Lipids ORGANIC COMPOUNDS Lipids Include fatty acids triglycerides



- Slides: 43

Lipids ORGANIC COMPOUNDS

Lipids Include fatty acids, triglycerides, phospholipids, steroids, eicosanoids, and vitamins A, E, and K Represent 18 -25% of your body mass Contain C, H, O, and sometimes P Most are hydrophobic Lipoproteins

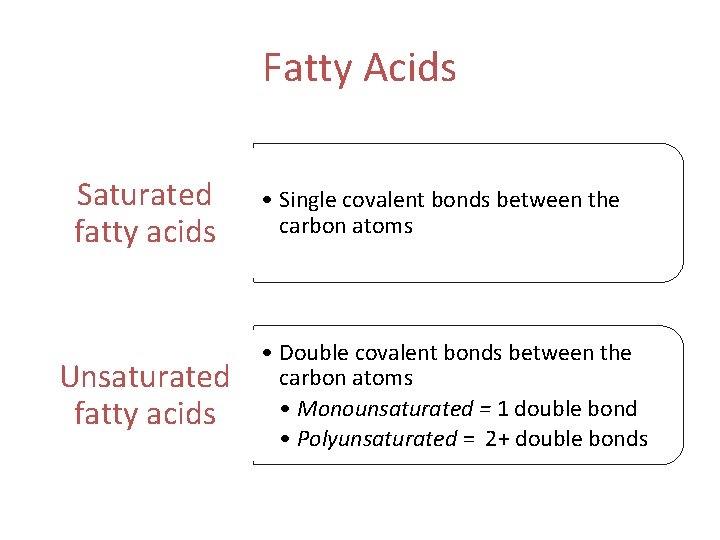
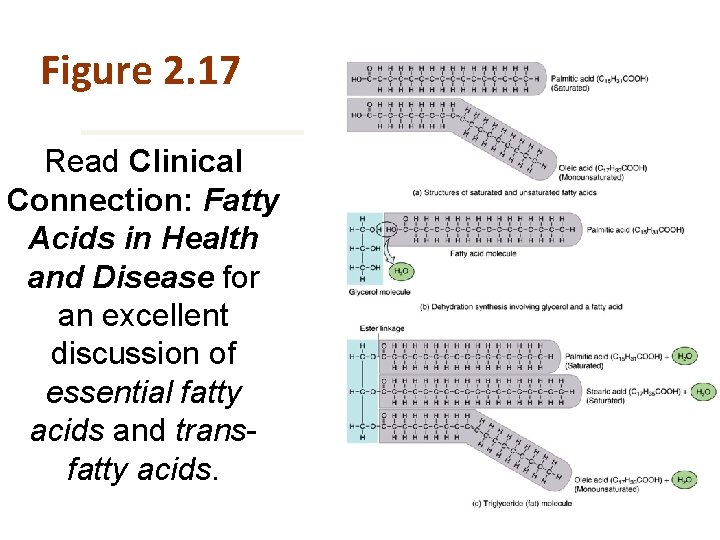
Fatty Acids The simplest lipids • Used to synthesize triglycerides and phospholipids • Can be catabolized to generate ATP • Composed of a carboxyl group and a hydrocarbon chain • Can either be saturated or unsaturated

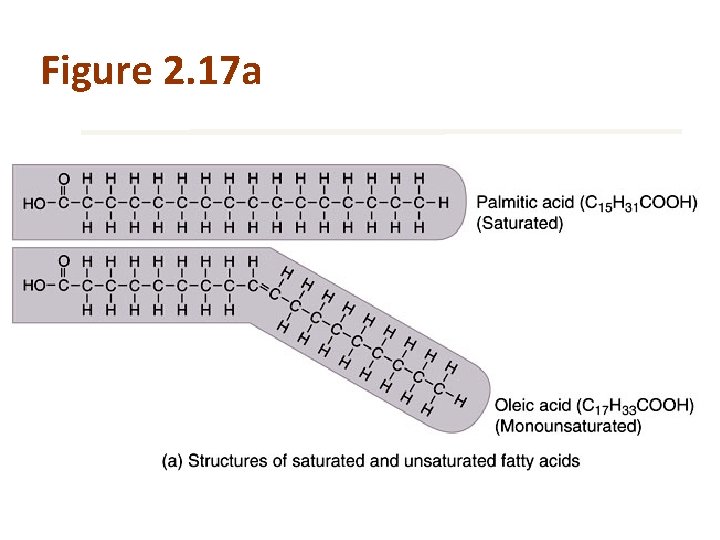
Fatty Acids Saturated fatty acids Unsaturated fatty acids • Single covalent bonds between the carbon atoms • Double covalent bonds between the carbon atoms • Monounsaturated = 1 double bond • Polyunsaturated = 2+ double bonds

Figure 2. 17 asynthesis

Triglycerides Structure • 1 molecule of glycerol and 3 fatty acids • Formed by dehydration synthesis • Broken down by hydrolysis Function • Energy storage (adipose tissue); can be catabolized to generate ATP • Protection and insulation

Triglycerides • Classified according to the fatty acids: Saturated • Solid at room temp • Common in animal fats and non-skim dairy • Cocoa butter, palm oil, coconut oil • Increase risk of heart disease and colorectal cancer Unsaturated • Liquid at room temp • Common in plant oils • Monounsaturated – olive, peanut, and canola oil, nuts, avocados • Polyunsaturated – corn, safflower, sunflower and soybean oil, fatty fish • Decrease risk of heart disease

Figure 2. 17 is Read Clinical Connection: Fatty Acids in Health and Disease for an excellent discussion of essential fatty acids and transfatty acids.

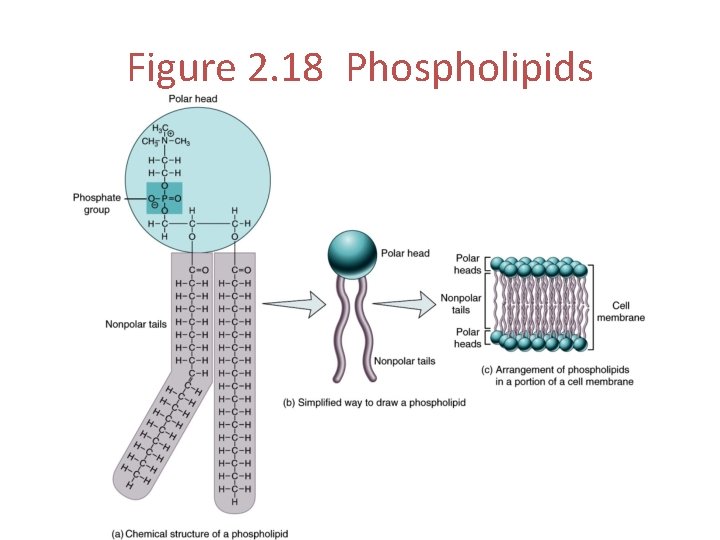
Phospholipids Structure • Composed of glycerol, 2 fatty acid molecules, and 1 phosphate group (PO 43 -) • The phosphate group is polar and can form hydrogen bonds with water - it is hydrophilic • The 2 fatty acid tails are nonpolar and cannot form hydrogen bonds with water - they are hydrophobic Function • Amphipathic phospholipids line up tail-to-tail in a double row to form cell membranes

Figure 2. 18 Phospholipids

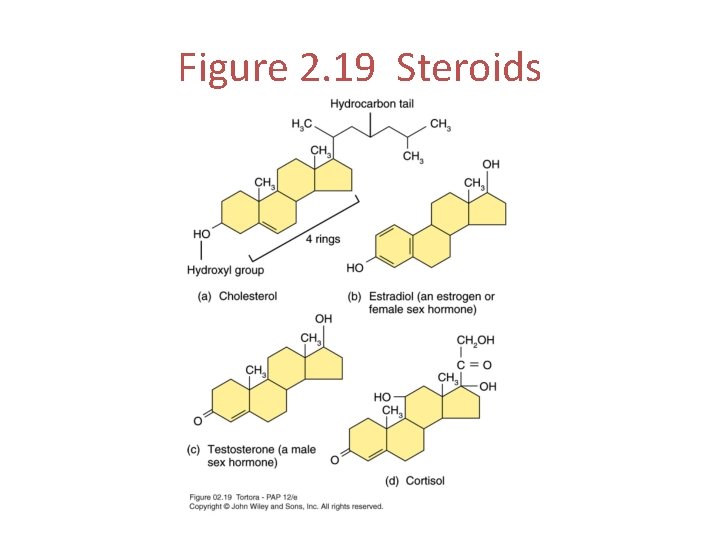
Steroids Composed of 4 rings of carbon atoms. Cholesterol Bile salts Vitamin D Adrenocortical hormones Sex hormones

Figure 2. 19 Steroids

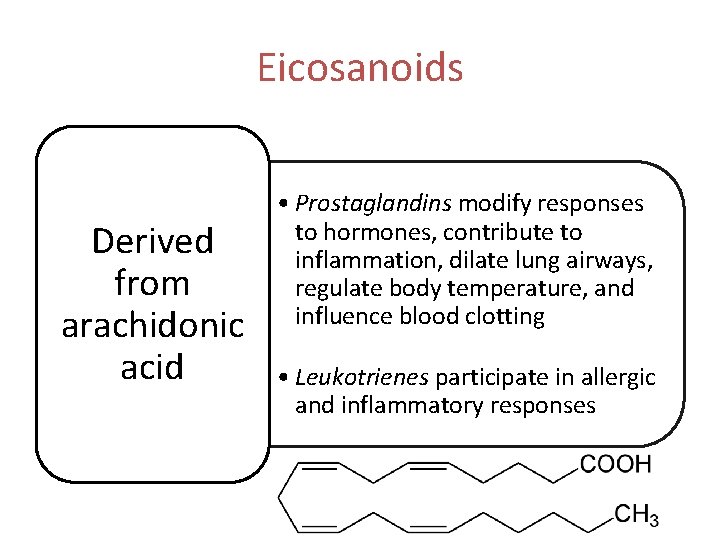
Eicosanoids Derived from arachidonic acid • Prostaglandins modify responses to hormones, contribute to inflammation, dilate lung airways, regulate body temperature, and influence blood clotting • Leukotrienes participate in allergic and inflammatory responses

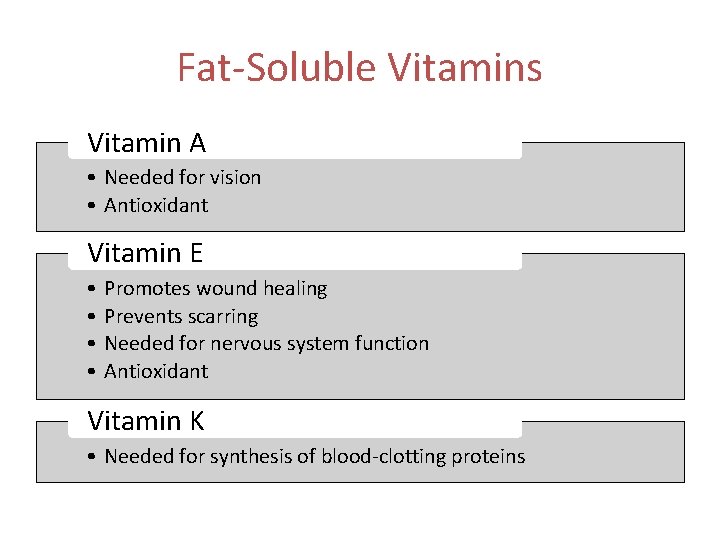
Fat-Soluble Vitamins Vitamin A • Needed for vision • Antioxidant Vitamin E • • Promotes wound healing Prevents scarring Needed for nervous system function Antioxidant Vitamin K • Needed for synthesis of blood-clotting proteins

Table 2. 7 Types of Lipids in the Body

Proteins ORGANIC COMPOUNDS

Proteins Represent about 12 -18% of your body mass Three major groups based on their sizes: amino acids, peptides, and polypeptides Contain C, H, O, and N (and sometimes S)

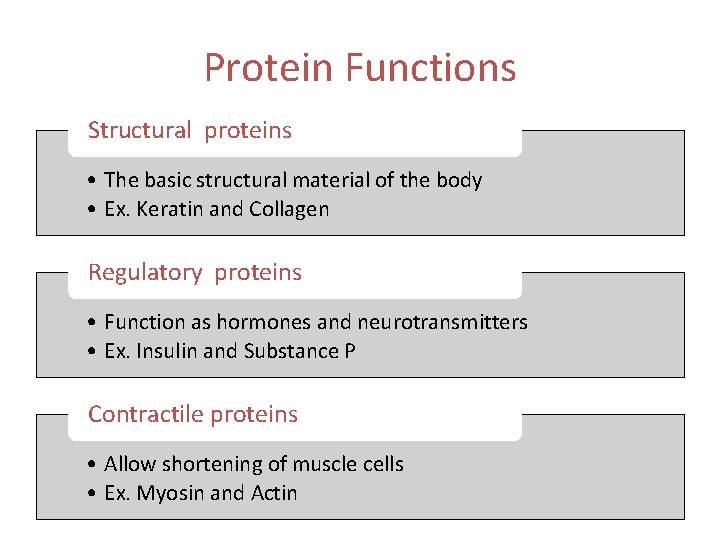
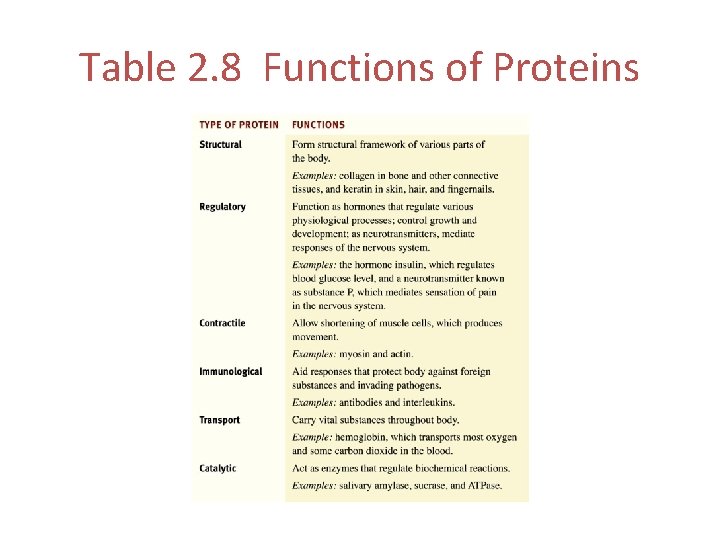
Protein Functions Structural proteins • The basic structural material of the body • Ex. Keratin and Collagen Regulatory proteins • Function as hormones and neurotransmitters • Ex. Insulin and Substance P Contractile proteins • Allow shortening of muscle cells • Ex. Myosin and Actin

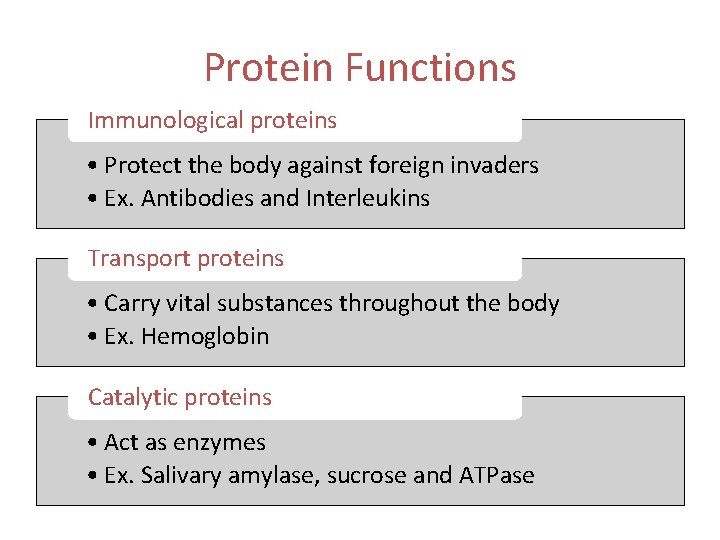
Protein Functions Immunological proteins • Protect the body against foreign invaders • Ex. Antibodies and Interleukins Transport proteins • Carry vital substances throughout the body • Ex. Hemoglobin Catalytic proteins • Act as enzymes • Ex. Salivary amylase, sucrose and ATPase

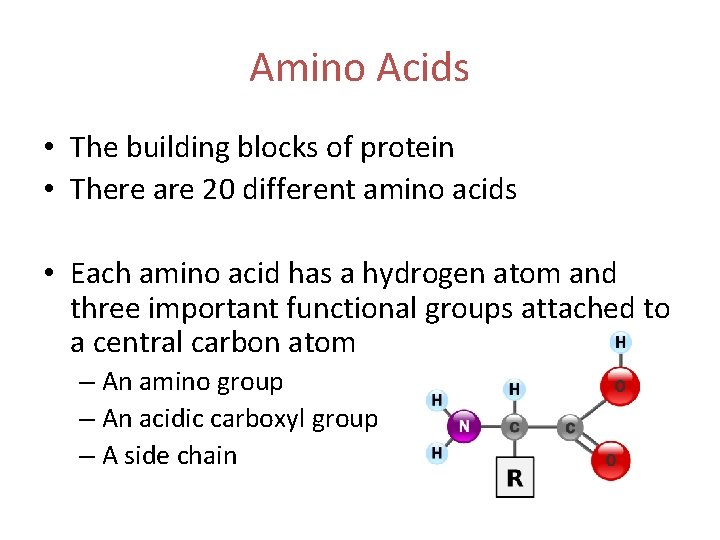
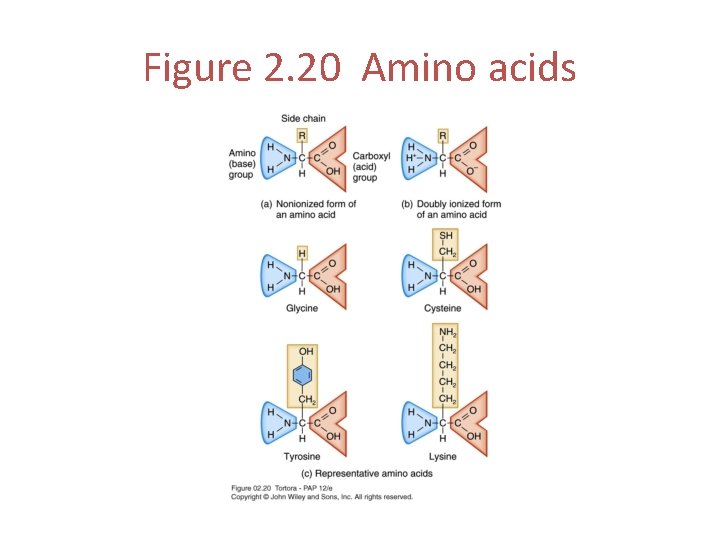
Amino Acids • The building blocks of protein • There are 20 different amino acids • Each amino acid has a hydrogen atom and three important functional groups attached to a central carbon atom – An amino group – An acidic carboxyl group – A side chain

Figure 2. 20 Amino acids

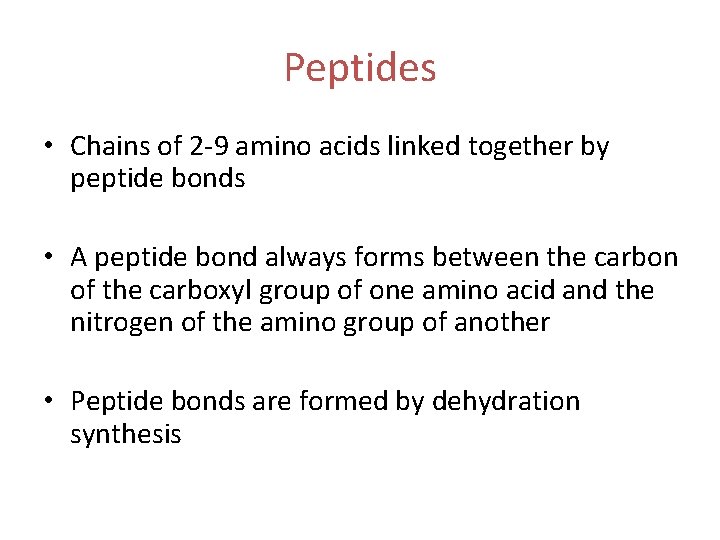
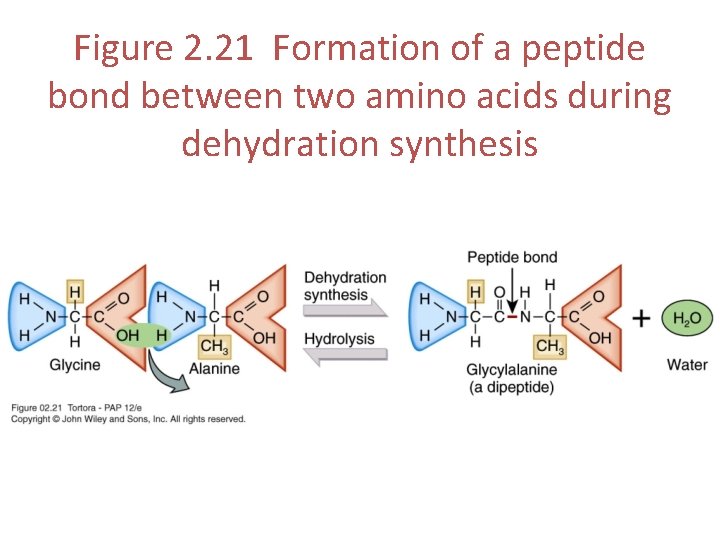
Peptides • Chains of 2 -9 amino acids linked together by peptide bonds • A peptide bond always forms between the carbon of the carboxyl group of one amino acid and the nitrogen of the amino group of another • Peptide bonds are formed by dehydration synthesis

Figure 2. 21 Formation of a peptide bond between two amino acids during dehydration synthesis

Polypeptides • Chains of 10 -99 amino acids linked together by peptide bonds • Some proteins are small (about 50 aa) • Most proteins are huge (100 – 2000 aa) and may consist of one polypeptide chain, or several folded together

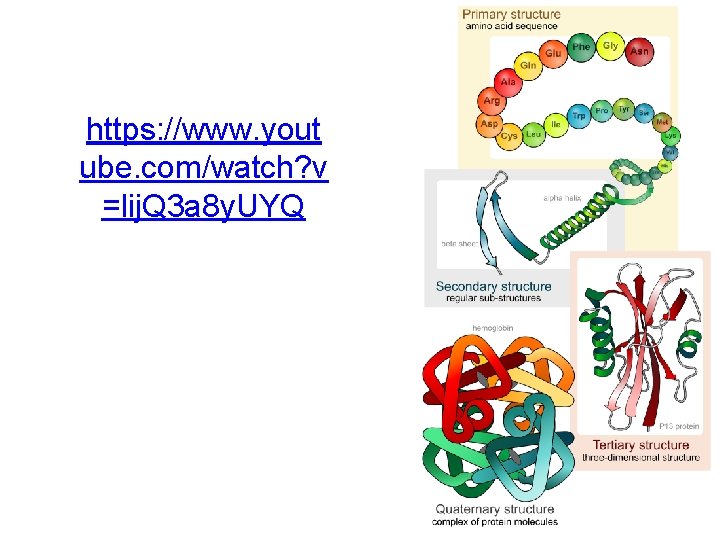
Protein Structure Quaternary Structure Tertiary Structure Secondary Structure Primary Structure

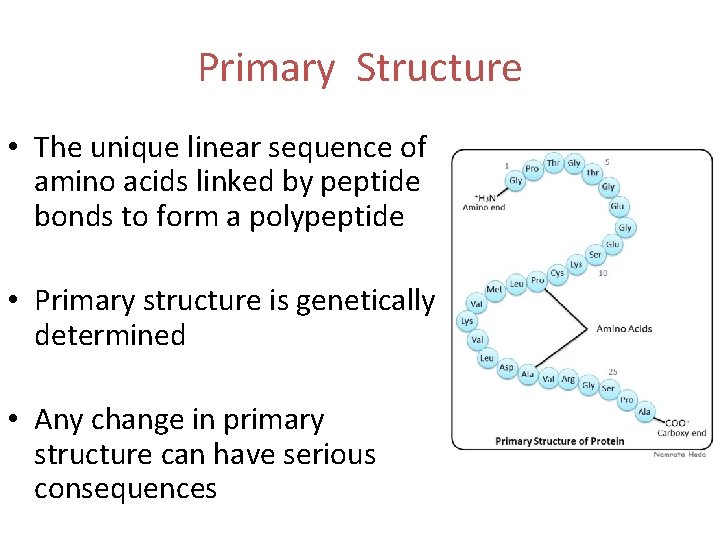
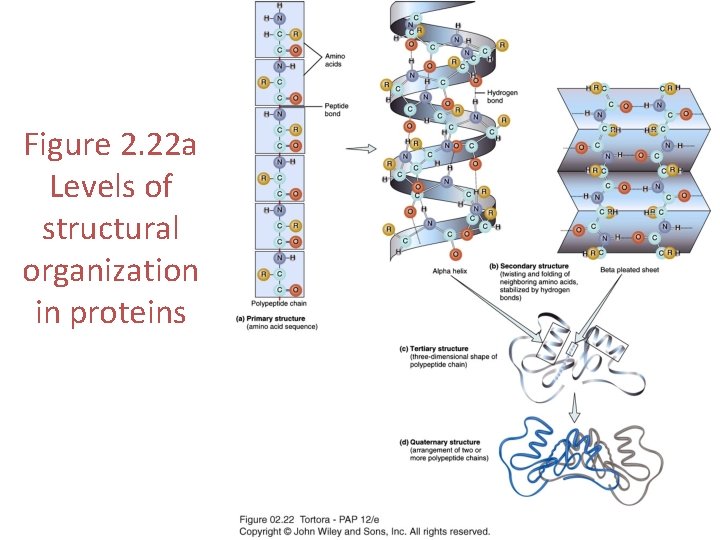
Primary Structure • The unique linear sequence of amino acids linked by peptide bonds to form a polypeptide • Primary structure is genetically determined • Any change in primary structure can have serious consequences

Figure 2. 22 a Levels of structural organization in proteins

Secondary Structure • The repeated twisting or folding of neighboring amino acids in the polypeptide chain – Alpha-helixes (clockwise spirals) – Beta-pleated sheets • A polypeptide can exhibit one or both types of secondary structure • Secondary structure is stabilized by hydrogen bonds – Form at regular intervals along the polypeptide backbone

Tertiary Structure • The 3 -dimensional shape of a polypeptide chain • A protein with tertiary structure is functional – It’s tertiary structure determines its function • Tertiary structure is stabilized by disulfide bonds, hydrogen bonds, ionic bonds, and hydrophobic interactions • Chaperones aid the folding process

Quaternary Structure • The arrangement of the individual polypeptide chains relative to one another in proteins that contain more than one polypeptide chain – Only some proteins contain more than one polypeptide chain. – Those chains are called protein subunits and cannot function on their own. • Quaternary structure is stabilized by the same bonds that maintain tertiary structure

https: //www. yout ube. com/watch? v =lij. Q 3 a 8 y. UYQ

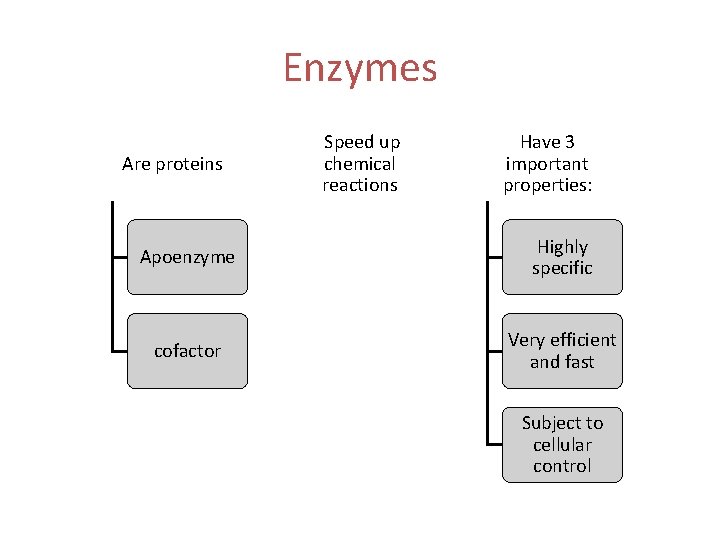
Enzymes Are proteins Speed up chemical reactions Have 3 important properties: Apoenzyme Highly specific cofactor Very efficient and fast Subject to cellular control

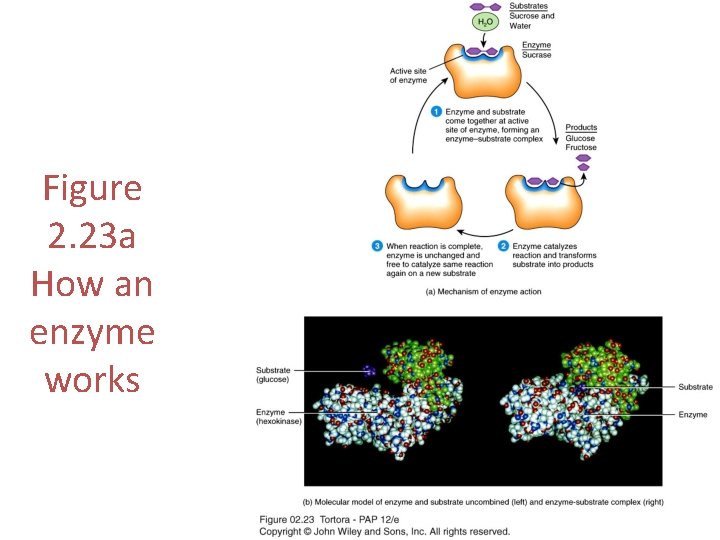
Figure 2. 23 a How an enzyme works

Table 2. 8 Functions of Proteins

Nucleic Acids ORGANIC COMPOUNDS

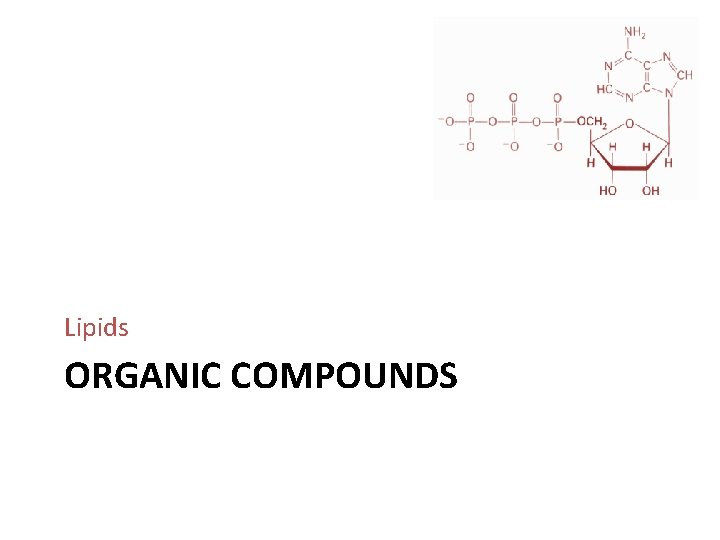
Nucleic Acids Huge organic molecules Deoxyribonucleic acid (DNA) • Forms the inherited genetic material (genes) inside each human cell Ribonucleic acid (RNA) • Relays instructions from the genes to guide protein synthesis Contain C, H, O, N, and P

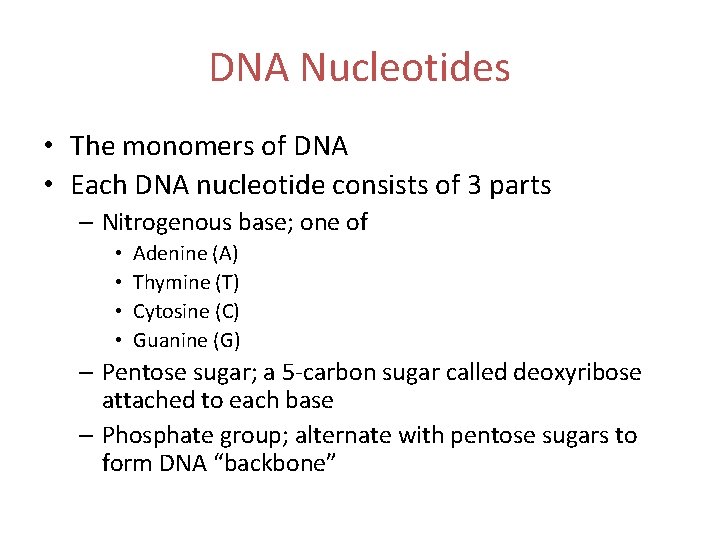
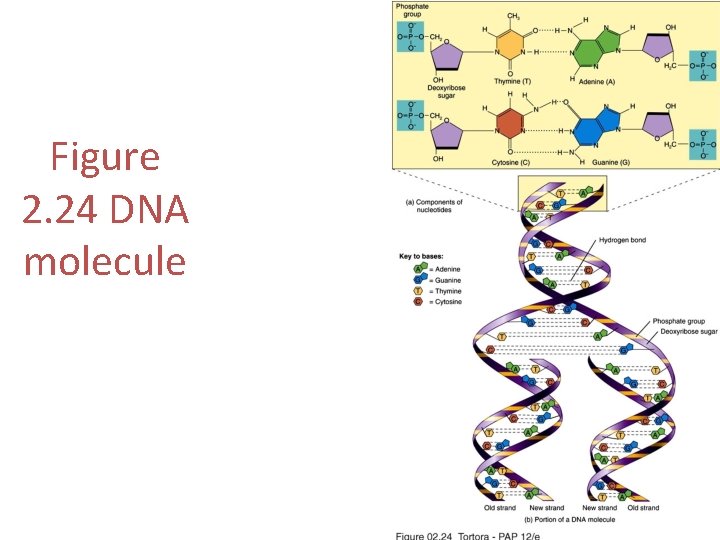
DNA Nucleotides • The monomers of DNA • Each DNA nucleotide consists of 3 parts – Nitrogenous base; one of • • Adenine (A) Thymine (T) Cytosine (C) Guanine (G) – Pentose sugar; a 5 -carbon sugar called deoxyribose attached to each base – Phosphate group; alternate with pentose sugars to form DNA “backbone”

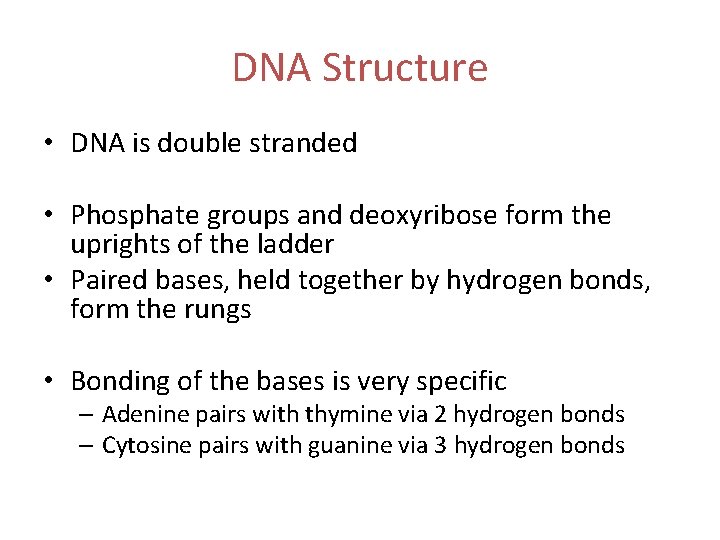
DNA Structure • DNA is double stranded • Phosphate groups and deoxyribose form the uprights of the ladder • Paired bases, held together by hydrogen bonds, form the rungs • Bonding of the bases is very specific – Adenine pairs with thymine via 2 hydrogen bonds – Cytosine pairs with guanine via 3 hydrogen bonds

Figure 2. 24 DNA molecule

RNA Nucleotides • The monomers of RNA • Each RNA nucleotide consists of 3 parts – Nitrogenous base; one of • • Adenine (A) Uracil (U) Cytosine (C) Guanine (G) – Pentose sugar; a 5 -carbon sugar called ribose attached to each base – Phosphate group; alternate with pentose sugars to form RNA “backbone”

RNA Structure • RNA is single stranded • Cells contain 3 different kinds of RNA, each of which has a specific role to perform during protein synthesis – Ribosomal RNA (r. RNA) – Messenger RNA (m. RNA) – Transfer RNA

Adenosine Triphosphate “Energy currency” Powers cellular activities such as Muscular contraction Organellar Movement within cells Membrane transport Protein synthesis

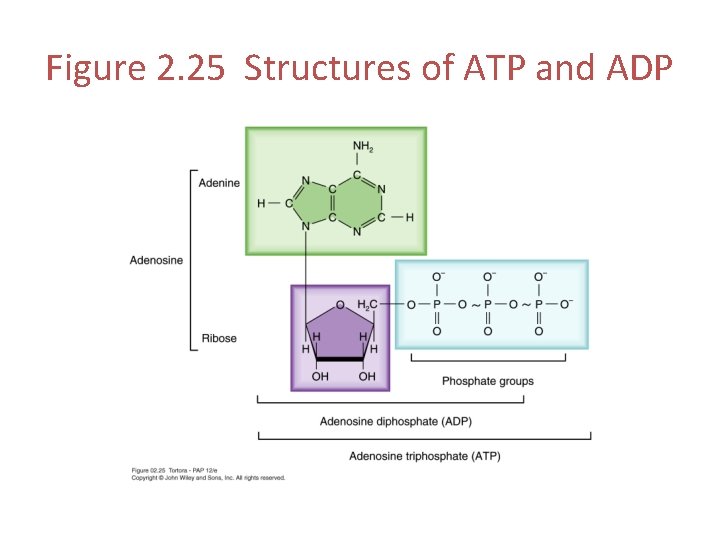
Figure 2. 25 Structures of ATP and ADP