OIL AND FAT TECHNOLOGY 1 st WEEK Food




- Slides: 32

OIL AND FAT TECHNOLOGY 1 st WEEK

Food Lipids • Definition: Nonpolar, hydrophobic, poorly water soluble components which are soluble in organic solvents such as alkanes (e. g. hexane) • Includes fats: solid at room temperature oils: liquid at room temperature • Definition based on physical properties Triglycerides Fatty Acids Primary topics Phospholipids Terpenes/Terpenoids Steroids Waxes

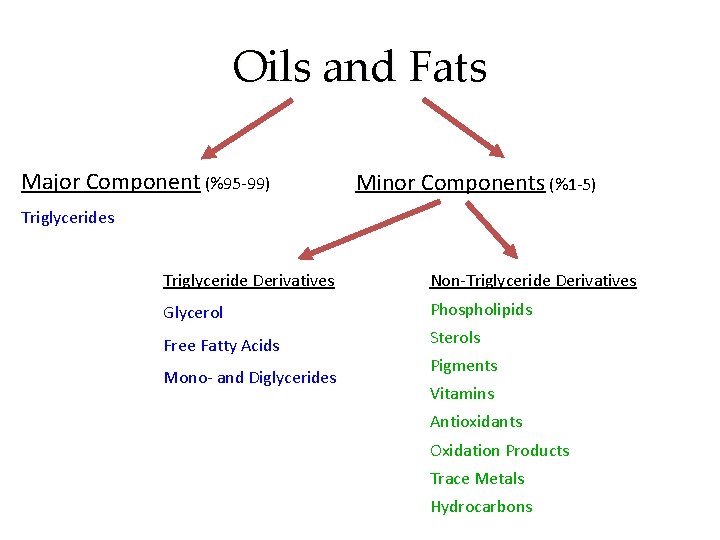
Oils and Fats Major Component (%95 -99) Minor Components (%1 -5) Triglycerides Triglyceride Derivatives Non-Triglyceride Derivatives Glycerol Phospholipids Free Fatty Acids Sterols Mono- and Diglycerides Pigments Vitamins Antioxidants Oxidation Products Trace Metals Hydrocarbons

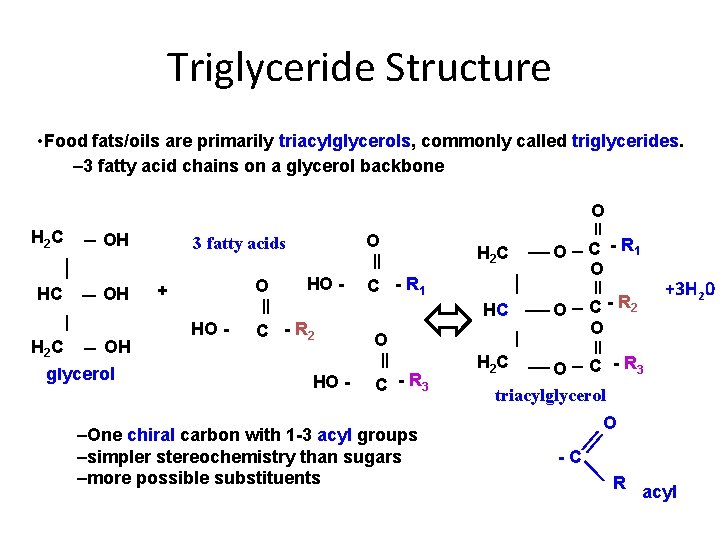
Triglyceride Structure • Food fats/oils are primarily triacylglycerols, commonly called triglycerides. – 3 fatty acid chains on a glycerol backbone O H 2 C OH glycerol O 3 fatty acids O + HO - C - R 2 HO - H 2 C O C - R 1 O O C - R 2 O C - R 1 HC O C - R 3 –One chiral carbon with 1 -3 acyl groups –simpler stereochemistry than sugars –more possible substituents +3 H 20 H 2 C O C - R 3 triacylglycerol O -C R acyl

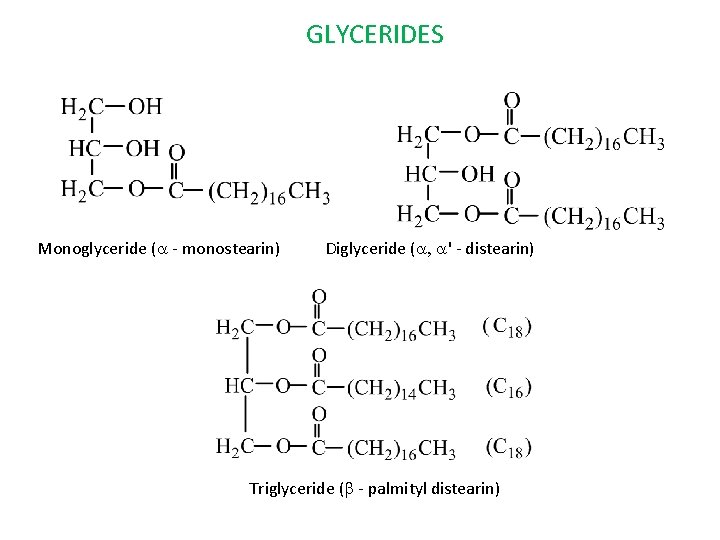
GLYCERIDES Monoglyceride (a - monostearin) Diglyceride (a, a' - distearin) Triglyceride (b - palmityl distearin)

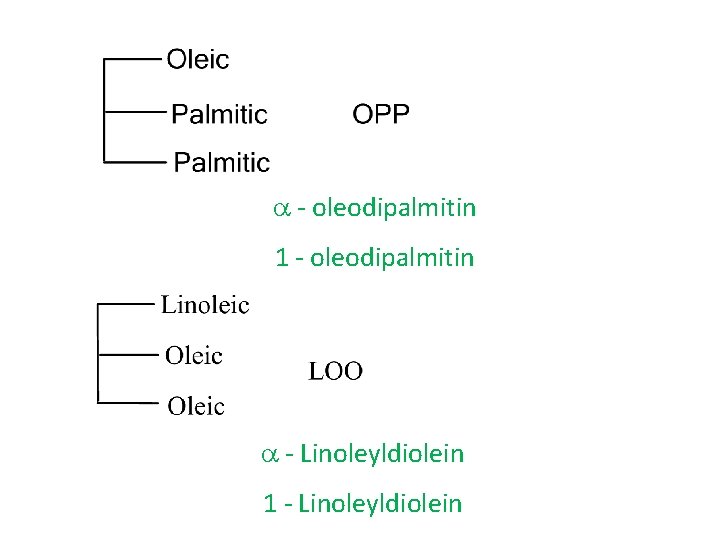
a - oleodipalmitin 1 - oleodipalmitin a - Linoleyldiolein 1 - Linoleyldiolein

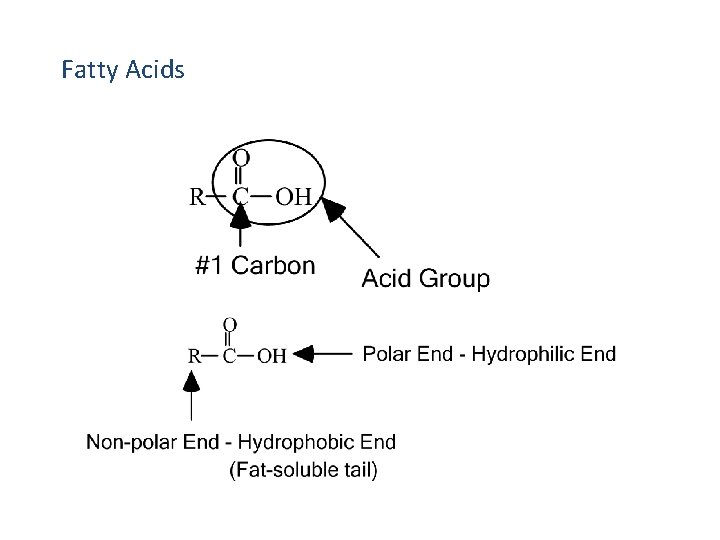
Fatty Acids

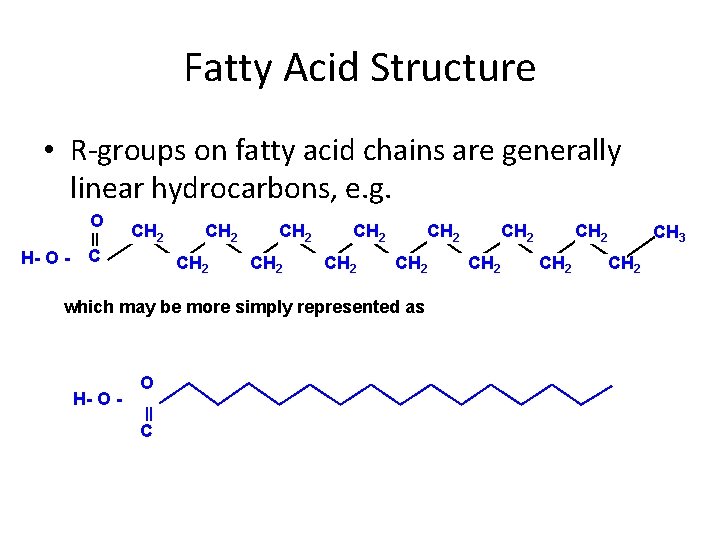
Fatty Acid Structure • R-groups on fatty acid chains are generally linear hydrocarbons, e. g. O H- O - CH 2 CH 2 CH 2 which may be more simply represented as H- O C CH 2 CH 3 CH 2

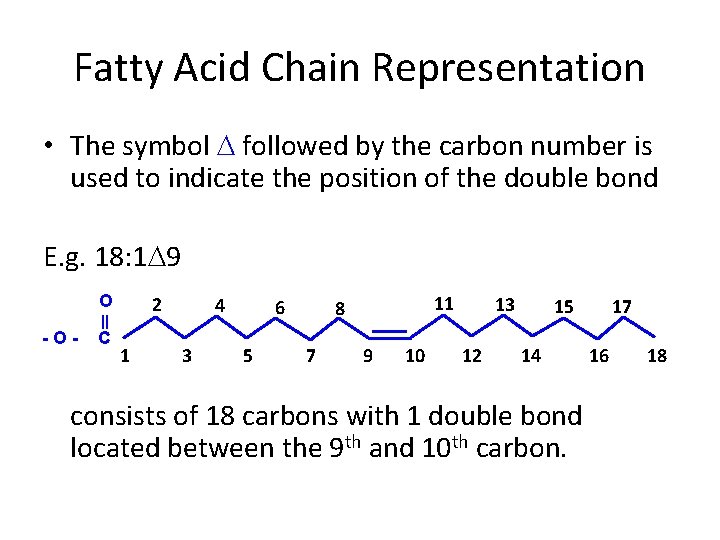
Fatty Acid Chain Representation • The symbol followed by the carbon number is used to indicate the position of the double bond E. g. 18: 1 9 O -O- C 2 1 4 3 6 5 11 8 7 9 10 13 12 15 14 17 16 consists of 18 carbons with 1 double bond located between the 9 th and 10 th carbon. 18

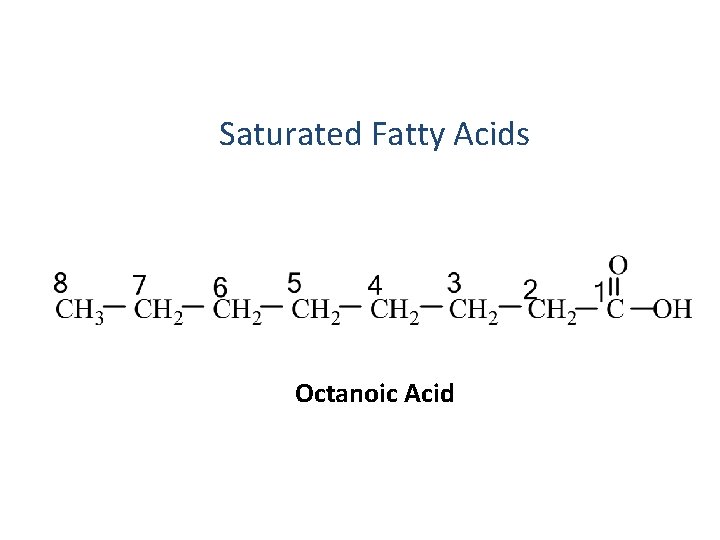
Saturated Fatty Acids Octanoic Acid

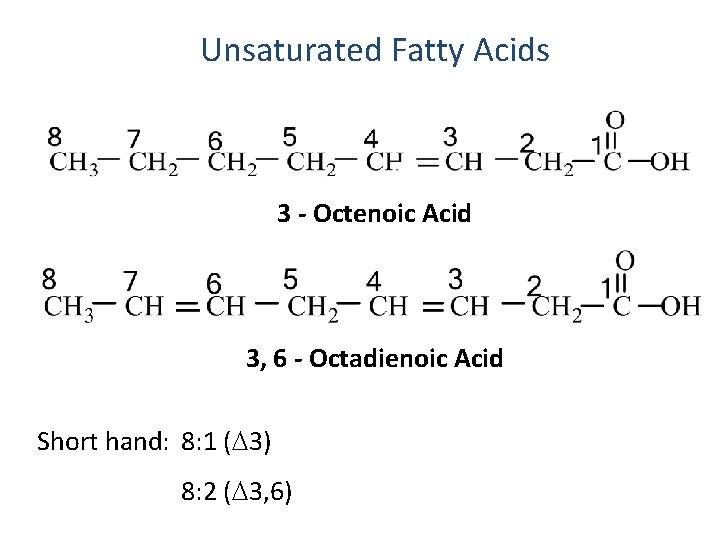
Unsaturated Fatty Acids 3 - Octenoic Acid 3, 6 - Octadienoic Acid Short hand: 8: 1 ( 3) 8: 2 ( 3, 6)

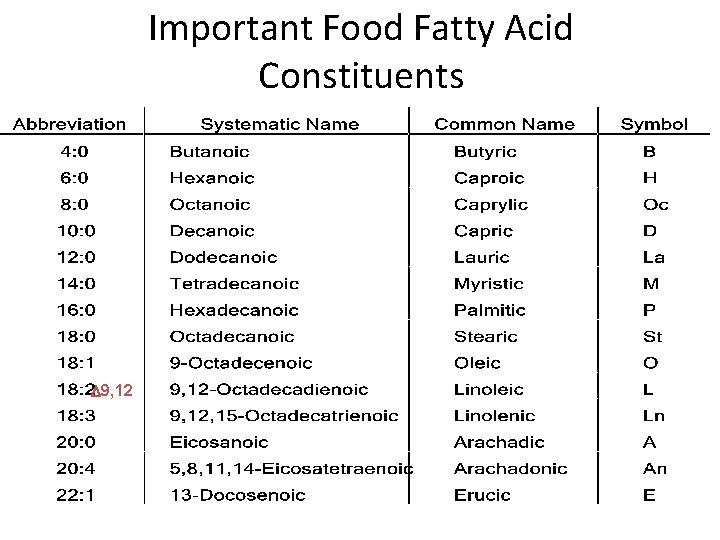
Important Food Fatty Acid Constituents D 9, 12

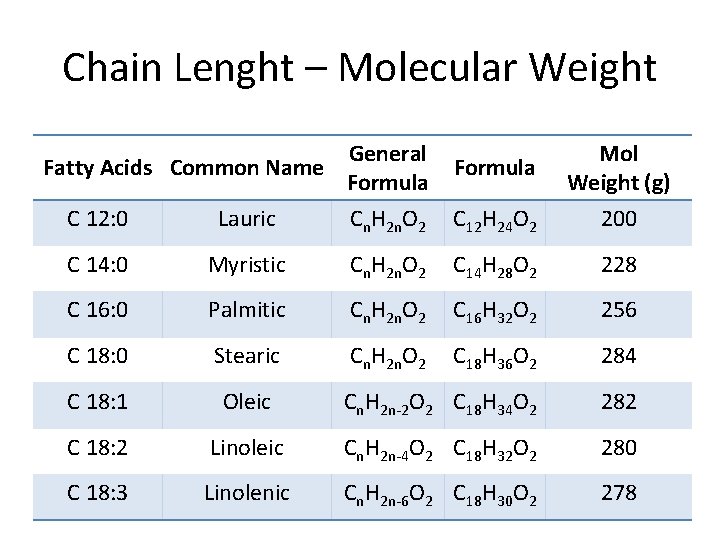
Chain Lenght – Molecular Weight Fatty Acids Common Name General Formula Mol Weight (g) C 12: 0 Lauric Cn. H 2 n. O 2 C 12 H 24 O 2 200 C 14: 0 Myristic Cn. H 2 n. O 2 C 14 H 28 O 2 228 C 16: 0 Palmitic Cn. H 2 n. O 2 C 16 H 32 O 2 256 C 18: 0 Stearic Cn. H 2 n. O 2 C 18 H 36 O 2 284 C 18: 1 Oleic Cn. H 2 n-2 O 2 C 18 H 34 O 2 282 C 18: 2 Linoleic Cn. H 2 n-4 O 2 C 18 H 32 O 2 280 C 18: 3 Linolenic Cn. H 2 n-6 O 2 C 18 H 30 O 2 278

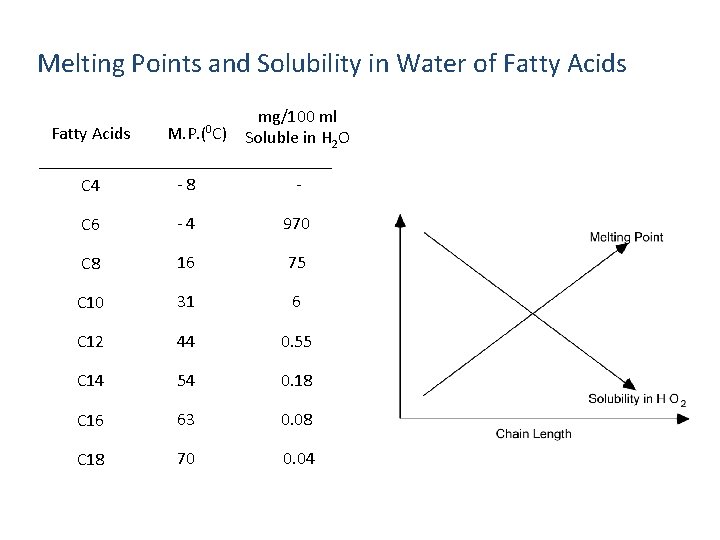
Melting Points and Solubility in Water of Fatty Acids M. P. (0 C) mg/100 ml Soluble in H 2 O C 4 - 8 - C 6 - 4 970 C 8 16 75 C 10 31 6 C 12 44 0. 55 C 14 54 0. 18 C 16 63 0. 08 C 18 70 0. 04

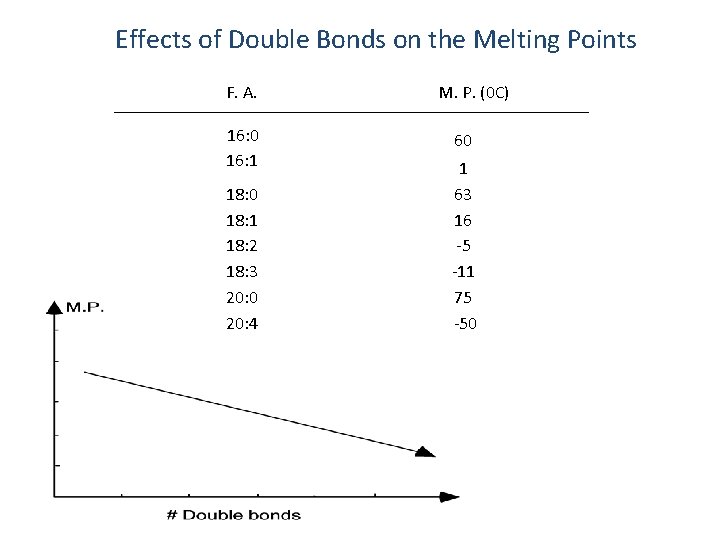
Effects of Double Bonds on the Melting Points F. A. 16: 0 16: 1 18: 0 18: 1 18: 2 18: 3 20: 0 20: 4 M. P. (0 C) 60 1 63 16 -5 -11 75 -50

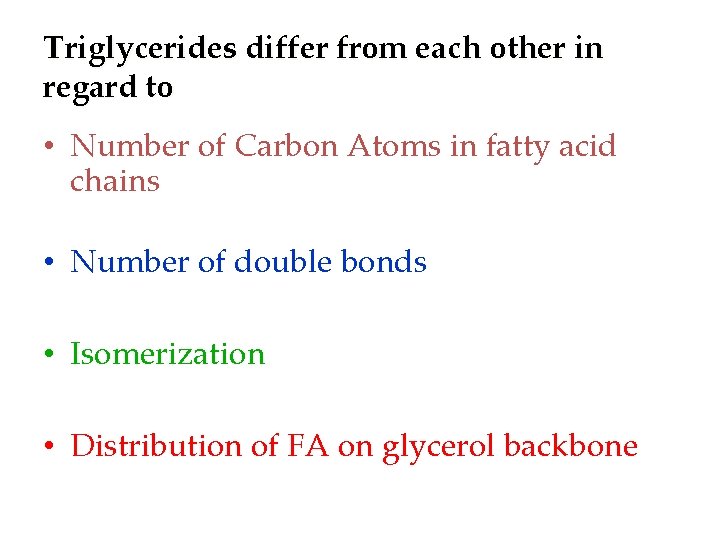
Triglycerides differ from each other in regard to • Number of Carbon Atoms in fatty acid chains • Number of double bonds • Isomerization • Distribution of FA on glycerol backbone

Analytical Methods • Saponification Value • Iodine Value • Gas Chromatographic Analysis for Fatty Acids • Liquid Chromatography

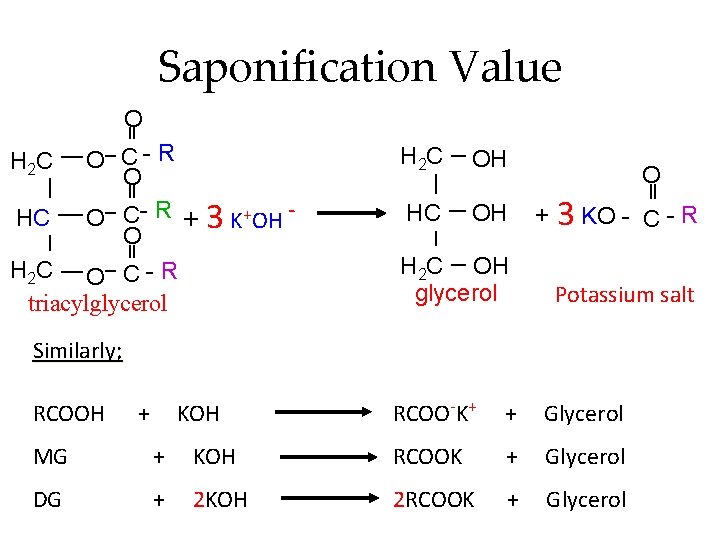
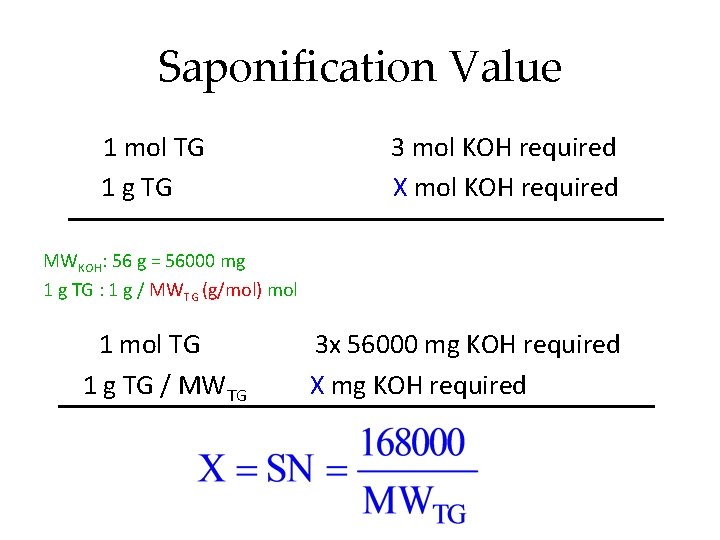
Saponification Value Saponification - hydrolysis of ester under alkaline condition. The saponification value of an oil or fat is defined as the number of mg of potassium hydroxide (KOH) required to neutralize the fatty acids resulting from the complete hydrolysis of 1 g of the sample.

Saponification Value O O C- R O HC O C- R + 3 K+OH O H 2 C O C - R triacylglycerol H 2 C OH HC OH H 2 C OH glycerol O + 3 KO - C-R Potassium salt Similarly; RCOOH + KOH RCOO-K+ + Glycerol MG + KOH RCOOK + Glycerol DG + 2 KOH 2 RCOOK + Glycerol

Saponification Value 1 mol TG 1 g TG 3 mol KOH required X mol KOH required MWKOH: 56 g = 56000 mg 1 g TG : 1 g / MWTG (g/mol) mol 1 mol TG 3 x 56000 mg KOH required 1 g TG / MWTG X mg KOH required

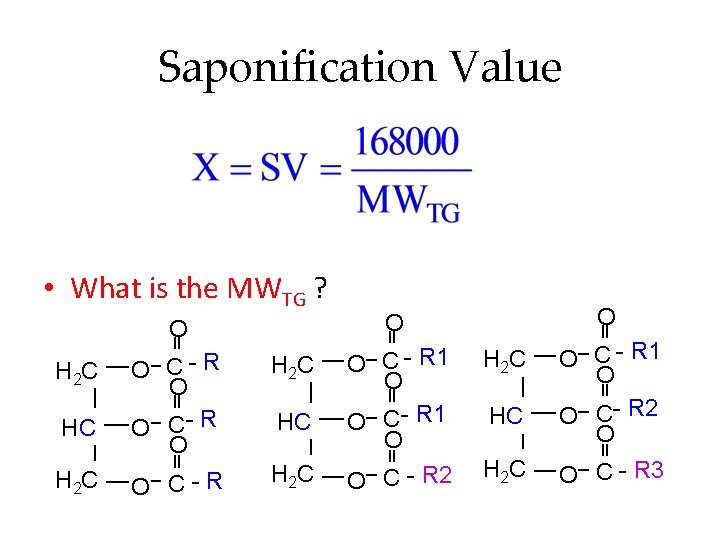
Saponification Value • What is the MWTG ? H 2 C HC H 2 C O O O C- R O O C-R O C - R 1 O O C - R 2 H 2 C HC H 2 C O C - R 1 O O C- R 2 O O C - R 3

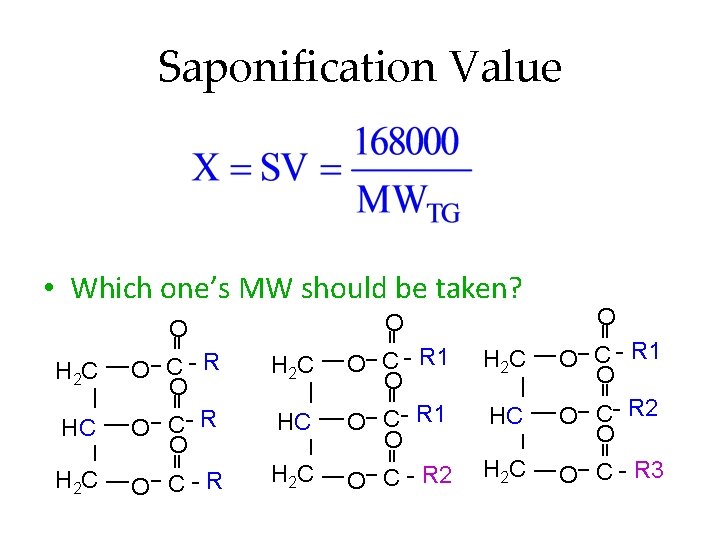
Saponification Value • Which one’s MW should be taken? H 2 C HC H 2 C O O O C- R O O C-R O C - R 1 O O C - R 2 H 2 C HC H 2 C O O C - R 1 O O C- R 2 O O C - R 3

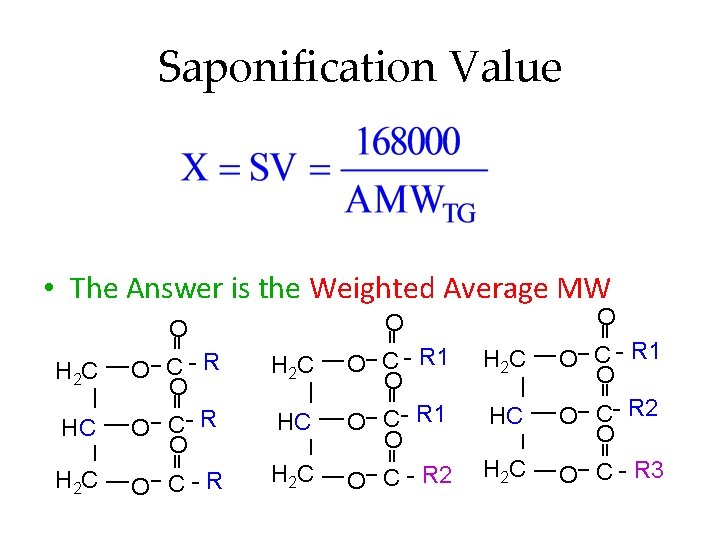
Saponification Value • The Answer is the Weighted Average MW H 2 C HC H 2 C O O O C- R O O C-R O C - R 1 O O C - R 2 H 2 C HC H 2 C O C - R 1 O O C- R 2 O O C - R 3

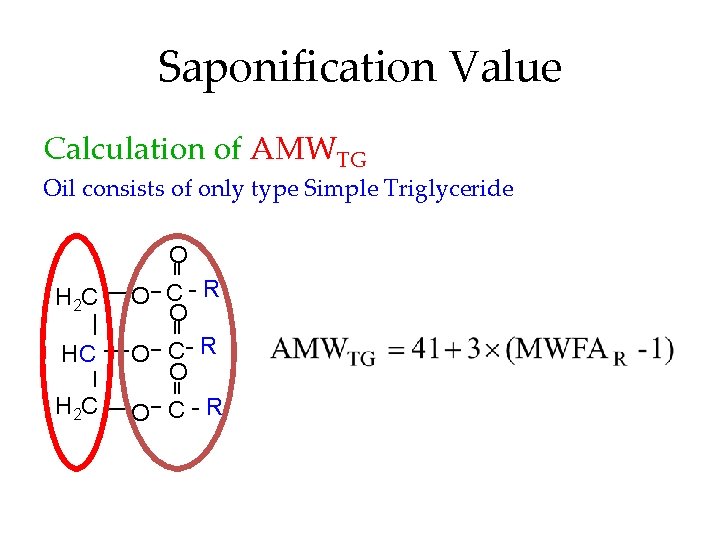
Saponification Value Calculation of AMWTG Oil consists of only type Simple Triglyceride O H 2 C HC H 2 C O C- R O O C-R

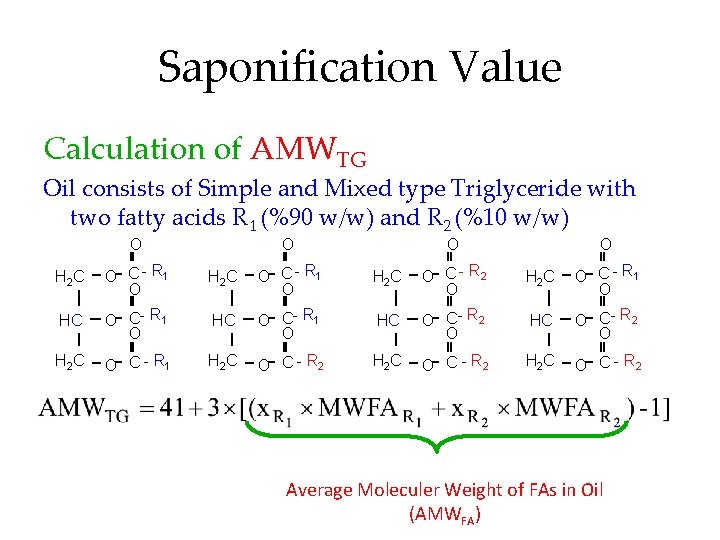
Saponification Value Calculation of AMWTG Oil consists of Simple and Mixed type Triglyceride with two fatty acids R 1 (%90 w/w) and R 2 (%10 w/w) O O H 2 C O C - R 1 O H 2 C O C - R 2 O H 2 C O C - R 1 O HC O C - R 2 O H 2 C O C - R 1 H 2 C O C - R 2 Average Moleculer Weight of FAs in Oil (AMWFA)

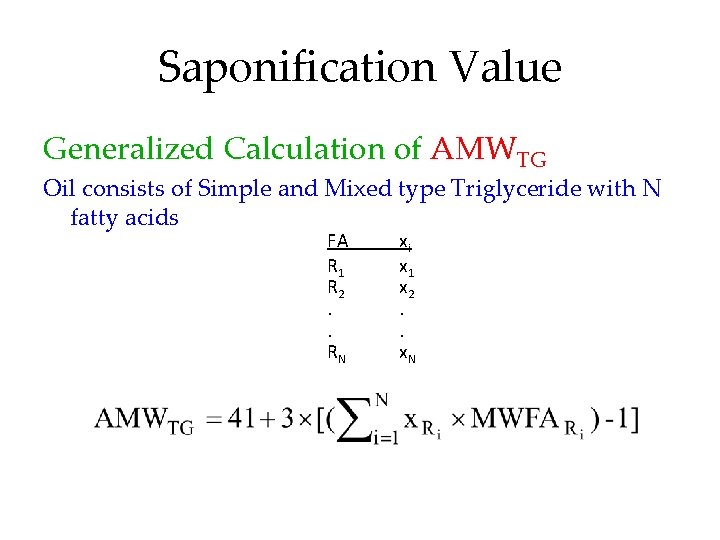
Saponification Value Generalized Calculation of AMWTG Oil consists of Simple and Mixed type Triglyceride with N fatty acids FA R 1 R 2. . RN xi x 1 x 2. . x. N

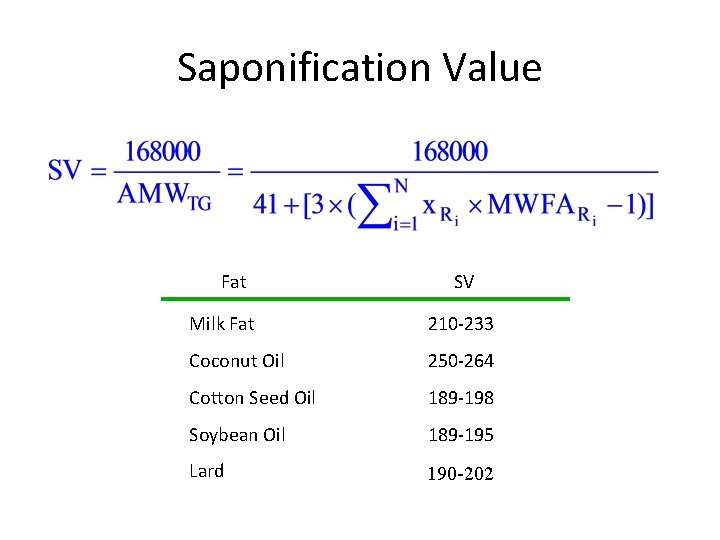
Saponification Value Fat SV Milk Fat 210 -233 Coconut Oil 250 -264 Cotton Seed Oil 189 -198 Soybean Oil 189 -195 Lard 190 -202

Iodine Number • The iodine value of an oil or fat is defined as the mass of iodine absorbed by 100 g of the sample. • The unsaturated fatty acid residues of the glycerides react with iodine, and thus the iodine value indicates the degree of unsaturation of the fatty acid residues of the glycerides. • It is constant for a particular oil or fat, but depends on the method used. Animal fats (butter, dripping, lard) 30 - 70 Iodine Value • Non-drying oils (olive, almond) 80 - 110 Iodine Value • Semi-drying oils (cottonseed, sesame, soya) 80 - 140 Iodine Value • Drying oils (linseed, sunflower) 120 - 200 Iodine Value • The iodine value is often most useful in identifying the source of an oil. Generally, the higher iodine values indicate oils and the lower values fats. Iodine values are normally determined using Wigs or Hanus methods.

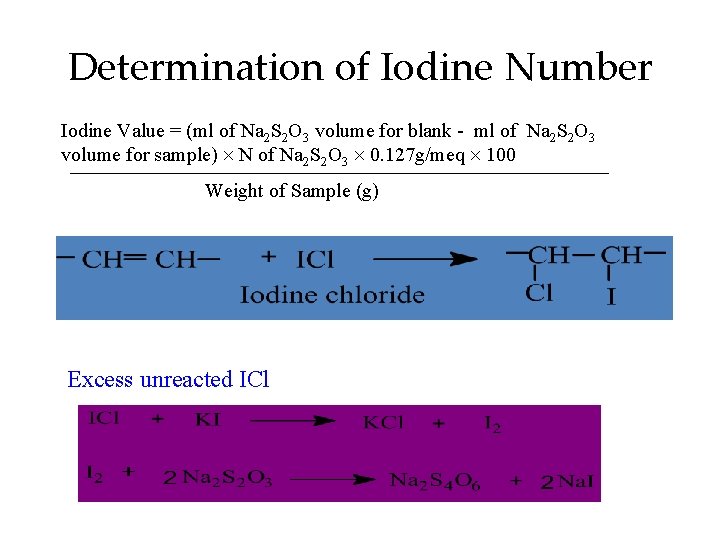
Determination of Iodine Number Iodine Value = (ml of Na 2 S 2 O 3 volume for blank - ml of Na 2 S 2 O 3 volume for sample) N of Na 2 S 2 O 3 0. 127 g/meq 100 Weight of Sample (g) Excess unreacted ICl

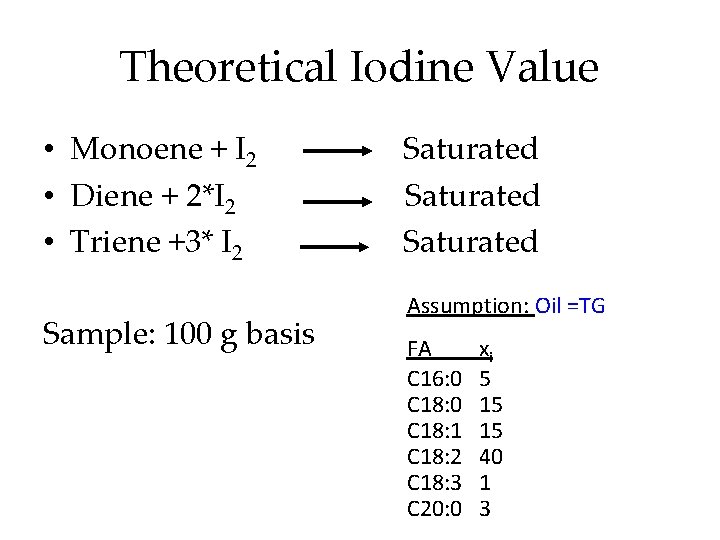
Theoretical Iodine Value • Monoene + I 2 • Diene + 2*I 2 • Triene +3* I 2 Sample: 100 g basis Saturated Assumption: Oil =TG FA C 16: 0 C 18: 1 C 18: 2 C 18: 3 C 20: 0 xi 5 15 15 40 1 3

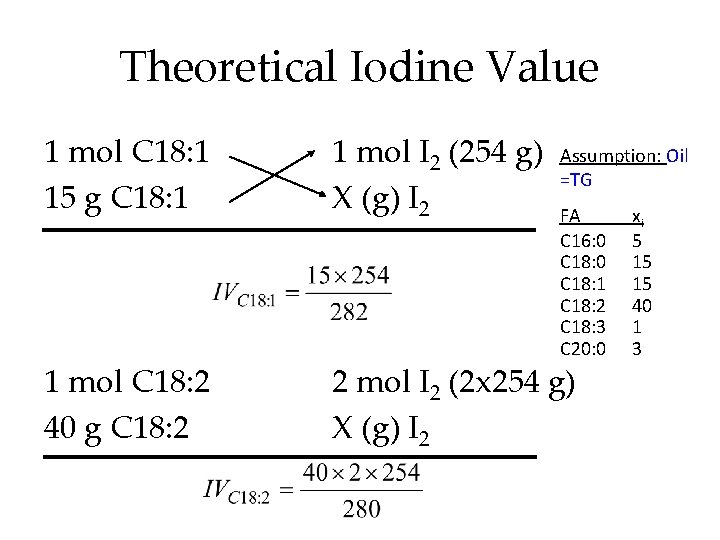
Theoretical Iodine Value 1 mol C 18: 1 15 g C 18: 1 1 mol I 2 (254 g) X (g) I 2 1 mol C 18: 2 40 g C 18: 2 2 mol I 2 (2 x 254 g) X (g) I 2 Assumption: Oil =TG FA C 16: 0 C 18: 1 C 18: 2 C 18: 3 C 20: 0 xi 5 15 15 40 1 3

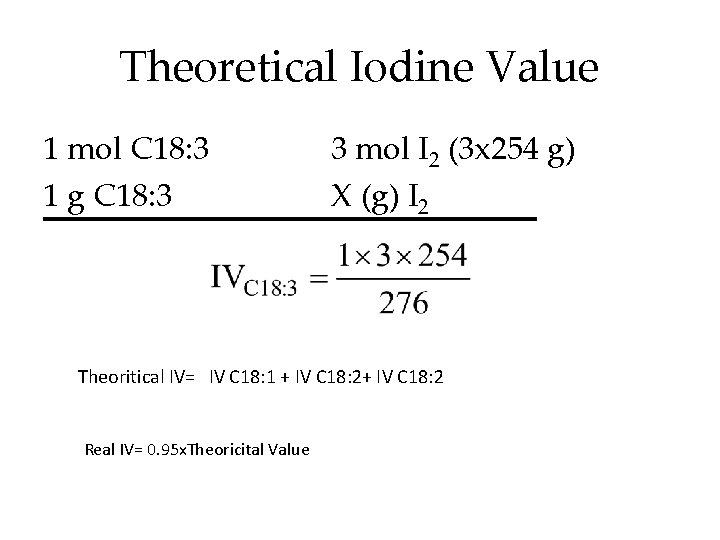
Theoretical Iodine Value 1 mol C 18: 3 1 g C 18: 3 3 mol I 2 (3 x 254 g) X (g) I 2 Theoritical IV= IV C 18: 1 + IV C 18: 2 Real IV= 0. 95 x. Theoricital Value
 To detect the presence of adulterants in fat oil and butter
To detect the presence of adulterants in fat oil and butter Creaming is a.......... process
Creaming is a.......... process Invisible fats examples
Invisible fats examples Trans fat vs cis fat
Trans fat vs cis fat Week by week plans for documenting children's development
Week by week plans for documenting children's development Unit 2 food food food
Unit 2 food food food Food chain sequence
Food chain sequence Your body uses carbohydrates by breaking them down into *
Your body uses carbohydrates by breaking them down into * Cooking food uncovered without adding liquid
Cooking food uncovered without adding liquid Basic cooking techniques
Basic cooking techniques Fat tom
Fat tom Sqa advanced higher english understanding standards
Sqa advanced higher english understanding standards Higher health and food technology
Higher health and food technology What is the third trophic level
What is the third trophic level Tertiary food web
Tertiary food web Food chain and food web
Food chain and food web A consumer that primarily eats one specific organism
A consumer that primarily eats one specific organism Difference between food webs and food chains
Difference between food webs and food chains Food chains, food webs and ecological pyramids
Food chains, food webs and ecological pyramids Food webs and energy pyramids
Food webs and energy pyramids Food chains food webs and energy pyramid worksheet
Food chains food webs and energy pyramid worksheet Desert ecosystem food web
Desert ecosystem food web Role play on healthy food and junk food
Role play on healthy food and junk food Of junk food
Of junk food Temperate rainforest food web
Temperate rainforest food web Junk food examples
Junk food examples Introduction of fast food
Introduction of fast food Year 7 food technology worksheets
Year 7 food technology worksheets Sensory analysis star diagram
Sensory analysis star diagram Food technology job
Food technology job Erasmus mundus scholarship for food technology
Erasmus mundus scholarship for food technology Food technology gcse revision
Food technology gcse revision Gcse food tech nea 2 example
Gcse food tech nea 2 example
























































