MASS SPECTROMETRY 1 MASS SPECTROMETRY Measures the mass






- Slides: 24

MASS SPECTROMETRY 1

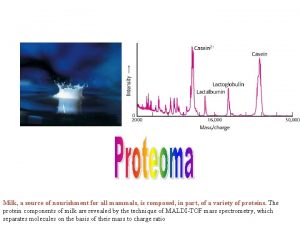
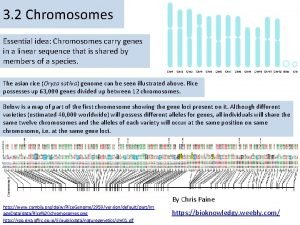
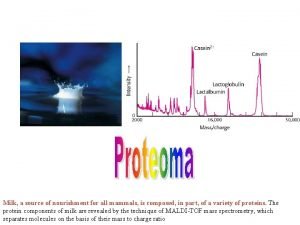
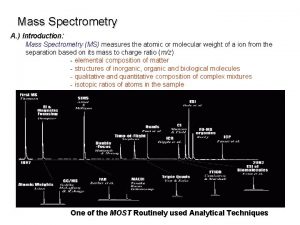
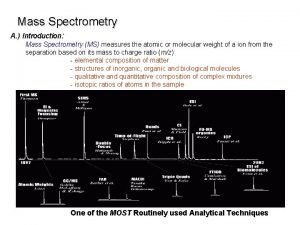
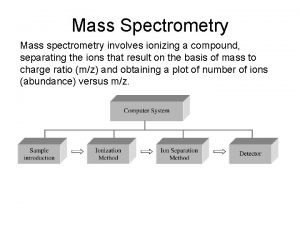
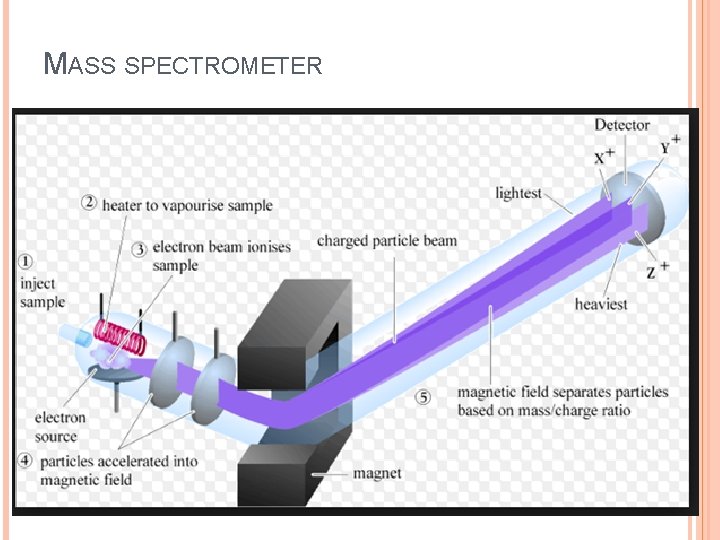
MASS SPECTROMETRY Measures the mass of the molecules Measures mass of the fragment particles which are broken apart from a molecule I. e. measures mass of a protein and also measures mass of the broken peptides of the protein This technique helps in the identification of the components of a chemical compound in detail and also allows the structure prediction of organic molecules by analyzing their molecular masses in detail 2

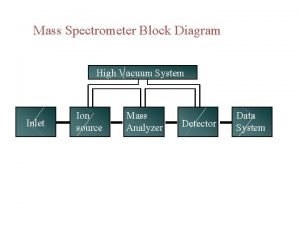
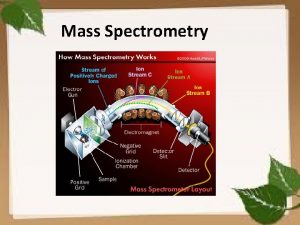
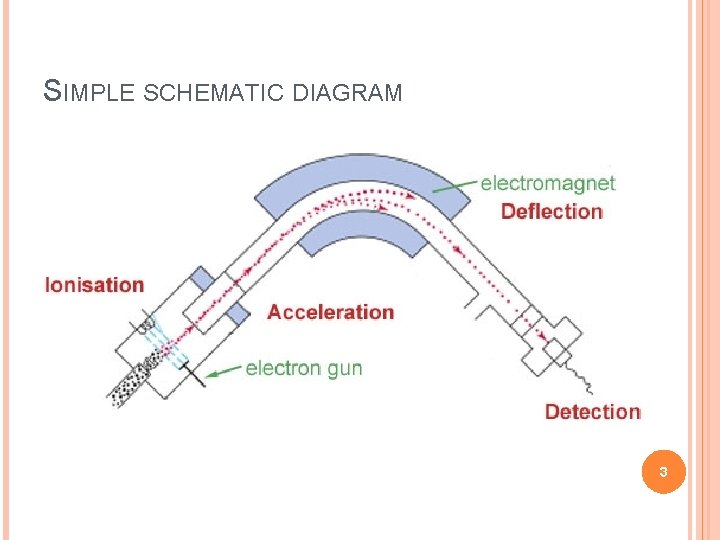
SIMPLE SCHEMATIC DIAGRAM 3

BASIC PRINCIPLE Formation of ions from the neutral molecules which undergoes decomposition into smaller ions which are then examined via the mass spectrometer The studies have more been done on the positive ions than the negative ions of a molecule This occurred due to the more potential behavior of the M+ radical cations to give an account of the information which negative ions cannot do Also the amount of positive fragment ions produced after bombardment of the neutrons is larger than the negative ions produced 4

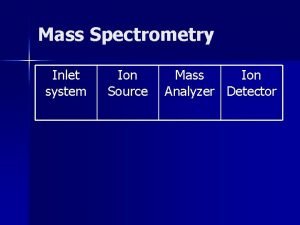
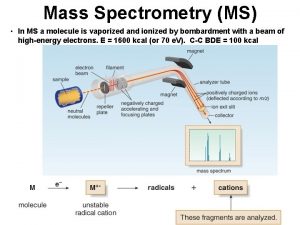
MASS SPECTROMETER There is no definite mass spectrometer which would be universally applicable but there are multiple combinations in which we could make and adjust the mass spectrometer as per requirement of the reaction The mass spectrometer ideally consist of three parts q The ionization source q A mass analyzer q Detector 5

1) SOURCES Formation of cations and anions is the basic aim of the ionization sources There are multiple ion sources which could be utilized in combination with the other two parts of analyzer and detector q Electron spray ionization ESI q Chemical ionization CI q Matrix assisted laser desorption ionization MALDI q Plasma desorption ionization PDI 6

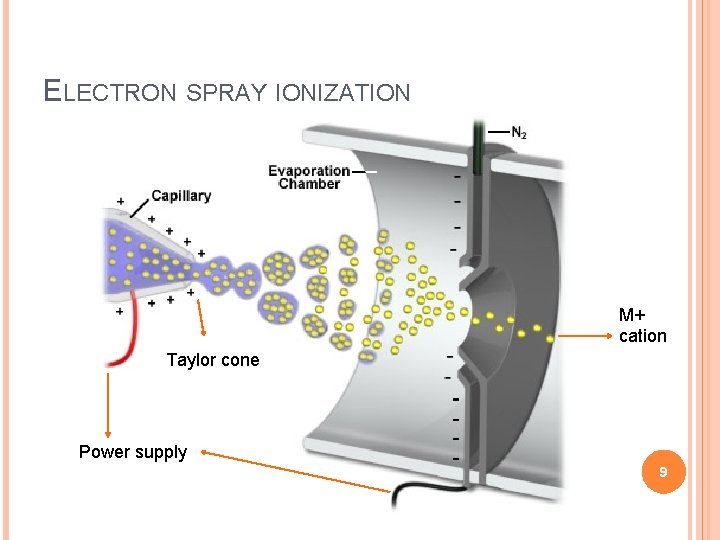
ELECTRON SPRAY IONIZATION (ESI) Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized. 7

The liquid containing the analyte(s) of interest is dispersed by electrospray into a fine aerosol. Because the ion formation involves extensive solvent evaporation (also termed desolvation), the typical solvents for electrospray ionization are prepared by mixing water with volatile organic compounds (e. g. methanol acetonitrile). To decrease the initial droplet size, compounds that increase the conductivity (e. g. acetic acid) are customarily added to the solution. These species also act to provide a source of protons to facilitate the ionization process. Large-flow electrosprays can benefit from additional nebulization by an inert gas such as nitrogen or carbon dioxide. 8

ELECTRON SPRAY IONIZATION M+ cation Taylor cone Power supply 9

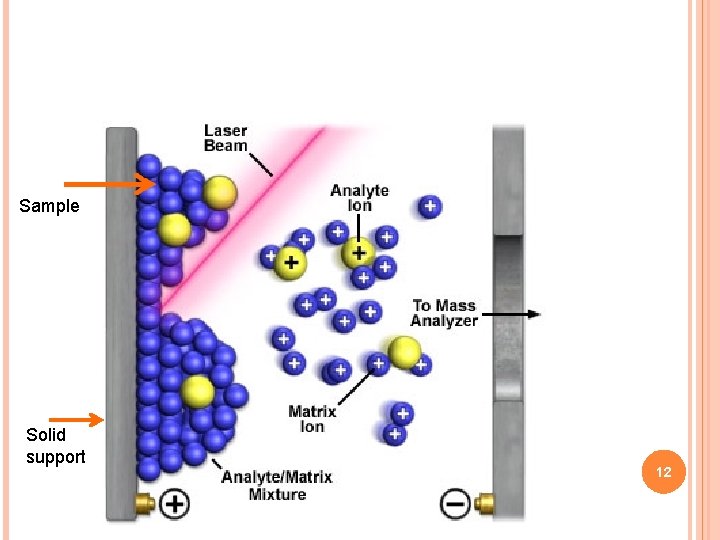
MATRIX ASSISTED LASER DESORPTION IONIZATION (MALDI) This is a soft ionization technique It allows the analysis of biomolecules (biopolymers such as DNA, proteins, peptides and sugars) and large organic molecules (such as polymers and other macromolecules), These tend to be fragile and fragment when ionized by more conventional ionization methods. It is similar to electrospray ionization (ESI) in that both techniques are relatively soft ways of obtaining large ions in the gas phase, though MALDI produces far fewer multiply charged ions. 10

MALDI is a two-step process. First, desorption is triggered by a UV laser beam. Matrix material heavily absorbs UV laser light, leading to the ablation of upper layer (~1 μm) of the matrix material. Many charged species termed as ablated species are produced. Second, the analyte molecules are ionized (more accurately protonated or deprotonated) in the hot plume. Ablated species may participate in the ionization of analyte, though the mechanism of MALDI is still debated. 11

Sample Solid support 12

13

14

CHEMICAL IONIZATION (CI) In a CI experiment, ions are produced through the collision of the analyte with ions of a reagent gas that are present in the ion source. Some common reagent gases include: methane, ammonia, and isobutane. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization plasma. Positive and negative ions of the analyte are formed by reactions with this plasma 15

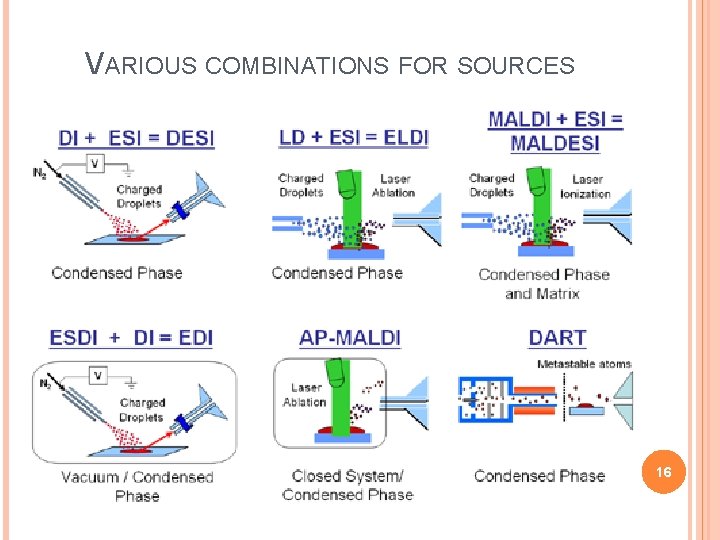
VARIOUS COMBINATIONS FOR SOURCES 16

2) MASS ANALYZER There are different kinds of mass analyzers one of which is the magnetic deflection mass analyzer The ions generated from the ion source are deflected as per their mass to charge ratios and are accelerated via the electrostatic slits And then led to an analyzer In the magnetic analyzer the ions are deflected in a strong magnetic field as per their mass to charge ratios The path followed by them is curved and it then lead to the detector 17

3) DETECTOR The three most common detectors used for the analysis of the different samples are q Faraday cup detector q Electron multiplier detector q TOFF time of flight 18

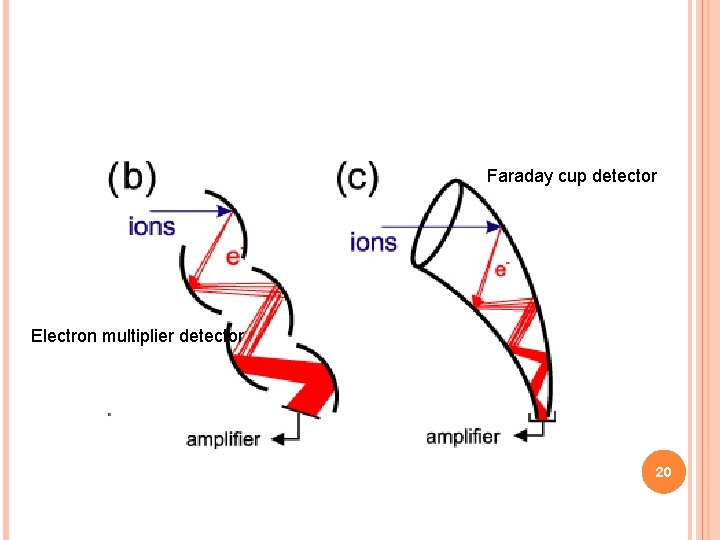
FARADAY CUP DETECTOR A Faraday cup is a metal (conductive) cup designed to catch charged particles in vacuum. The resulting current can be measured and used to determine the number of ions or electrons hitting the cup. The Faraday cup is named after Michael Faraday who first theorized ions around 1830. 19

Faraday cup detector Electron multiplier detector 20

ELECTRON MULTIPLIER DETECTOR An electron multiplier is a vacuum-tube structure that multiplies incident charges In a process called secondary emission, a single electron can, when bombarded on secondary emissive material, induce emission of roughly 1 to 3 electrons. If an electric potential is applied between this metal plate and yet another, the emitted electrons will accelerate to the next metal plate and induce secondary emission of still more electrons. This can be repeated a number of times, resulting in a large shower of electrons all collected by a metal anode, all having been triggered by just one. 21

MASS SPECTROMETER 22

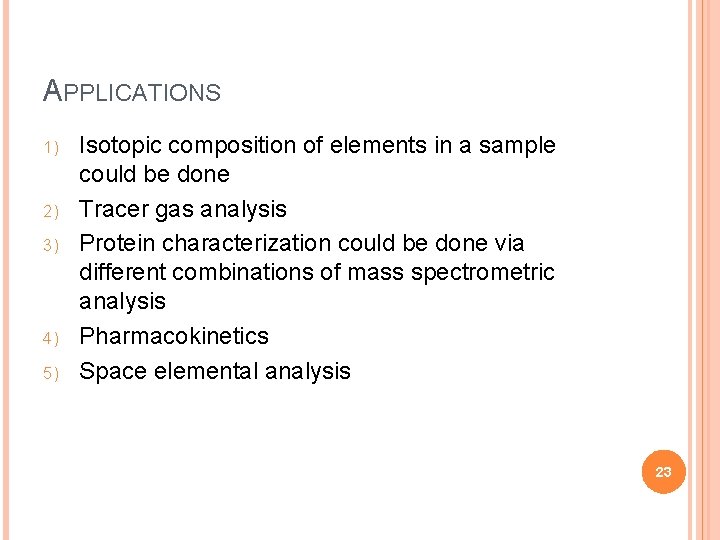
APPLICATIONS 1) 2) 3) 4) 5) Isotopic composition of elements in a sample could be done Tracer gas analysis Protein characterization could be done via different combinations of mass spectrometric analysis Pharmacokinetics Space elemental analysis 23

THANKS 24
 Butyl isopropyl ether
Butyl isopropyl ether Mass spectrometry
Mass spectrometry Rule of thirteen mass spectrometry
Rule of thirteen mass spectrometry Inlet system in mass spectrometry
Inlet system in mass spectrometry Deflection in mass spectrometry
Deflection in mass spectrometry Mass spectrometry
Mass spectrometry Mass spectrometry lecture
Mass spectrometry lecture Mass spectrometry a level
Mass spectrometry a level Mass spectrometry
Mass spectrometry Mass spectrometer block diagram
Mass spectrometer block diagram Mass spectrometry data acquisition for gc/ms
Mass spectrometry data acquisition for gc/ms Ms
Ms Mass spectrometry in forensic science
Mass spectrometry in forensic science Drosophila definition
Drosophila definition Dating
Dating Mass spectrometry
Mass spectrometry Mass spectrum graph
Mass spectrum graph Khan academy mass spectrometry
Khan academy mass spectrometry Mass spectrometry ionization
Mass spectrometry ionization Nitrogen rule in mass spectrometry
Nitrogen rule in mass spectrometry Swath mass spectrometry
Swath mass spectrometry Spectroscopy problem set
Spectroscopy problem set Principle mass spectroscopy
Principle mass spectroscopy Mass spectrometry
Mass spectrometry Mass spectrometry exam questions
Mass spectrometry exam questions