Mass Spectrometry MS In MS a molecule is
- Slides: 11

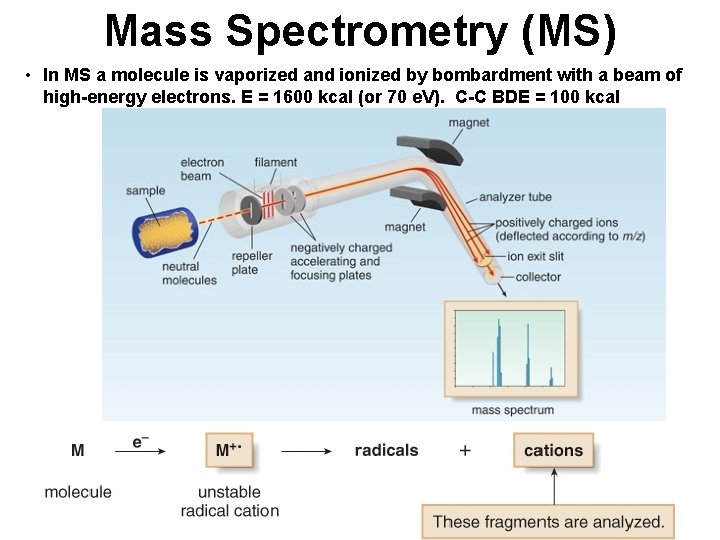
Mass Spectrometry (MS) • In MS a molecule is vaporized and ionized by bombardment with a beam of high-energy electrons. E = 1600 kcal (or 70 e. V). C-C BDE = 100 kcal

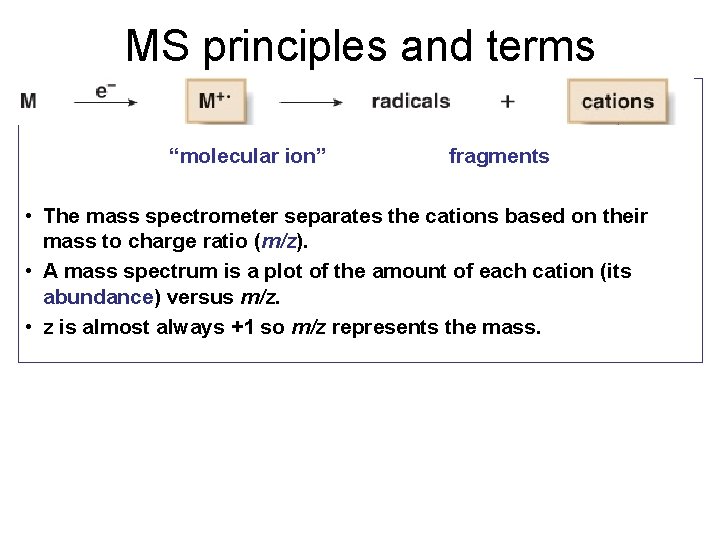
MS principles and terms “molecular ion” fragments • The mass spectrometer separates the cations based on their mass to charge ratio (m/z). • A mass spectrum is a plot of the amount of each cation (its abundance) versus m/z. • z is almost always +1 so m/z represents the mass.

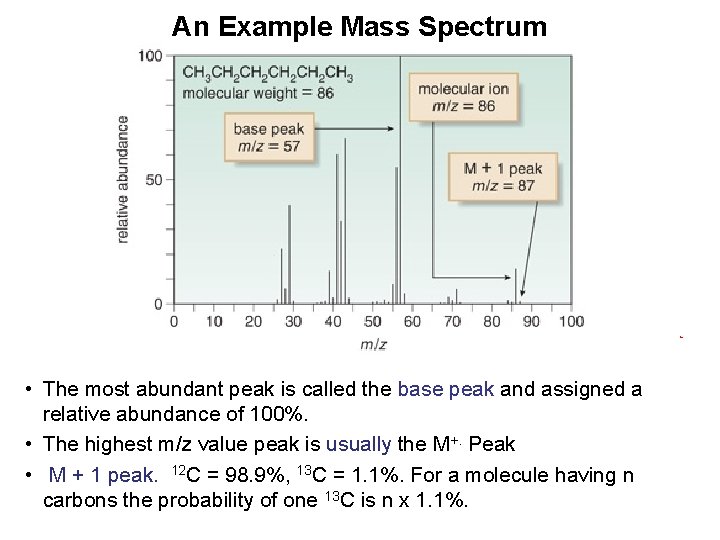
An Example Mass Spectrum • The most abundant peak is called the base peak and assigned a relative abundance of 100%. • The highest m/z value peak is usually the M+. Peak • M + 1 peak. 12 C = 98. 9%, 13 C = 1. 1%. For a molecule having n carbons the probability of one 13 C is n x 1. 1%.

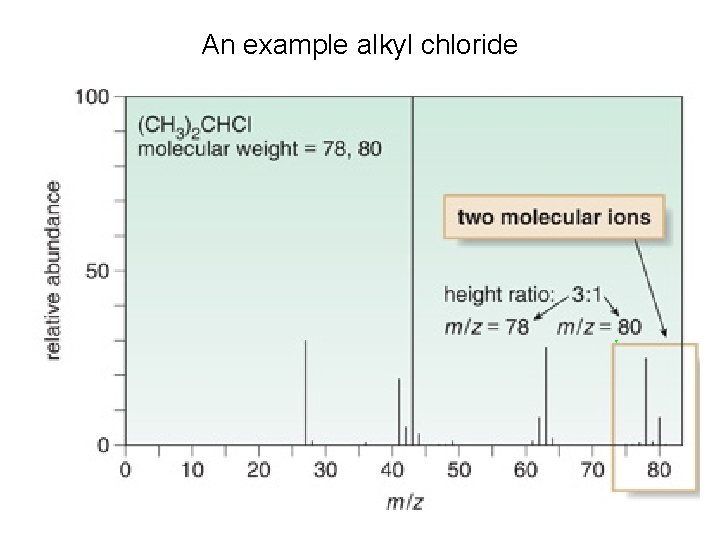
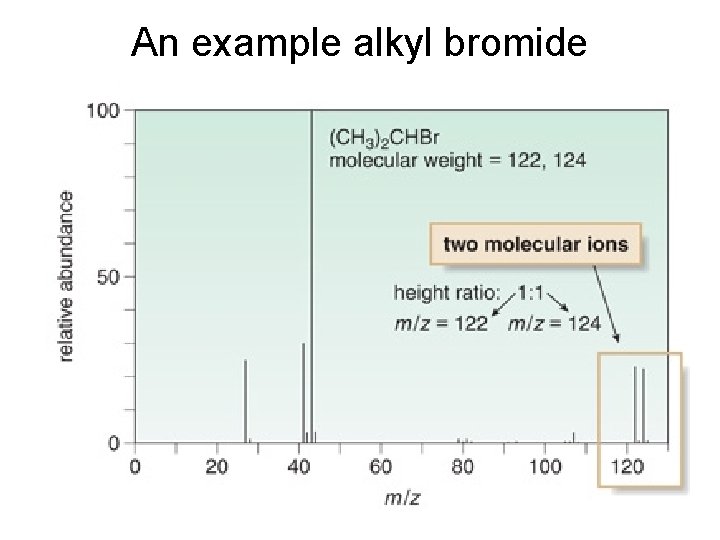
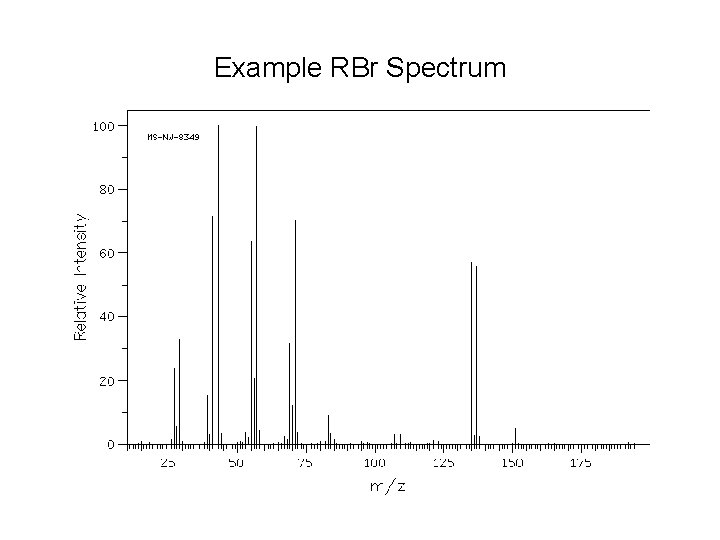
Alkyl Halides • Most common atoms (C, H, O, N, I, P) are monoisotopic. • Chlorine is diisotopic: 35 Cl = 75% and 37 Cl = 25%. § Thus, the molecular ion of an alkyl chloride shows two peaks separated by two m/z units in a 3: 1 ratio. § The larger peak corresponds to molecules of the compound containing the 35 Cl. The smaller peak corresponds to the molecules containing 37 Cl. • Br is also diisotopic. 79 Br and 81 Br occur in a ratio of ~1: 1. • Thus, the molecular ion of an alkyl bromide shows two peaks separated by two m/z units in a 1: 1 ratio. • We can use these facts to confirm or disprove the presence of Cl or Br in a compound.

An example alkyl chloride

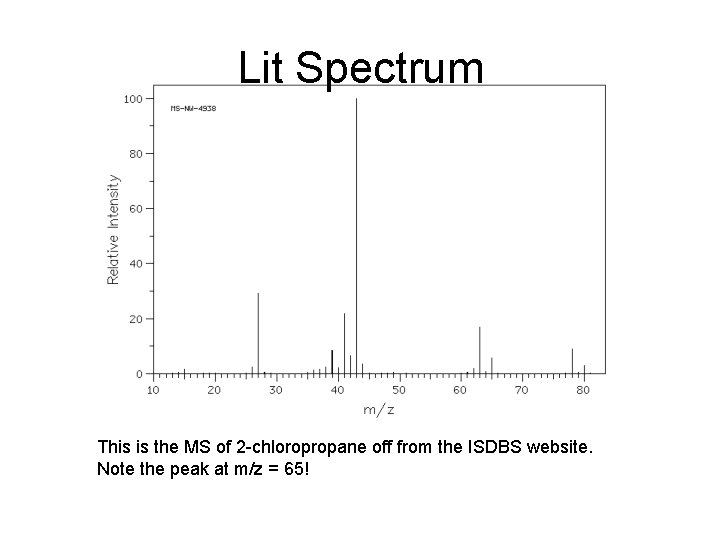
Lit Spectrum This is the MS of 2 -chloropropane off from the ISDBS website. Note the peak at m/z = 65!

An example alkyl bromide

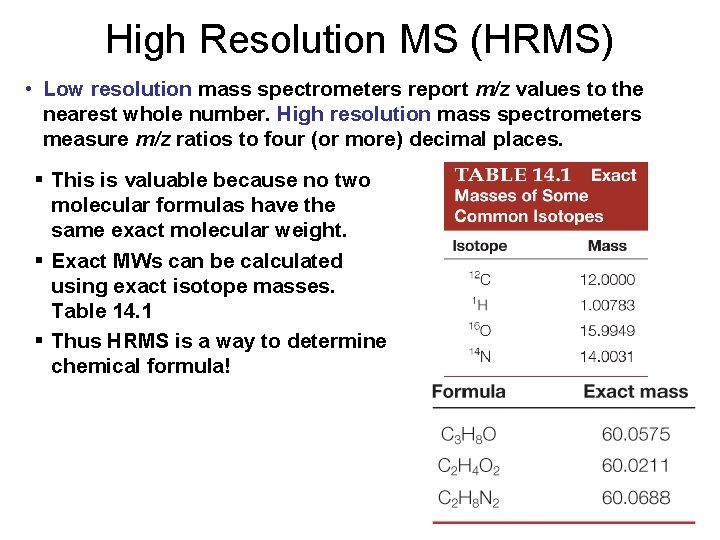
High Resolution MS (HRMS) • Low resolution mass spectrometers report m/z values to the nearest whole number. High resolution mass spectrometers measure m/z ratios to four (or more) decimal places. § This is valuable because no two molecular formulas have the same exact molecular weight. § Exact MWs can be calculated using exact isotope masses. Table 14. 1 § Thus HRMS is a way to determine chemical formula!

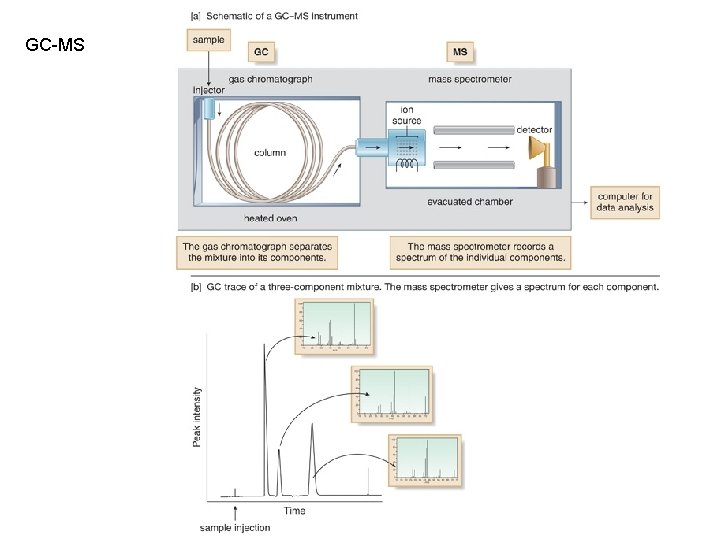
GC-MS

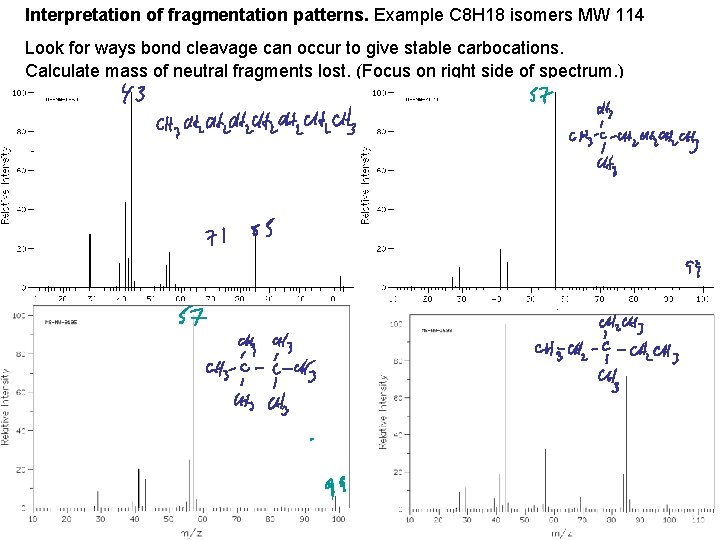
Interpretation of fragmentation patterns. Example C 8 H 18 isomers MW 114 Look for ways bond cleavage can occur to give stable carbocations. Calculate mass of neutral fragments lost. (Focus on right side of spectrum. )

Example RBr Spectrum