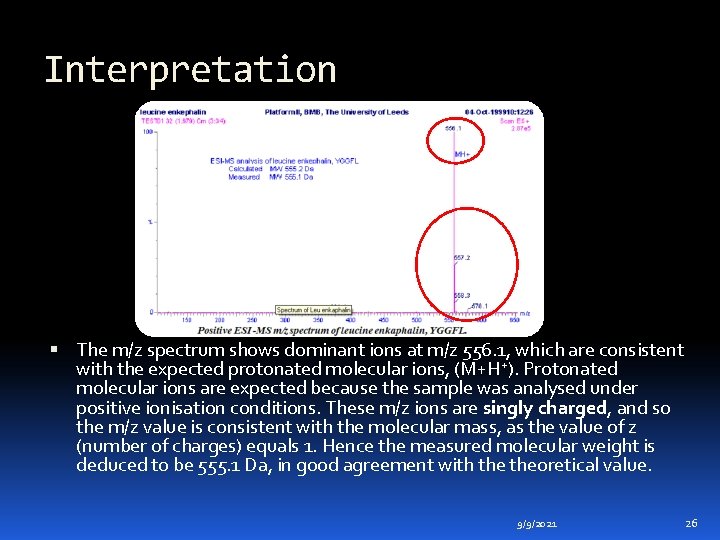
Example An example of this type of sample analysis is shown in the m/z spectrum of the pentapeptide leucine enkephalin, YGGFL. The molecular formula for this compound is C 28 H 37 N 5 O 7 and the calculated monoisotopic molecular weight is 555. 2692 Da. 9/9/2021 25

Interpretation The m/z spectrum shows dominant ions at m/z 556. 1, which are consistent with the expected protonated molecular ions, (M+H+). Protonated molecular ions are expected because the sample was analysed under positive ionisation conditions. These m/z ions are singly charged, and so the m/z value is consistent with the molecular mass, as the value of z (number of charges) equals 1. Hence the measured molecular weight is deduced to be 555. 1 Da, in good agreement with theoretical value. 9/9/2021 26

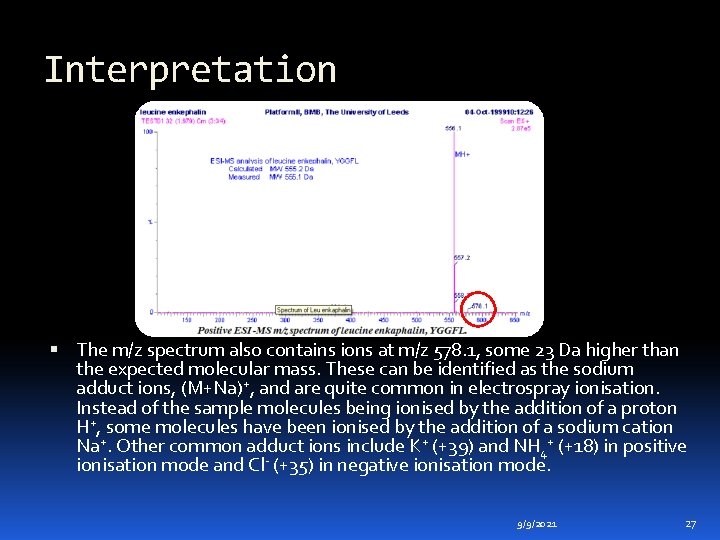
Interpretation The m/z spectrum also contains ions at m/z 578. 1, some 23 Da higher than the expected molecular mass. These can be identified as the sodium adduct ions, (M+Na)+, and are quite common in electrospray ionisation. Instead of the sample molecules being ionised by the addition of a proton H+, some molecules have been ionised by the addition of a sodium cation Na+. Other common adduct ions include K+ (+39) and NH 4+ (+18) in positive ionisation mode and Cl- (+35) in negative ionisation mode. 9/9/2021 27

Multiple charged species Electrospray ionisation is known as a "soft" ionisation method as the sample is ionised by the addition or removal of a proton, with very little extra energy remaining to cause fragmentation of the sample ions. Samples (M) with molecular weights greater than ca. 1200 Da give rise to multiply charged molecular-related ions such as (M+n. H)n+ in positive ionisation mode and (M-n. H)n- in negative ionisation mode. Proteins have many suitable sites for protonation as all of the backbone amide nitrogen atoms could be protonated theoretically, as well as certain amino acid side chains such as lysine and arginine which contain primary amine functionalities. 9/9/2021 28

An example of multiple charging An example of multiple charging, which is practically unique to electrospray ionisation, is presented in the positive ionisation m/z spectrum of the protein hen egg white lysozyme. 9/9/2021 29

Interpretation The m/z values can be expressed as follows: m/z = (MW + n. H+)/n where m/z = the mass-to-charge ratio marked on the abscissa of the spectrum; MW = the molecular mass of the sample n = the integer number of charges on the ions H = the mass of a proton = 1. 008 Da. If the number of charges on an ion is known, then it is simply a matter of reading the m/z value from the spectrum and solving the above equation to determine the molecular weight of the sample. Usually the number of charges is not known, but can be calculated if the assumption is made that any two adjacent members in the series of multiply charged ions differ by one charge. 9/9/2021 30

Interpretation For example, if the ions appearing at m/z =1431. 6 in the lysozyme spectrum have "n" charges, then the ions at m/z= 1301. 4 will have "n+1" charges, and the above equation can be written again for these two ions: 1431. 6 = (MW + n. H+)/n and 1301. 4 = [MW + (n+1)H+] /(n+1) These simultaneous equations can be rearranged to exclude the MW term: and so: n(1431. 6) - n. H+ = (n+1)1301. 4 - (n+1)H+ n(1431. 6) = n(1301. 4) +1301. 4 - H+ therefore: n(1431. 6 - 1301. 4) = 1301. 4 - H+ and so: n = (1301. 4 - H+) / (1431. 6 - 1301. 4) hence the number of charges on the ions at m/z 1431. 6 = 1300. 4/130. 2 = 10. Putting the value of n back into the equation: 1431. 6 = (MW + n. H+) n gives 1431. 6 x 10 = MW + (10 x 1. 008) and so MW = 14, 316 - 10. 08 therefore MW = 14, 305. 9 Da 9/9/2021 31

MS, an Overview 9/9/2021 32

Different Analyzers General: The effect of electromagnetic fields on ions All commonly used mass analyzers use electric and magnetic fields to apply a force on charged particles (ions). The relationship between force, mass, and the applied fields can be summarized in Newton's second law and the Lorentz force law: 9/9/2021 33

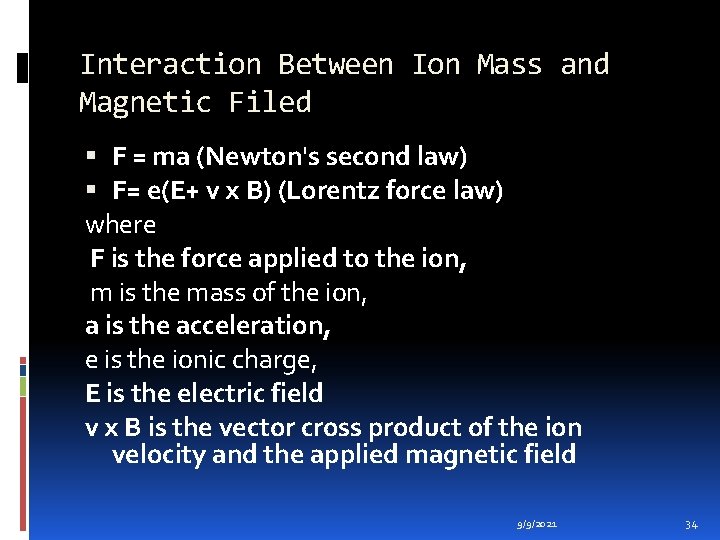
Interaction Between Ion Mass and Magnetic Filed F = ma (Newton's second law) F= e(E+ v x B) (Lorentz force law) where F is the force applied to the ion, m is the mass of the ion, a is the acceleration, e is the ionic charge, E is the electric field v x B is the vector cross product of the ion velocity and the applied magnetic field 9/9/2021 34

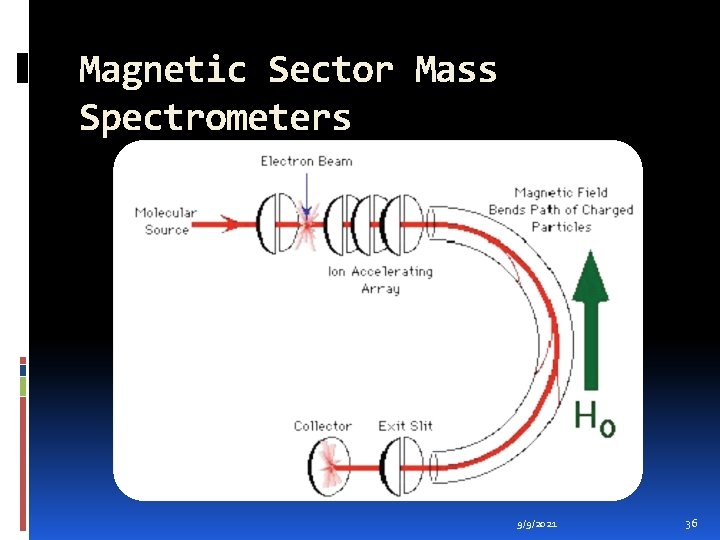
Magnetic Sector Mass Spectrometers In a magnetic deflection mass spectrometer, ions leaving the ion source are accelerated to a high velocity. The ions then pass through a magnetic sector in which the magnetic field is applied in a direction perpendicular to the direction of ion motion. From physics, we know that when acceleration is applied perpendicular to the direction of motion of an object, the object's velocity remains constant, but the object travels in a circular path. Therefore, the magnetic sector follows an arc; the radius and angle of the arc vary with different ions. 9/9/2021 35

Magnetic Sector Mass Spectrometers 9/9/2021 36

Magnetic Sector Mass Spectrometers A magnetic sector alone will separate ions according to their mass-to-charge ratio. However, the resolution will be limited by the fact that ions leaving the ion source do not all have exactly the same energy and therefore do not have exactly the same velocity. This is analogous to the chromatic aberration in optical spectroscopy. To achieve better resolution, it is necessary to add an electric sector that focuses ions according to their kinetic energy. Like the magnetic sector, the electric sector applies a force perpendicular to the direction of ion motion, and therefore has the form of an arc. 9/9/2021 37

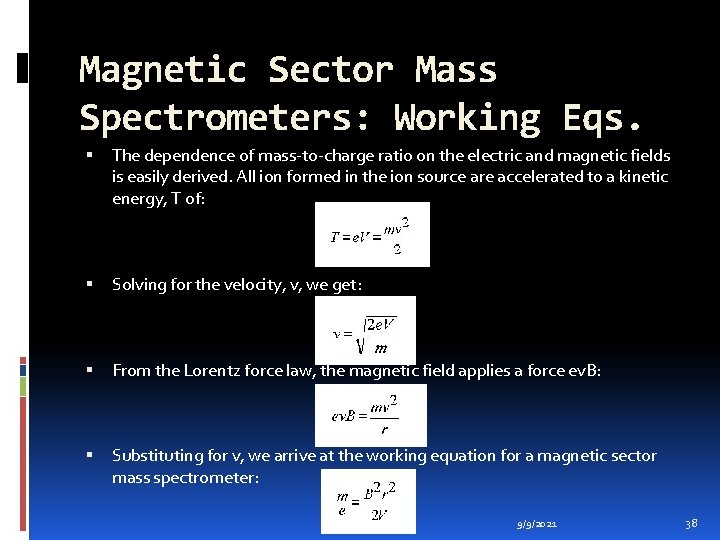
Magnetic Sector Mass Spectrometers: Working Eqs. The dependence of mass-to-charge ratio on the electric and magnetic fields is easily derived. All ion formed in the ion source are accelerated to a kinetic energy, T of: Solving for the velocity, v, we get: From the Lorentz force law, the magnetic field applies a force ev. B: Substituting for v, we arrive at the working equation for a magnetic sector mass spectrometer: 9/9/2021 38

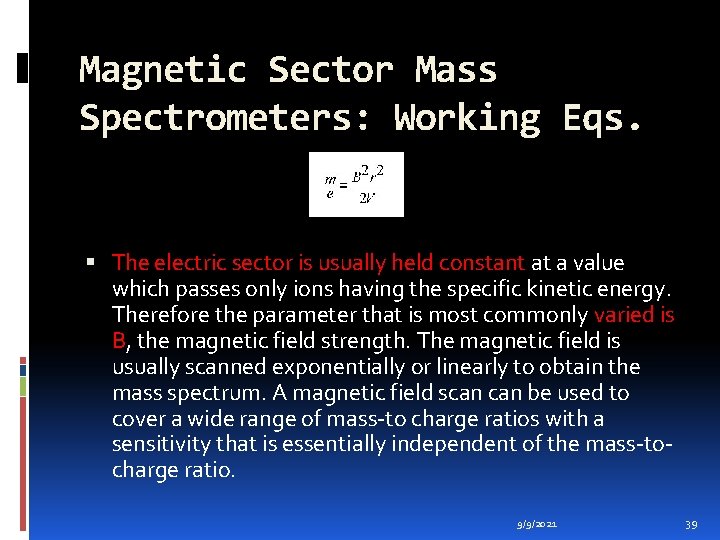
Magnetic Sector Mass Spectrometers: Working Eqs. The electric sector is usually held constant at a value which passes only ions having the specific kinetic energy. Therefore the parameter that is most commonly varied is B, the magnetic field strength. The magnetic field is usually scanned exponentially or linearly to obtain the mass spectrum. A magnetic field scan be used to cover a wide range of mass-to charge ratios with a sensitivity that is essentially independent of the mass-tocharge ratio. 9/9/2021 39

Magnetic Sector Mass Spectrometers Benefits Classical mass spectra Very high reproducibility Best quantitative performance of all mass spectrometer analyzers High resolution High sensitivity High dynamic range Linked scan MS/MS does not require another analyzer High-energy CID (Collision-induced dissociation) MS/MS spectra are very reproducible 9/9/2021 40

Magnetic Sector Mass Spectrometers Limitations Not well-suited for pulsed ionization methods (e. g. MALDI) Usually larger and higher cost than other mass analyzers Applications All organic MS analysis methods Accurate mass measurements Quantitation Isotope ratio measurements 9/9/2021 41

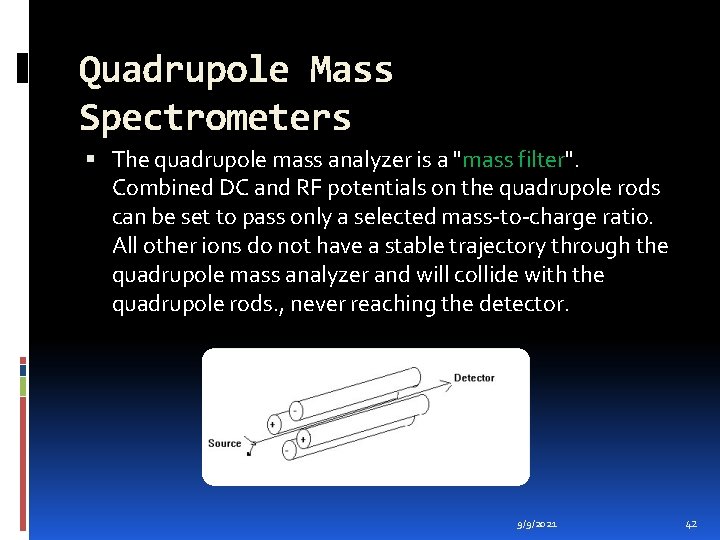
Quadrupole Mass Spectrometers The quadrupole mass analyzer is a "mass filter". Combined DC and RF potentials on the quadrupole rods can be set to pass only a selected mass-to-charge ratio. All other ions do not have a stable trajectory through the quadrupole mass analyzer and will collide with the quadrupole rods. , never reaching the detector. 9/9/2021 42

The strength and frequency of the RF field determines whether or not an ion of a certain mass passes through the rods (and is counted by the detector) or smashes into a nearby surface. For example, in a 120 volt field at a radio frequency of 2 MHz, only ions of 16 daltons (Da) will navigate through the rods and into the detector. Heavier or lighter ions do not survive the journey to the detector. In this manner, scientists can control the mass of the ions that the detector collects. 9/9/2021 43

Quadrupole Mass Spectrometers Benefits Limitations Classical mass spectra Good reproducibility Relatively small and low-cost systems Low-energy collision-induced dissociation (CID) MS/MS spectra in triple quadrupole and hybrid mass spectrometers have efficient conversion of precursor to product Limited resolution Peak heights variable as a function of mass (mass discrimination). Peak height vs. mass response must be 'tuned'. Not well suited for pulsed ionization methods Low-energy collision-induced dissociation (CID) MS/MS spectra in triple quadrupole and hybrid mass spectrometers depend strongly on energy, collision gas, pressure, and other factors. Applications Majority of bench top GC/MS and LC/MS systems Triple quadrupole MS/MS systems Sector / quadrupole hybrid MS/MS systems 9/9/2021 45
 Spectroscopy problem set
Spectroscopy problem set Mass spectrometry
Mass spectrometry Applications of mass spectroscopy
Applications of mass spectroscopy Past paper
Past paper 4-heptanone
4-heptanone Mass spectrometry
Mass spectrometry Sdbsweb
Sdbsweb Batch inlet system in mass spectrometry
Batch inlet system in mass spectrometry Deflection in mass spectrometry
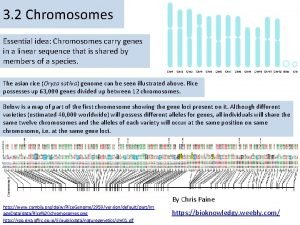
Deflection in mass spectrometry Chromosomes and alleles
Chromosomes and alleles Mass spectrometry lecture
Mass spectrometry lecture Mass spectrometry a level
Mass spectrometry a level Mass spectrometry
Mass spectrometry Esi, apci 차이
Esi, apci 차이 Mass spectrometry data acquisition for gc/ms
Mass spectrometry data acquisition for gc/ms Mass spectrometry in forensic science
Mass spectrometry in forensic science Mass spectrum of 2 chloropropane
Mass spectrum of 2 chloropropane Assortative
Assortative Accelerator mass spectrometry
Accelerator mass spectrometry Mass spectrometry
Mass spectrometry Mass spectrometry
Mass spectrometry Khan academy mass spectrometry
Khan academy mass spectrometry Nitrogen rule in mass spectrometry
Nitrogen rule in mass spectrometry Mass spectrometry ionization
Mass spectrometry ionization Swath mass spectrometry
Swath mass spectrometry Cold vapor atomic fluorescence spectrometry
Cold vapor atomic fluorescence spectrometry Cold vapor atomic fluorescence spectrometry
Cold vapor atomic fluorescence spectrometry Ion mobility spectrometry
Ion mobility spectrometry Table of contents for project proposal
Table of contents for project proposal Jcp račice
Jcp račice Edp course contents
Edp course contents Contents of inquiry
Contents of inquiry Ark of the covenant lampstand
Ark of the covenant lampstand Elbow joint
Elbow joint Work portfolio examples
Work portfolio examples Democritus atom modeli
Democritus atom modeli Table of contents for company profile
Table of contents for company profile Ffp clotting factors
Ffp clotting factors How are metamorphic rocks classified
How are metamorphic rocks classified Specimen of cost audit report
Specimen of cost audit report Sds document in software engineering
Sds document in software engineering Mediastinum anatomy
Mediastinum anatomy Science logbook example
Science logbook example Composition notebook table of contents
Composition notebook table of contents Content of portfolio
Content of portfolio