RADIOMETRIC DATING THE AGE OF THE EARTH Why




















![MIXING THEORY [with subsequent decay] The mixing equations now become: The isochron plots then MIXING THEORY [with subsequent decay] The mixing equations now become: The isochron plots then](https://slidetodoc.com/presentation_image_h/a2bc9578b1f36ab6b0df048283502c7c/image-47.jpg)













- Slides: 75

RADIOMETRIC DATING & THE AGE OF THE EARTH

Why is this topic in any way important? Theory of Evolution requires very long time spans for the proposed changes in organisms to take place. This is due to the number of generations needed to allow very small changes to accumulate and produce species/species transitions, if this is possible. Therefore, scientists who believe in evolution need to be able to show that the Earth is very old in order to validate their ideas. If the Earth is young, then evolution cannot happen. It is not straightforward to estimate the age of the Earth [or the universe] as huge assumptions have to be made to do so. Radiometric dating has been used by geologists to estimate the age of rocks in the Earth’s crust.

Radiometric dating is used to estimate the age of some rocks There are THREE basic rock types: IGNEOUS [cooled from molten rock] SEDIMENTARY [mixed particles of igneous rock] METAMORPHIC [reheated sedimentary rock] Only igneous and metamorphic rocks can be used for radiometric dating as the radioactive materials are “frozen” in when the rock cools BUT… the vast majority of fossils are found in sedimentary rock layers About 75% of the Earth’s land surface is covered by sedimentary rock Therefore, fossils cannot normally be dated using the rocks in which they are buried – only adjacent strata where applicable

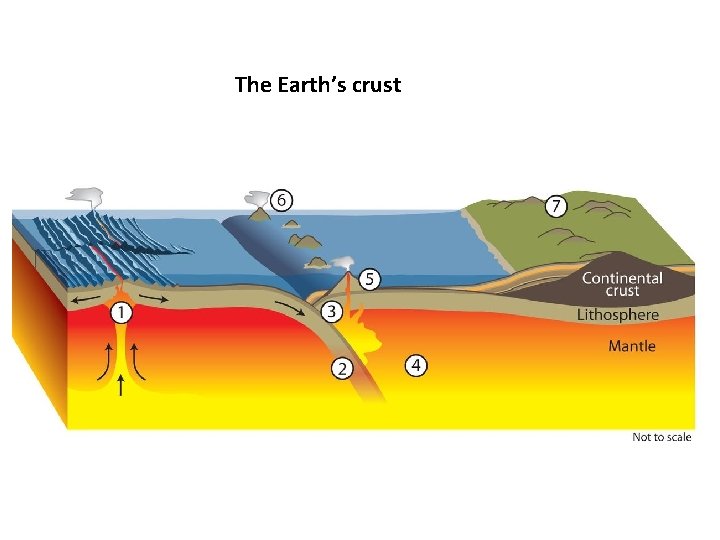
The Earth’s crust

How has the age of rocks been estimated in the past? Traditional geology has used an idea popularised by Charles Lyell. 1797 – 1875 Uniformitarianism This is a geological doctrine. It states that current geologic processes, occurring at the same rates observed today, in the same manner, account for all of Earth's geological features. “The present is the key to the past” Modern geologists are moving away from this model and many now think that the sedimentary rock layers were the result of a series of catastrophic events. The 1980 eruption of Mount St. Helens is a recent catastrophic event NOAH’S FLOOD IS A BIBLICAL CATASTROPHIC EVENT! So now the situation has changed and scientific - rather than purely dogmatic dating methods are sought to ascertain the age of rocks and fossils. Lyell said his goal was to: "free the science from Moses“, when he wrote his “Principles of Geology”

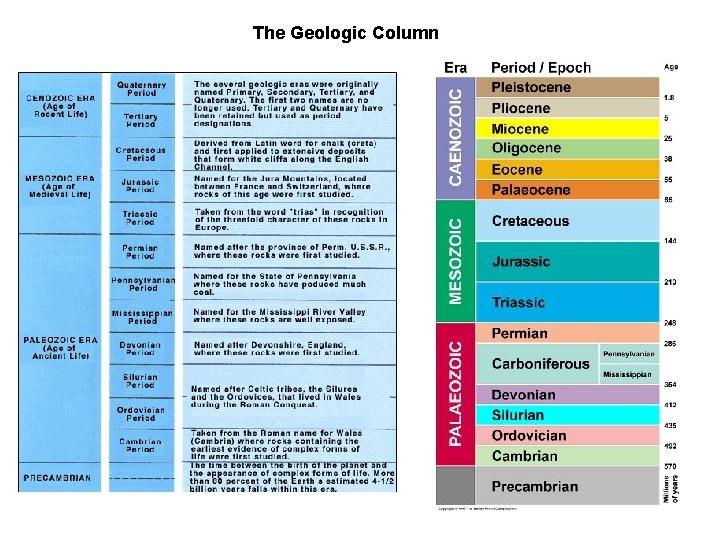
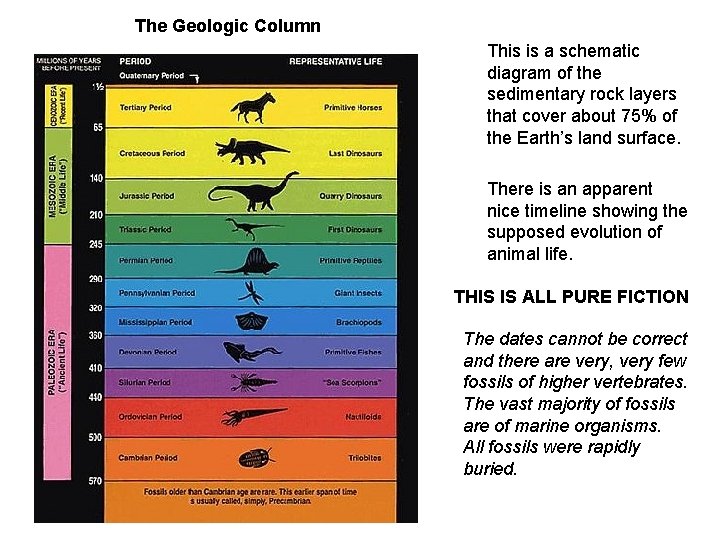
The Geologic Column

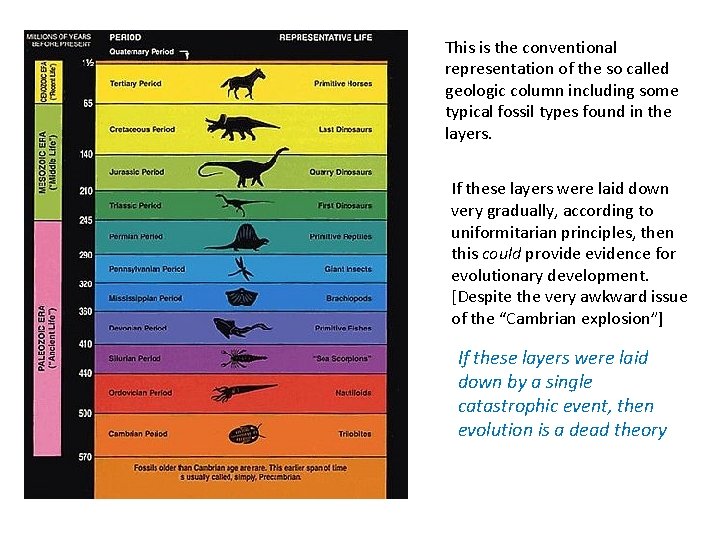
This is the conventional representation of the so called geologic column including some typical fossil types found in the layers. If these layers were laid down very gradually, according to uniformitarian principles, then this could provide evidence for evolutionary development. [Despite the very awkward issue of the “Cambrian explosion”] If these layers were laid down by a single catastrophic event, then evolution is a dead theory

THE METAPHYSICAL ASSUMPTIONS There is no God, only the material world exists. God exists and he created the material world. We are here and simpler life forms exist. God created all life forms. Therefore we must have evolved from simpler life forms as the human race is very recent. We have NOT evolved from simpler life forms and the human race is a very recent creation. The biochemical and physiological similarities between higher organisms provide evidence of descent. The biochemical and physiological similarities between higher organisms provide evidence of a common design. Evolution is therefore a FACT as there is no other possible explanation for the data, apart from alien intervention. Therefore, all other proposed causes of life are not correct. According to the neo-Darwinian theory, evolution requires millions of years. Therefore, the fossil bearing rocks containing simple organisms MUST be millions of years old. Millions of years are NOT required for creation so the fossil bearing rocks are not ancient and were laid down by a recent flood as described in the Bible. Any data that indicates a young age for fossil bearing rocks MUST BE WRONG Any data that indicates a very old age for fossil bearing rocks MUST BE WRONG

The age of the igneous and metamorphic rocks underneath the sedimentary layers does not matter in terms of evolution! These may indicate the age of the Earth. The underlying rocks do not contain fossils so can never provide evidence of any proposed evolutionary timeline. Sedimentary rocks are most often dated using “index fossils”. This is called relative dating. The sedimentary layers containing the fossils cannot easily be given an absolute date. Therefore, nearby rock intrusive layers are used i. e. hardened lava and “tuff”, which is compacted volcanic ash. These layers are dated by radiometric means, often potassium/argon dating.

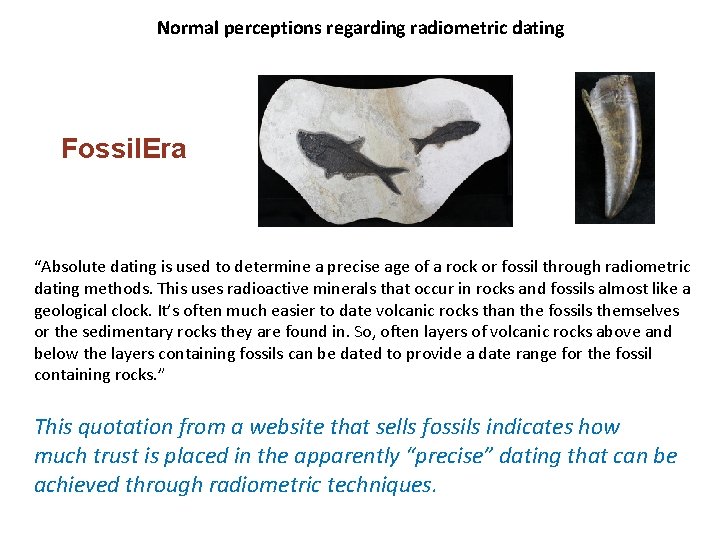
Normal perceptions regarding radiometric dating Fossil. Era “Absolute dating is used to determine a precise age of a rock or fossil through radiometric dating methods. This uses radioactive minerals that occur in rocks and fossils almost like a geological clock. It’s often much easier to date volcanic rocks than the fossils themselves or the sedimentary rocks they are found in. So, often layers of volcanic rocks above and below the layers containing fossils can be dated to provide a date range for the fossil containing rocks. ” This quotation from a website that sells fossils indicates how much trust is placed in the apparently “precise” dating that can be achieved through radiometric techniques.

“For religious reasons, some students believe that the earth is young (on the order of 10, 000 years old); they are antievolution at least in part because a young earth would provide inadequate time for evolution to occur. Therefore, it becomes important for them to invalidate radiometric dating as a way to disprove evolution. Although one cannot deal with the religious ideas promoting such views, students need to understand how isotopic age dating works and that it is very accurate—usually less than 1% error of measurement. Methods used for radiometric dating are based on the same physics that put people on the moon—it is not unreliable science! Commonly students are familiar only with carbon-14 dating, and are unaware of the large number of isotopes available for dating different periods of time. The multiplicity of isotopic dating techniques resulting in the same or very close dates also supports the reliability of radiometric dating. ” We are told here that the same physics that put men on the moon can date rocks better than 1% Here is how to get reliable rock ages…………

STEP 1 Process the rock with sophisticated equipment. A pure rock sample is thus obtained.

STEP 2 Look at the rock under a microscope, after sophisticated processing.

STEP 3 Carefully examine the rock to discover its age.

STEP 4 Turn up the magnification to discover the age of the rock. MAGMA PRODUCTS DCLXVI

THE THREE KEY QUESTIONS How does radiometric dating work and what can it tell us? How accurate is radiometric dating? Can we place confidence in radiometric dating?



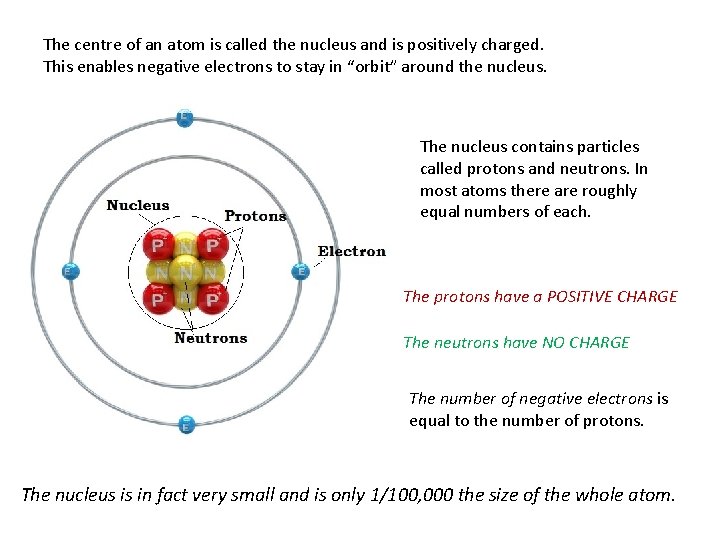
The centre of an atom is called the nucleus and is positively charged. This enables negative electrons to stay in “orbit” around the nucleus. The nucleus contains particles called protons and neutrons. In most atoms there are roughly equal numbers of each. The protons have a POSITIVE CHARGE The neutrons have NO CHARGE The number of negative electrons is equal to the number of protons. The nucleus is in fact very small and is only 1/100, 000 the size of the whole atom.

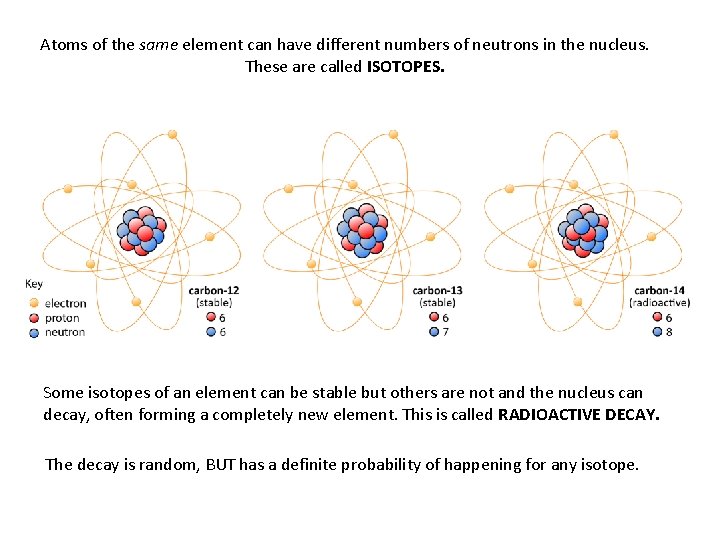
Atoms of the same element can have different numbers of neutrons in the nucleus. These are called ISOTOPES. Some isotopes of an element can be stable but others are not and the nucleus can decay, often forming a completely new element. This is called RADIOACTIVE DECAY. The decay is random, BUT has a definite probability of happening for any isotope.

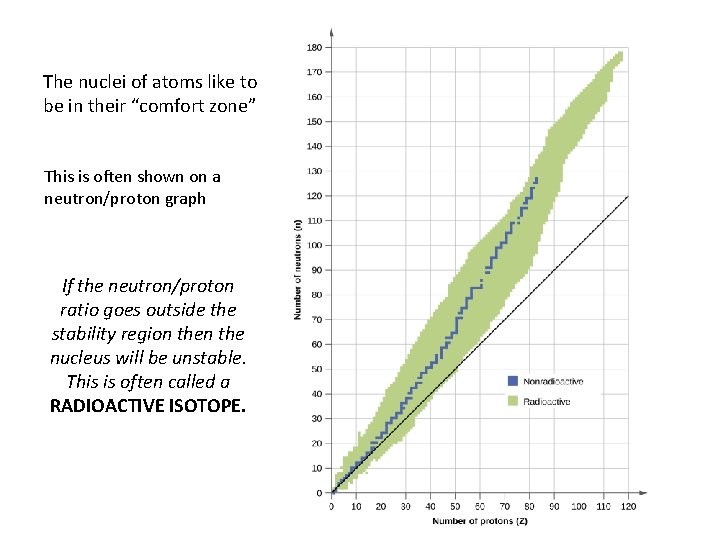
The nuclei of atoms like to be in their “comfort zone” This is often shown on a neutron/proton graph If the neutron/proton ratio goes outside the stability region the nucleus will be unstable. This is often called a RADIOACTIVE ISOTOPE.

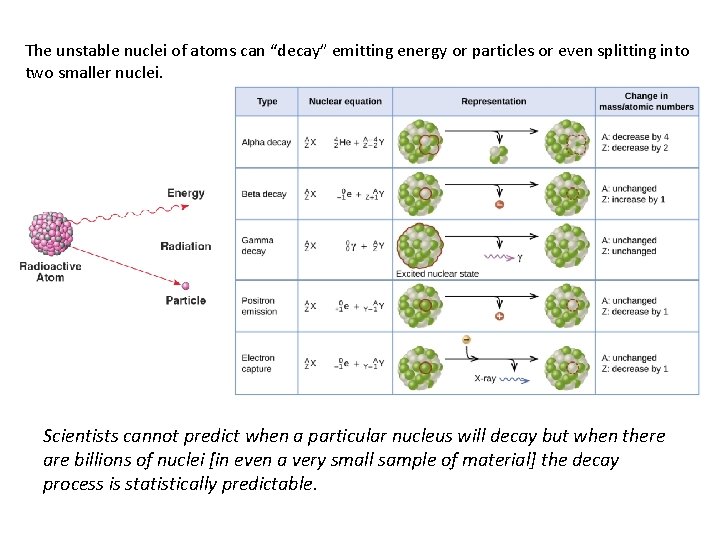
The unstable nuclei of atoms can “decay” emitting energy or particles or even splitting into two smaller nuclei. Scientists cannot predict when a particular nucleus will decay but when there are billions of nuclei [in even a very small sample of material] the decay process is statistically predictable.

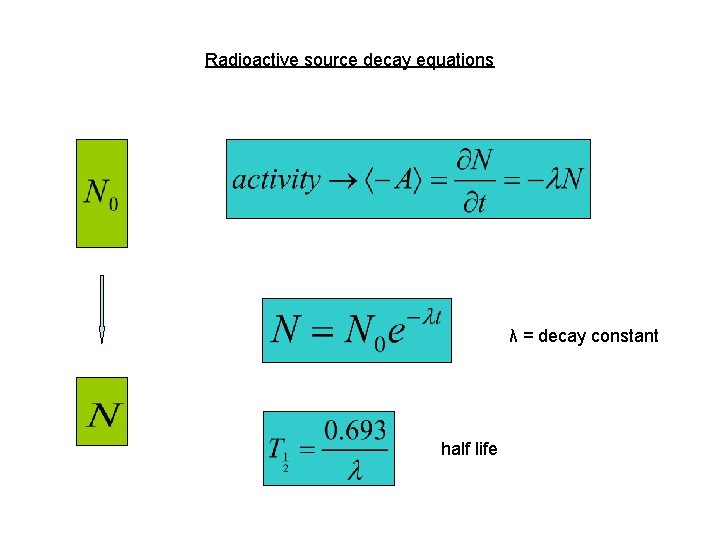
Radioactive source decay equations λ = decay constant half life

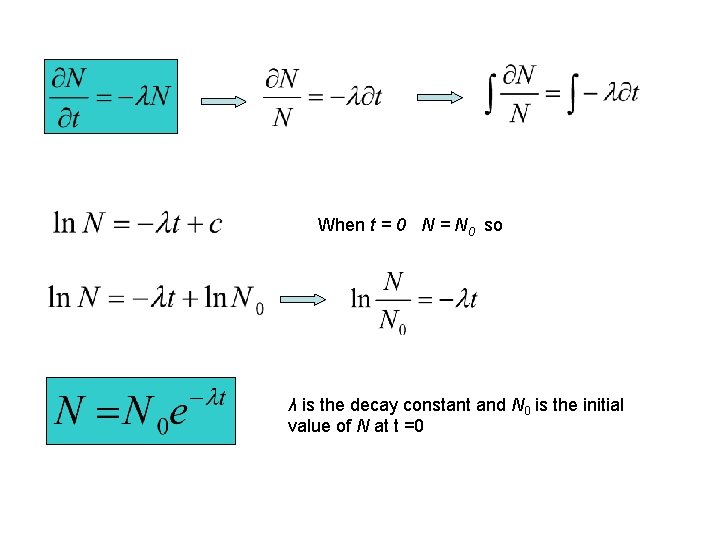
When t = 0 N = N 0 so λ is the decay constant and N 0 is the initial value of N at t =0

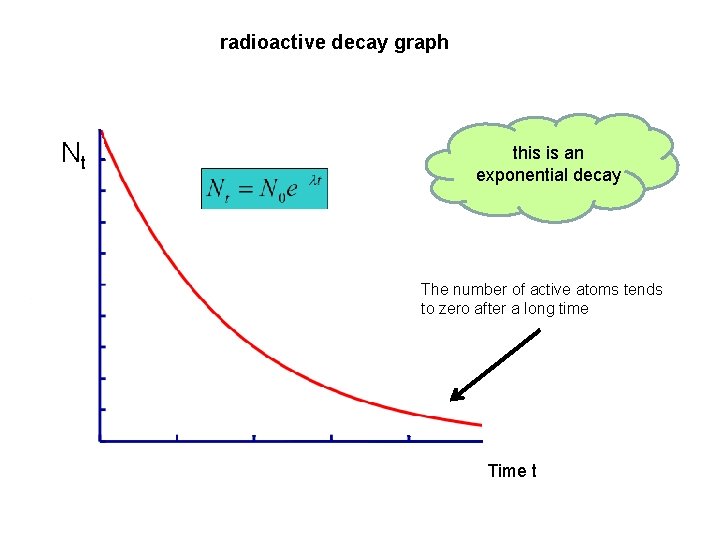
radioactive decay graph Nt this is an exponential decay The number of active atoms tends to zero after a long time Time t

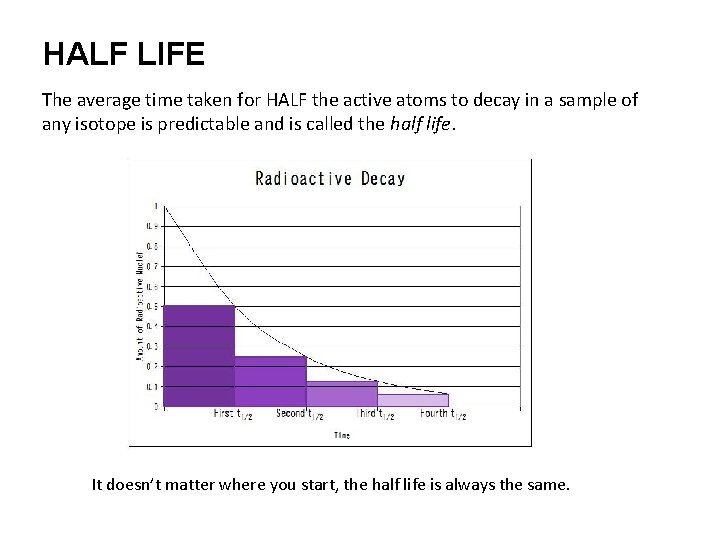
HALF LIFE The average time taken for HALF the active atoms to decay in a sample of any isotope is predictable and is called the half life. It doesn’t matter where you start, the half life is always the same.

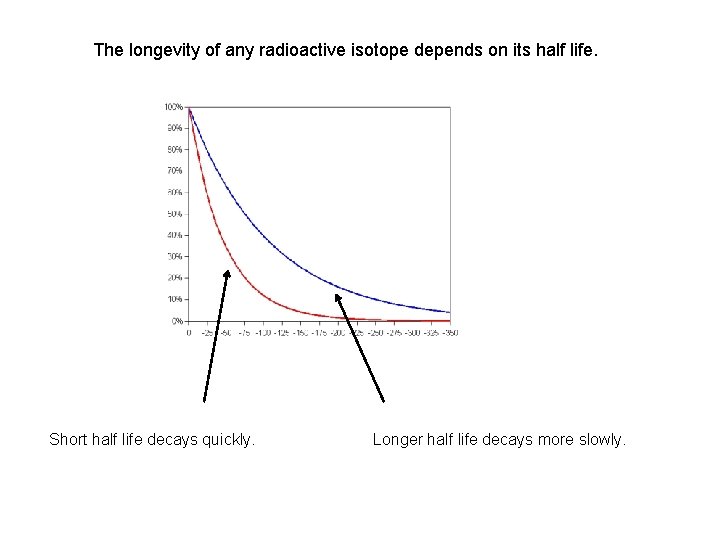
The longevity of any radioactive isotope depends on its half life. Short half life decays quickly. Longer half life decays more slowly.

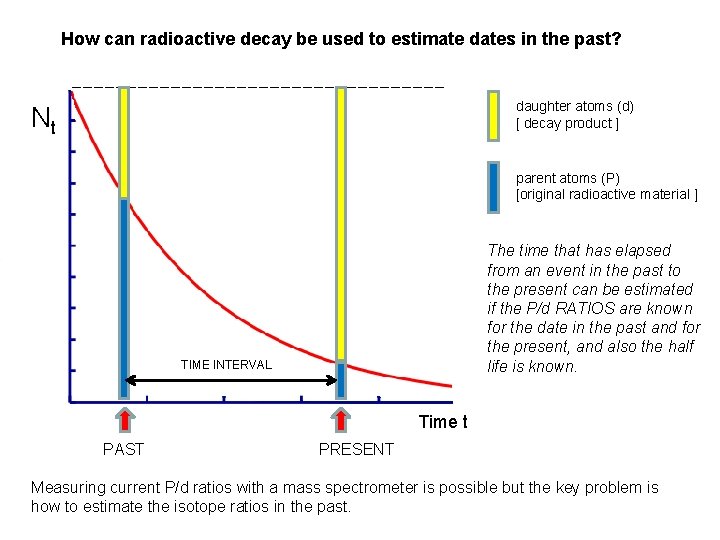
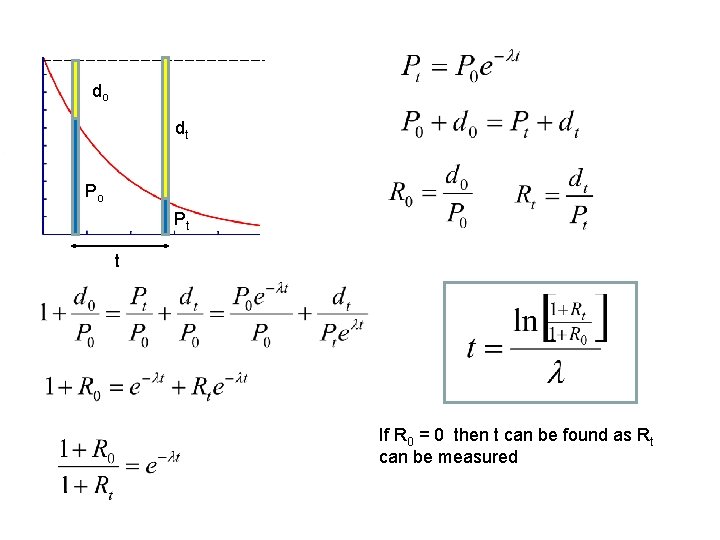
How can radioactive decay be used to estimate dates in the past? daughter atoms (d) [ decay product ] Nt parent atoms (P) [original radioactive material ] The time that has elapsed from an event in the past to the present can be estimated if the P/d RATIOS are known for the date in the past and for the present, and also the half life is known. TIME INTERVAL Time t PAST PRESENT Measuring current P/d ratios with a mass spectrometer is possible but the key problem is how to estimate the isotope ratios in the past.

do dt Po Pt t If R 0 = 0 then t can be found as Rt can be measured

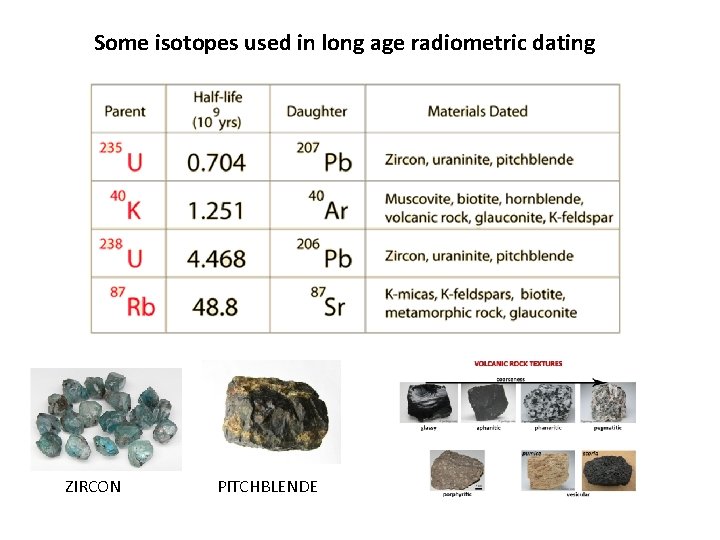
Some isotopes used in long age radiometric dating ZIRCON PITCHBLENDE

Intrinsic problems with simple radiometric dating 1. The initial isotope ratio cannot be measured directly. 2. Some of the daughter product may have been in the rock already before any decay of the parent took place. 3. The rock may not over time be a closed system and could gain or lose parent or daughter atoms. 4. The radioactive decay rate may not have been constant. N. B. Measuring current half-lives is not difficult and can be done with reasonable accuracy in most cases.

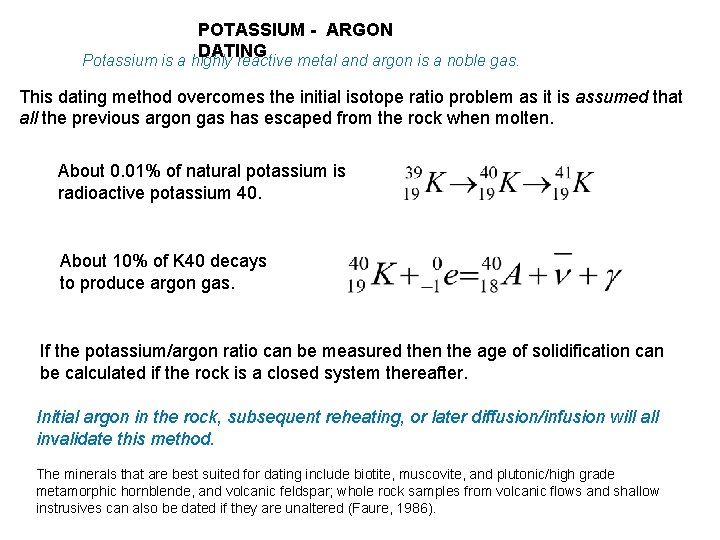
POTASSIUM - ARGON DATING Potassium is a highly reactive metal and argon is a noble gas. This dating method overcomes the initial isotope ratio problem as it is assumed that all the previous argon gas has escaped from the rock when molten. About 0. 01% of natural potassium is radioactive potassium 40. About 10% of K 40 decays to produce argon gas. If the potassium/argon ratio can be measured then the age of solidification can be calculated if the rock is a closed system thereafter. Initial argon in the rock, subsequent reheating, or later diffusion/infusion will all invalidate this method. The minerals that are best suited for dating include biotite, muscovite, and plutonic/high grade metamorphic hornblende, and volcanic feldspar; whole rock samples from volcanic flows and shallow instrusives can also be dated if they are unaltered (Faure, 1986).

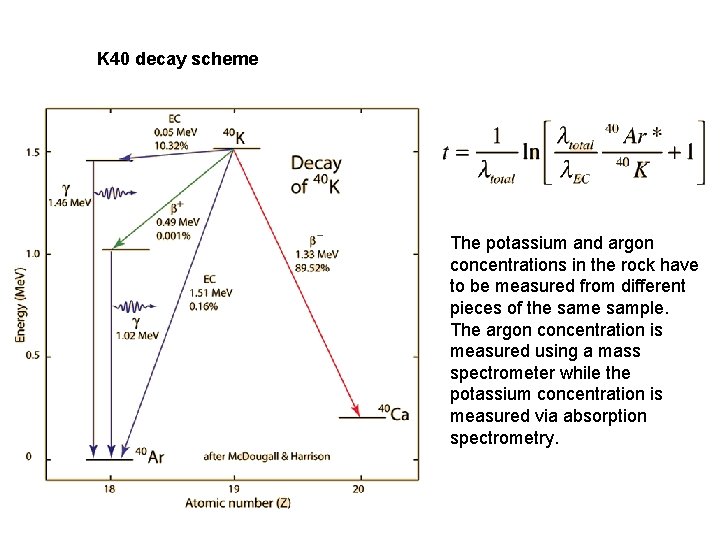
K 40 decay scheme The potassium and argon concentrations in the rock have to be measured from different pieces of the sample. The argon concentration is measured using a mass spectrometer while the potassium concentration is measured via absorption spectrometry.

Argon – Argon dating This is a variation on potassium-argon dating where the measurement of the K/Ar ratio is from the same rock sample. The rock sample is irradiated with fast neutrons in a nuclear reactor in order to convert the potassium 39 to argon 39. The natural K 40/K 39 ratio is known, so a ratio of argon 39/argon 40 measured in a mass spectrometer can reveal the original K 40/Ar 40 ratio. This technique has to be calibrated against a known reference which is mostly dated with the original K/Ar method. Hence only relative dates can be obtained.

Argon–argon (or 40 Ar/39 Ar) dating is a radiometric dating method invented to supersede potassium-argon (K/Ar) dating in accuracy. Dating minerals may provide age information on a rock, but assumptions must be made. Minerals usually only record the last time they cooled down below the closure temperature, and this may not represent all of the events which the rock has undergone, and may not match the age of intrusion. Thus, discretion and interpretation of age dating is essential. 40 Ar/39 Ar geochronology assumes that a rock retains all of its 40 Ar after cooling past the closing temperature and that this was properly sampled during analysis. The 40 Ar/39 Ar method only measures relative dates. In order for an age to be calculated by the 40 Ar/39 Ar technique, the J parameter must be determined by irradiating the unknown sample along with a sample of known age for a standard. Because this (primary) standard ultimately cannot be determined by 40 Ar/39 Ar, it must be first determined by another dating method. The method most commonly used to date the primary standard is the conventional K/Ar technique. [1] An alternative method of calibrating the used standard is astronomical tuning (also known as orbital tuning), which arrives at a slightly different age. [2]

Why can potassium-argon dates be unreliable? 1. The original molten rock contained argon which heating had not removed. 2. The rock cooled slowly allowing argon to diffuse into the rock. 3. Over time, argon diffused out of the rock as it is inert and has a small atomic radius. 4. Atmospheric argon entered the rock at some stage (argon is about 1% by volume of the Earth’s atmosphere and this argon is 99. 6% 40 Ar). If K – Ar dates are to be trusted to indicate “millions of years” then the rock must have been a completely closed and stable system after cooling, and also have contained no argon 40 at all. K – Ar dating is used to estimate the ages of lava and tuff layers which are found in the sedimentary rock layers.

The KBS TUFF controversy Leakey supplied samples from the KBS tuff to Jack Miller, a geophysicist at Cambridge University, to determine the feasibility of radiometrically dating them. Jack Miller and his partner Frank Fitch preliminarily dated the rocks at 212 to 230 million years old. In conventional geochronology, those dates correspond to the Triassic (the lower dinosaur strata), but Leakey was working near the boundary of the Tertiary and the Quaternary, which is thought to be only around two million years old. In 1980, Ian Mc. Dougall of Australian National University weighed in on the KBS tuff controversy, reporting a date of 1. 88 million years. Again, much more interestingly, Mc. Dougall revealed that Fitch and Miller had achieved a scatter of 0. 52 to 2. 64 million years on one set of samples, but on another set, they had a scatter of 8. 43 to 17. 5 million years! Thus, the total scatter was from half a million years to 17. 5 million years, a range of 17 million years (not including the preliminary finding of 230 million years) on a layer of rock supposedly around two million years old. There is a HUGE difference between the intrinsic accuracy of mass spectrometer measurements and the variability in the rock itself due to a range of unpredictable and unknown interfering factors.

Dalrymple's early work on 26 historic lava flows showed that many of them had excess argon and were not set to zero at the eruption of the volcano. The following is the data from these tests: 5 • • Hualalai basalt, Hawaii (AD 1800 -1801) 1. 05 to 1. 19 million years Mt. Etna basalt, Sicily (122 BC) 100, 000 years Mt. Etna basalt, Sicily (AD 1972) 150, 000 years Mt. Lassen plagioclase, California (AD 1915) 130, 000 years Sunset Crater basalt, Arizona (AD 1064 -1065) 210, 000 to 220, 000 years Glass Mountain (BP 130 -390) 130, 000 years in the future Mt. Mihara (AD 1951) 70, 000 years in the future Sakurajima (AD 1946) 200, 000 years in the future It seems that rocks whose ages are very recent cannot be accurately dated by K-Ar techniques just because of the relatively wide ranges of error.

“A few years ago I took a course in the ‘Evolution of Desert Environments’. We were standing on the Simi Volcanic flow, about 80 miles south of the south end of Death Valley. The instructor was a well known geologist and evolutionist from Cal. State Long Beach. He told us that the upper end of the flow was dated at 100, 000 years, the middle of the flow was dated at 50, 000 years, and the toe of the flow was dated at 20, 000 years. He then noted that the whole flow probably occurred and solidified (the surface at least) within weeks. He then said, based on his observation of the rates of evolution of desert environments, he thought the flow was less than 10, 000 years of age. He then said, ‘radiometric dating is the cornerstone of modern historical geology and we get this kind of variation? ’ Clearly he was not happy with the published dates on the Simi flow”. The Simi flow would have been dated by the K/Ar method Physics cannot date rocks! It can only be used to measure isotope ratios in any sample of rock. This ratio has to be interpreted in terms of the assumed formation and history of the rock. The measured ratio may be very accurate indeed but the calculated age of the rock depends upon the model(s) used.

Rubidium strontium dating Rubidium and strontium are both reactive metals. This uses a very simple decay process, which has very long half life. Therefore the dating method is only suitable for rocks which are thought to be very old indeed. Half life = 48, 800, 000 years The problem with using Rb/Sr dating is that the rocks in question already have strontium present before cooling. Therefore the initial conditions are not known. This means that a special technique has to be used which is called ISOCHRON DATING.

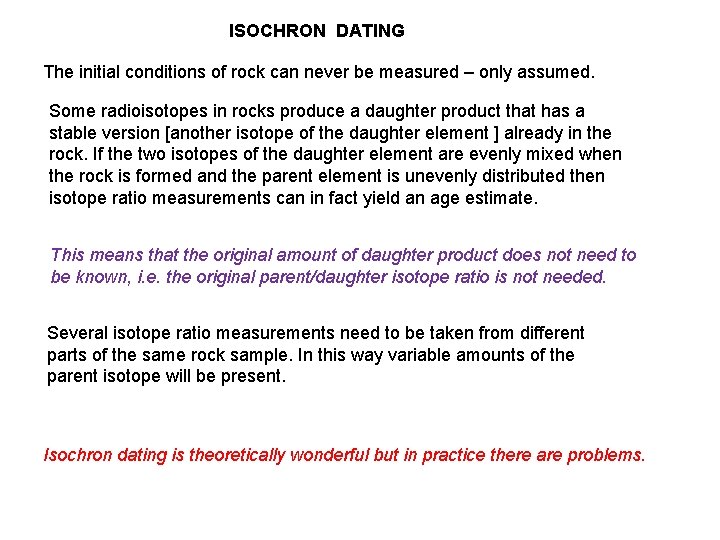
ISOCHRON DATING The initial conditions of rock can never be measured – only assumed. Some radioisotopes in rocks produce a daughter product that has a stable version [another isotope of the daughter element ] already in the rock. If the two isotopes of the daughter element are evenly mixed when the rock is formed and the parent element is unevenly distributed then isotope ratio measurements can in fact yield an age estimate. This means that the original amount of daughter product does not need to be known, i. e. the original parent/daughter isotope ratio is not needed. Several isotope ratio measurements need to be taken from different parts of the same rock sample. In this way variable amounts of the parent isotope will be present. Isochron dating is theoretically wonderful but in practice there are problems.

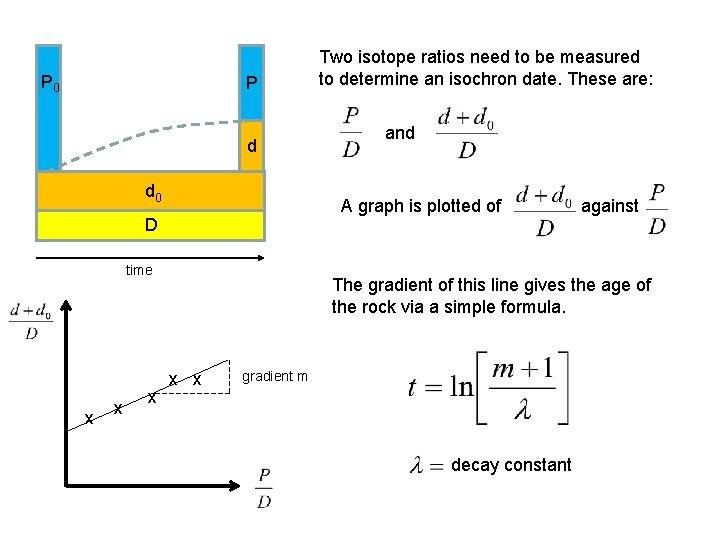
P 0 P d d 0 time x x and A graph is plotted of against D x Two isotope ratios need to be measured to determine an isochron date. These are: The gradient of this line gives the age of the rock via a simple formula. x x gradient m decay constant

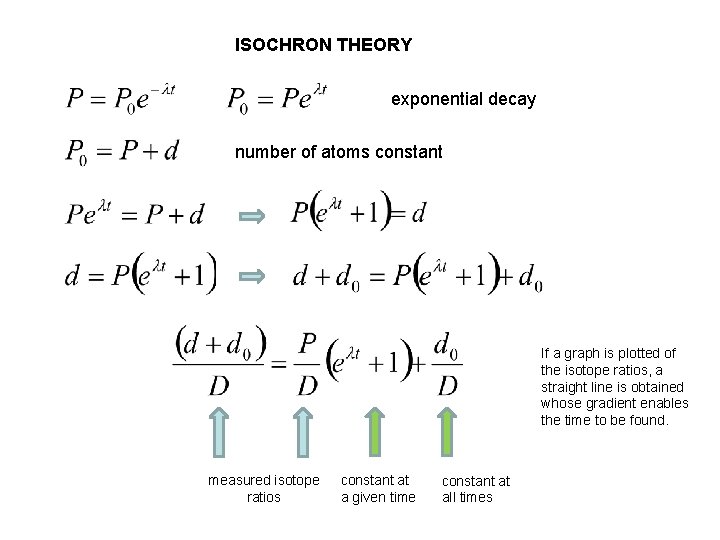
ISOCHRON THEORY exponential decay number of atoms constant If a graph is plotted of the isotope ratios, a straight line is obtained whose gradient enables the time to be found. measured isotope ratios constant at a given time constant at all times

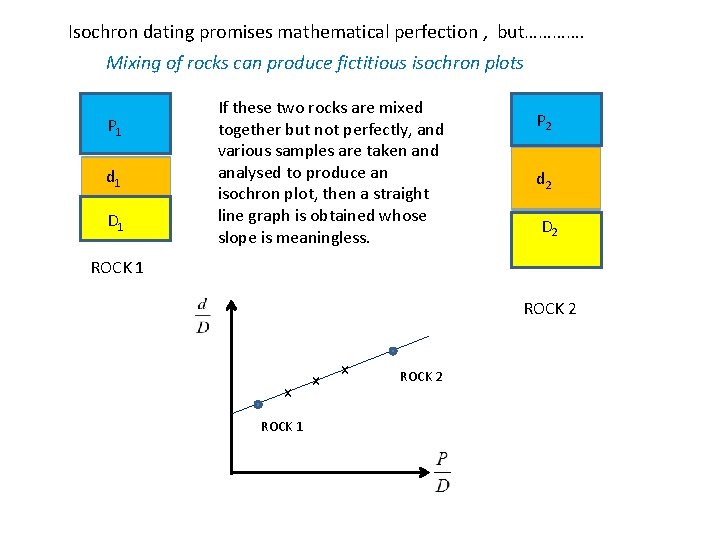
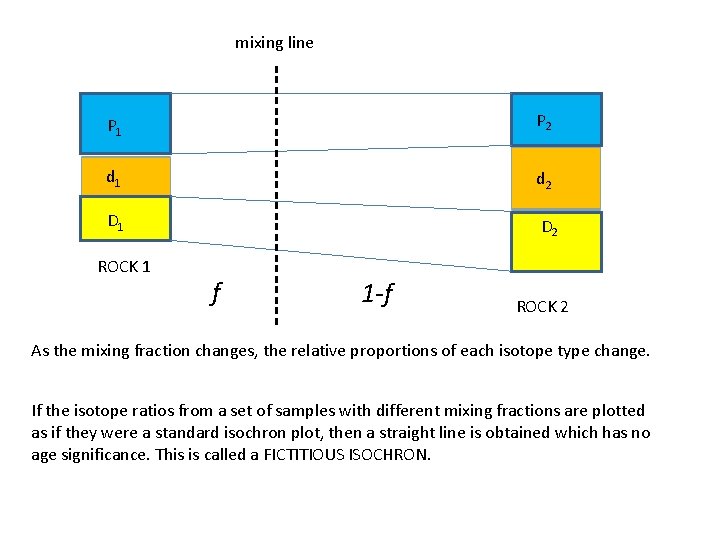
Isochron dating promises mathematical perfection , but…………. Mixing of rocks can produce fictitious isochron plots P 1 d 1 D 1 If these two rocks are mixed together but not perfectly, and various samples are taken and analysed to produce an isochron plot, then a straight line graph is obtained whose slope is meaningless. P 2 d 2 D 2 ROCK 1 ROCK 2 x ROCK 1 x x ROCK 2

mixing line P 1 P 2 d 1 d 2 D 1 D 2 ROCK 1 f 1 -f ROCK 2 As the mixing fraction changes, the relative proportions of each isotope type change. If the isotope ratios from a set of samples with different mixing fractions are plotted as if they were a standard isochron plot, then a straight line is obtained which has no age significance. This is called a FICTITIOUS ISOCHRON.

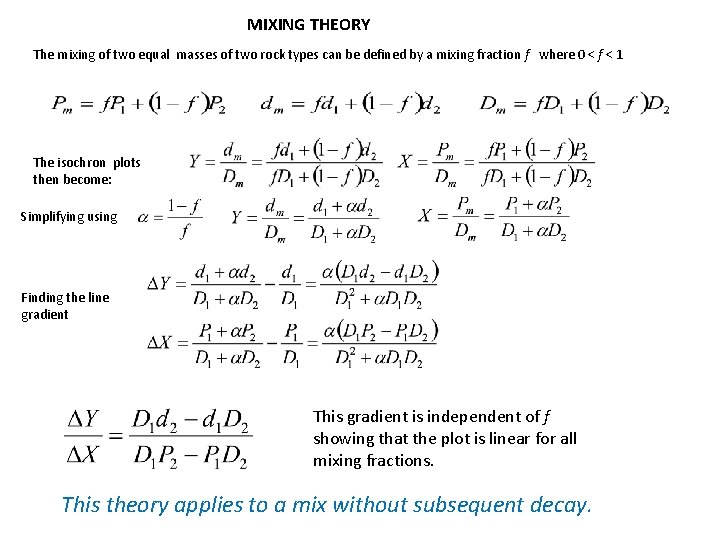
MIXING THEORY The mixing of two equal masses of two rock types can be defined by a mixing fraction f where 0 < f < 1 The isochron plots then become: Simplifying using Finding the line gradient This gradient is independent of f showing that the plot is linear for all mixing fractions. This theory applies to a mix without subsequent decay.
![MIXING THEORY with subsequent decay The mixing equations now become The isochron plots then MIXING THEORY [with subsequent decay] The mixing equations now become: The isochron plots then](https://slidetodoc.com/presentation_image_h/a2bc9578b1f36ab6b0df048283502c7c/image-47.jpg)
MIXING THEORY [with subsequent decay] The mixing equations now become: The isochron plots then become: the line gradient is This gradient is independent of f showing that the plot is linear for all mixing fractions. The gradient of the line cannot be used to calculate an age for the rock. This theory applies to a mix with subsequent decay.

Detecting rock mixing This is not at all easy and can only be done with a simple two component mix, assuming that the reference daughter isotope concentration is the same in each sample. This criterion is not met for general mixing. Conclusion Isochron dating must be considered as being unreliable simply because a straight line plot is indistinguishable from a mixing line, and there is no foolproof method of distinguishing between a true isochron and a mixing line. Geologists therefore can “choose” isochron derived dates that are in agreement with the “expected” age of the rock.

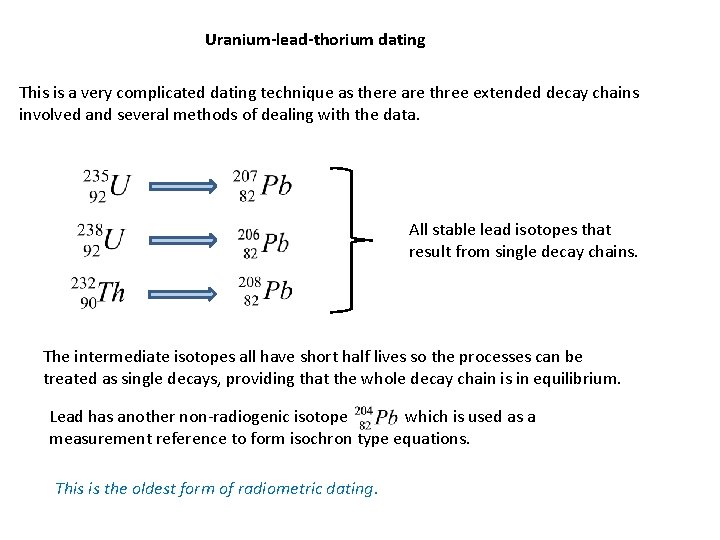
Uranium-lead-thorium dating This is a very complicated dating technique as there are three extended decay chains involved and several methods of dealing with the data. All stable lead isotopes that result from single decay chains. The intermediate isotopes all have short half lives so the processes can be treated as single decays, providing that the whole decay chain is in equilibrium. Lead has another non-radiogenic isotope which is used as a measurement reference to form isochron type equations. This is the oldest form of radiometric dating.

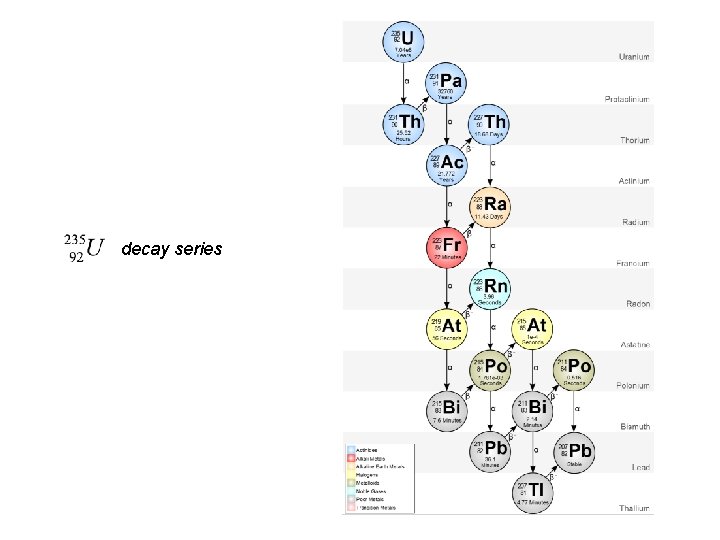
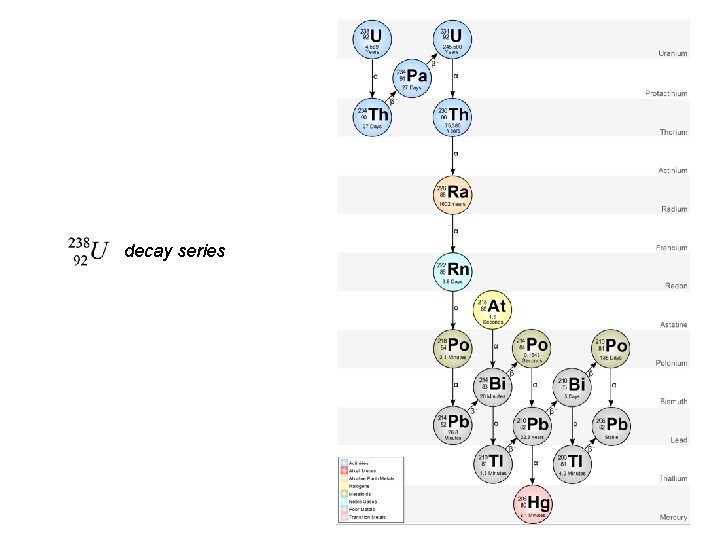
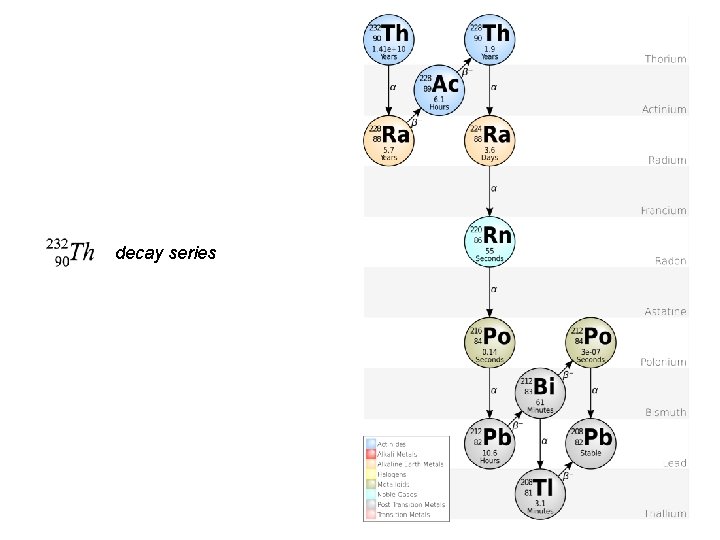
decay series

decay series

decay series

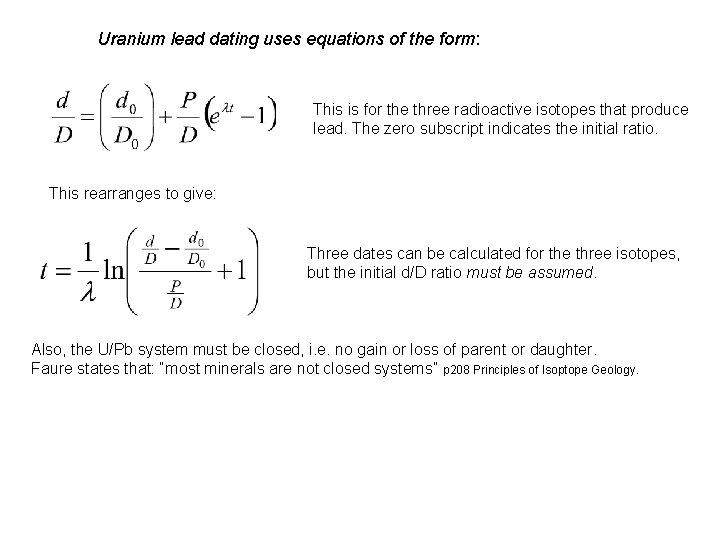
Uranium lead dating uses equations of the form: This is for the three radioactive isotopes that produce lead. The zero subscript indicates the initial ratio. This rearranges to give: Three dates can be calculated for the three isotopes, but the initial d/D ratio must be assumed. Also, the U/Pb system must be closed, i. e. no gain or loss of parent or daughter. Faure states that: “most minerals are not closed systems” p 208 Principles of Isoptope Geology.

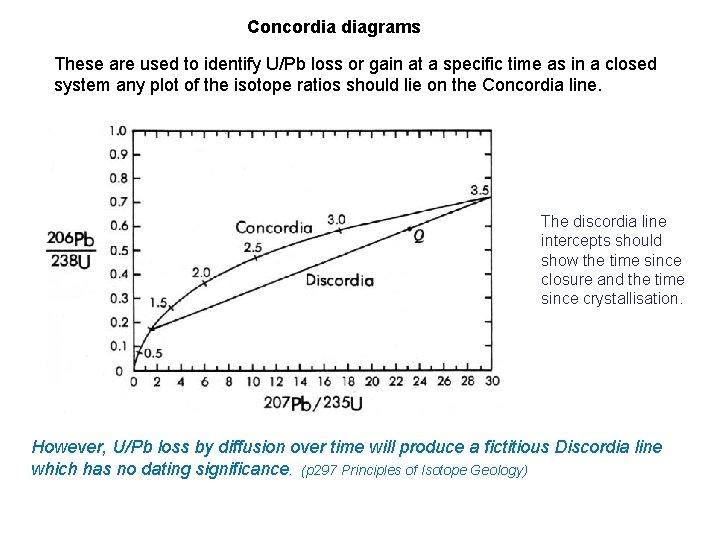
Concordia diagrams These are used to identify U/Pb loss or gain at a specific time as in a closed system any plot of the isotope ratios should lie on the Concordia line. The discordia line intercepts should show the time since closure and the time since crystallisation. However, U/Pb loss by diffusion over time will produce a fictitious Discordia line which has no dating significance. (p 297 Principles of Isotope Geology)

LEAD-LEAD DATING This technique uses isotope ratio measurements of lead 204, 206 and 207 to estimate the age of the Earth and also meteorites. The classic is the “Holmes-Houtermans model”, which assumes: 1. 2. 3. 4. 5. 6. The Earth was originally molten and homogenous (evenly mixed). U, Th and Pb were uniformly distributed. The isotopic composition of lead was the same everywhere. After solidification “small regional differences” arose in the U/Pb ratio (how? ). Subsequently in any given region the U/Pb ratio only changed due to decay. At the time of formation of a mineral (e. g. galena) the Pb was separated from U and Th and its isotopic composition has remained constant ever since. This model does not produce very good results when used. The next version is the “Stacey-Kramers” model which assumes: 1. The “evolution” of lead started 4. 57 Ga years ago. 2. At a more recent date (3. 7 Ga) the U/Pb ratio was changed by geochemical differentiation and has remained constant since then. This model produces better results when used, but still relies upon huge assumptions.

All the main radioisotope dating techniques highlighted so far have common features: 1. 2. 3. 4. A long age history of the Earth is assumed. The formation mechanism and history of the rocks is assumed. The isotope ratio measurements are interpreted in terms of 1. & 2. The calculated ages are very large. An analytical method such as this is a classic case of circular reasoning. The calculated age of a rock will depend uniquely on the mathematical model used, which in turn will depend uniquely on the assumptions made about the rock formation, history and likely age of the rock. Radiometric dating is therefore not normal operational science but uses a variety of forensic techniques [based on accurate physics measurements now], which can only suggest rock ages based on geological models of rock history. In fairness to other evidence; If the Earth started as a molten blob then it would have taken at least 20 Ma to cool, apart from any internal radioactive heating.

What is the conflict between conventional geology and Biblical teaching? GEOLOGY Old universe Old solar system Old Earth BIBLE Young universe Young solar system Young Earth There is disagreement among sincere Christians regarding the true interpretation of the Genesis account of creation. Some Christians who are qualified scientists think that the days in Genesis 1 refer to long periods of time whereas Christians who adopt a literal translation of Genesis will believe that God created everything in six 24 hr periods. THE FLOOD & NOAH If there was a global flood about 4000 years ago, then this would have caused severe disruption to the Earth’s crustal rocks. The Genesis account indicates that the “fountains of the great deep were broken up”. Almost all sedimentary rocks would have been deposited at this time. The geological significance of this is that all conventional radiometric dating assumptions would be changed.

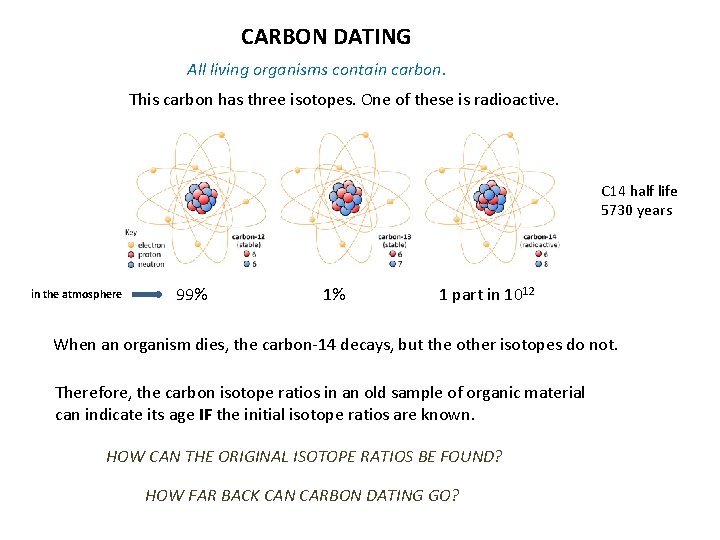
CARBON DATING All living organisms contain carbon. This carbon has three isotopes. One of these is radioactive. C 14 half life 5730 years in the atmosphere 99% 1% 1 part in 1012 When an organism dies, the carbon-14 decays, but the other isotopes do not. Therefore, the carbon isotope ratios in an old sample of organic material can indicate its age IF the initial isotope ratios are known. HOW CAN THE ORIGINAL ISOTOPE RATIOS BE FOUND? HOW FAR BACK CAN CARBON DATING GO?

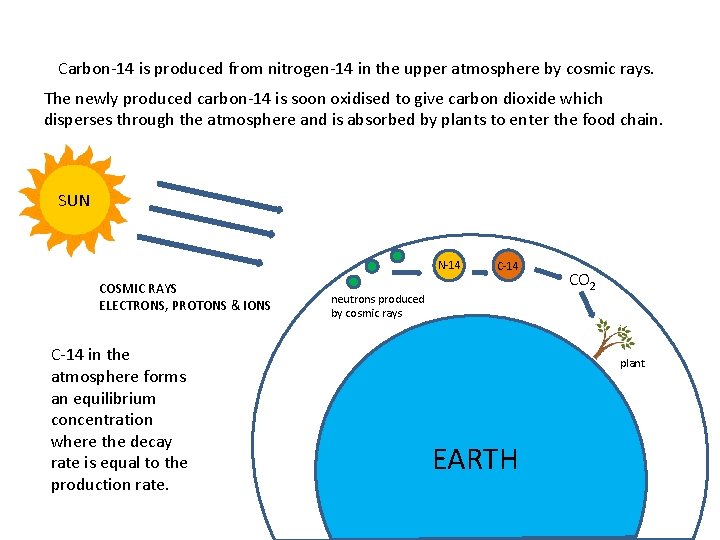
Carbon-14 is produced from nitrogen-14 in the upper atmosphere by cosmic rays. The newly produced carbon-14 is soon oxidised to give carbon dioxide which disperses through the atmosphere and is absorbed by plants to enter the food chain. SUN N-14 COSMIC RAYS ELECTRONS, PROTONS & IONS C-14 in the atmosphere forms an equilibrium concentration where the decay rate is equal to the production rate. C-14 neutrons produced by cosmic rays CO 2 plant EARTH

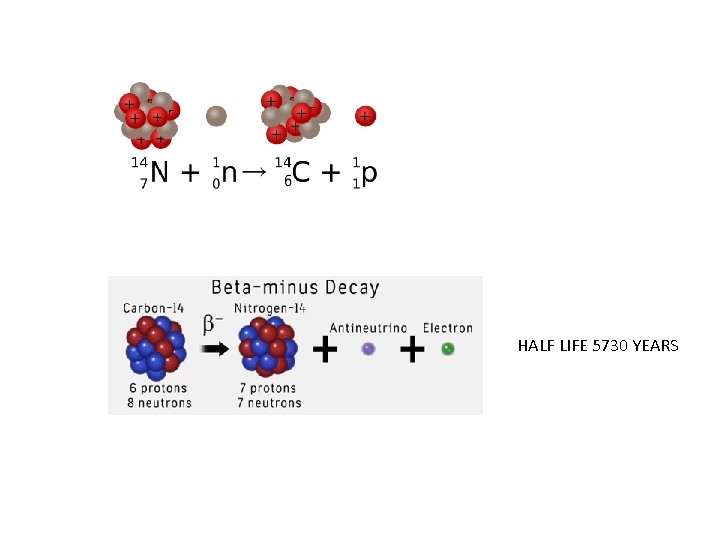
HALF LIFE 5730 YEARS

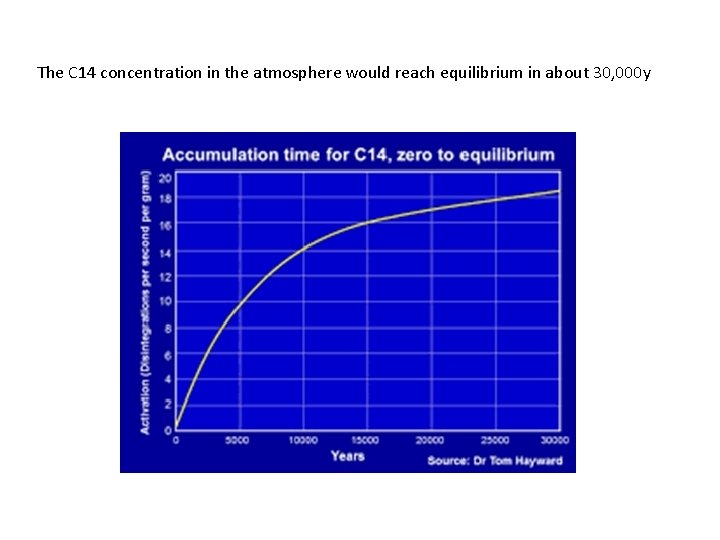
The C 14 concentration in the atmosphere would reach equilibrium in about 30, 000 y

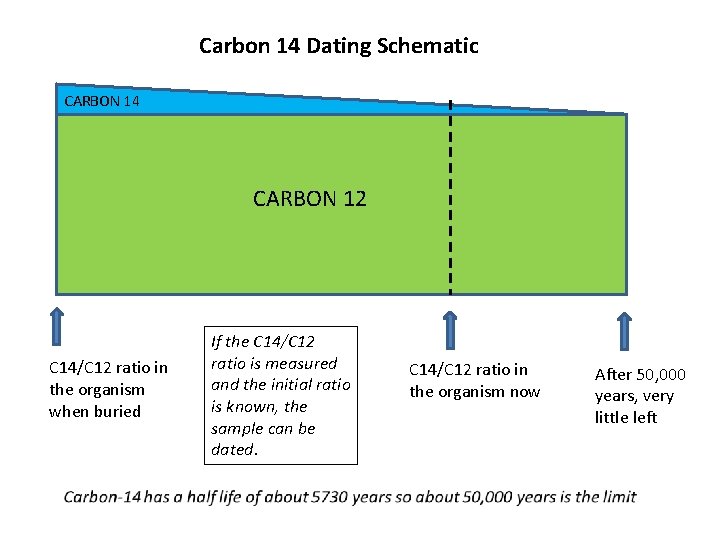
Carbon 14 Dating Schematic CARBON 14 CARBON 12 C 14/C 12 ratio in the organism when buried If the C 14/C 12 ratio is measured and the initial ratio is known, the sample can be dated. C 14/C 12 ratio in the organism now After 50, 000 years, very little left

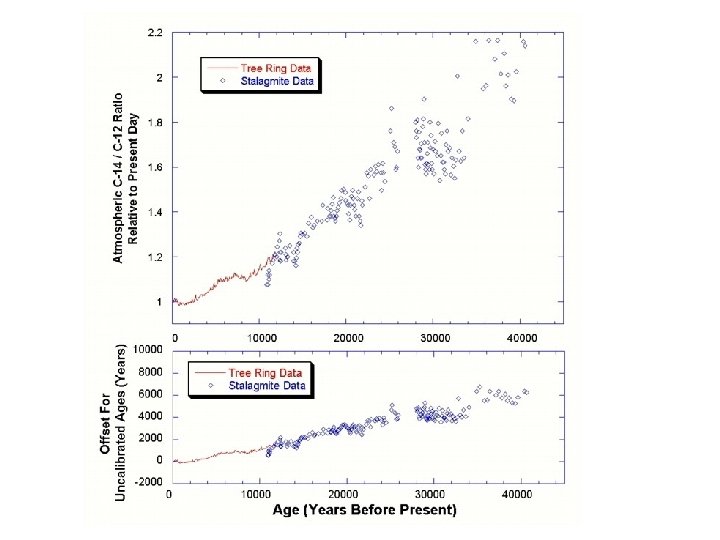
If the carbon isotope ratio is the same in plants as it is in the atmosphere, then the initial conditions are known for any dead organic matter, and dating can be estimated by measuring the current carbon isotope ratio. What are the problems with carbon dating? 1. The atmospheric concentration of C-14 has not been the same in the past as it is now. 2. Plants do not absorb the different carbon isotopes at equal rates. 3. The very low concentration of C-14 means that it is difficult to detect, and any contamination by more recent organic matter can greatly affect results. HOWEVER – the dating technique has been refined to account for systematic errors by calibrating it using artefacts of known age – as well as tree rings [dendrochronology ] and stalagmites. each ring represents one year stalagmites are carbonate deposits


A Simple Mass Spectrometer

Simple Mass Spectrometer Schematic

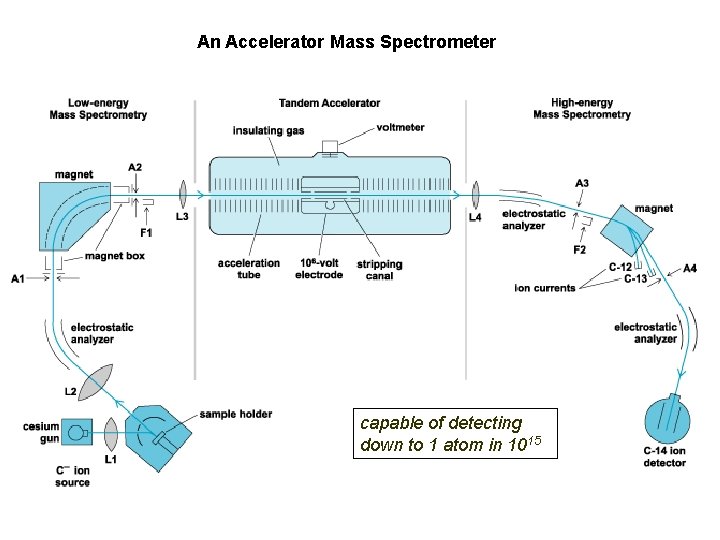
An Accelerator Mass Spectrometer capable of detecting down to 1 atom in 1015


How good is carbon dating in practice? Up to 4000 years: quite good as the results can be compared with artefacts and trees etc of a known age enabling empirical calibration. Beyond 4000 years: the technique is still used but is not as reliable as there are far fewer artefacts/trees etc of known age to act as references for atmospheric C 14 concentration.

Can carbon dating give us any information about the age of rocks or the Earth? Not directly – but……… C -14 has been detected in diamonds which are thought to have been formed millions of years ago. [25 x machine threshold]. C -14 has been detected in coal. [450 x machine threshold]. Coal is thought to have been formed at least 2 million years ago [mostly 60 MYA]. C -14 has been detected in fossil wood from sandstone in Australia. This sandstone is thought to have been formed 200 -250 million years ago. The conclusion from this scientific evidence is that many rock dates presupposed by secular geologists must be wrong – the presence of measurable C-14 in these examples absolutely invalidates previous date estimates. There should be almost none left after 50, 000 years. This means that the dating of the so called geologic column is wrong and that the embedded fossils cannot be millions of years old.

The Geologic Column This is a schematic diagram of the sedimentary rock layers that cover about 75% of the Earth’s land surface. There is an apparent nice timeline showing the supposed evolution of animal life. THIS IS ALL PURE FICTION The dates cannot be correct and there are very, very few fossils of higher vertebrates. The vast majority of fossils are of marine organisms. All fossils were rapidly buried.

GENERAL CONCLUSIONS K/Ar dating gives very unreliable results, especially for younger rocks. Rb/Sr isochrons cannot be distinguished from meaningless mixing lines. All isochron techniques are therefore suspect. U/Pb dating methods cannot rely on closed rock systems so all dating techniques attempt to account for this with very variable success. Long age dating is not an exact science and relies on assumed rock histories and invariant decay rates. It may or may not be correct. Carbon dating has revealed the young age of the geologic column and has therefore invalidated the so-called fossil record as proof of evolution.

THE RATE PROJECT Radioisotopes and the Age of the Earth (RATE) Recent experiments commissioned by the RATE project 1 indicate that "1. 5 billion years" worth of nuclear decay took place in one or more short episodes between 4, 000 and 14, 000 years ago. The results strongly support our accelerated decay hypothesis, that episodes with billion-fold speed-ups of nuclear decay occurred in the recent past, such as during the Genesis flood, the Fall of Adam, or early Creation week. Such accelerations would shrink the alleged 4. 5 billion year radioisotope age of the Earth down to the 6, 000 years that a straightforward reading of the Bible gives. These experimental results are as yet provisional evidence, as a sound theoretical model for accelerated decay has not yet been finalised.

Can radiometric dating………. Prove that the earth is 4. 6 billion years old? NO Prove that the earth is 6000 years old? NO Prove that most fossils are very old? NO What you believe about the past depends on your assumptions. Scientific methods cannot tell us exactly what happened in the past – they can only provide data that is or is not consistent with any theory about past events.

Thank you for listening. I hope that your understanding of radiometric dating is better than it was.