Lipids Lipids commonly referred to as fats are





- Slides: 30


Lipids • Lipids, commonly referred to as fats, are substances of biological origin. • Soluble in organic solvents such as chloroform and methanol. • Fats, oils, certain vitamins and hormones, and most non protein membrane components are lipids. 2

Functions of Lipids § Lipids play three major roles in human biochemistry: 1. Store energy within fat cells. Because they are composed of mostly carbon-hydrogen (C—H) bonds, they are a rich source of energy and an efficient way for the body to store excess calories. 2. Parts of membranes that separate compartments of aqueous solutions from each other. Because of their unique physical properties, lipids are also an integral part of cell membranes and, therefore, also play an important structural role in cells. 3. Serve as chemical messengers such as steroid hormones. 3

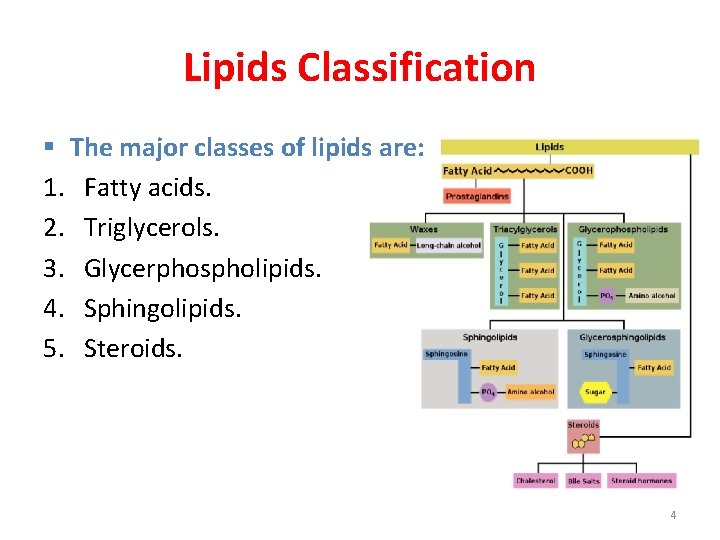
Lipids Classification § The major classes of lipids are: 1. Fatty acids. 2. Triglycerols. 3. Glycerphospholipids. 4. Sphingolipids. 5. Steroids. 4

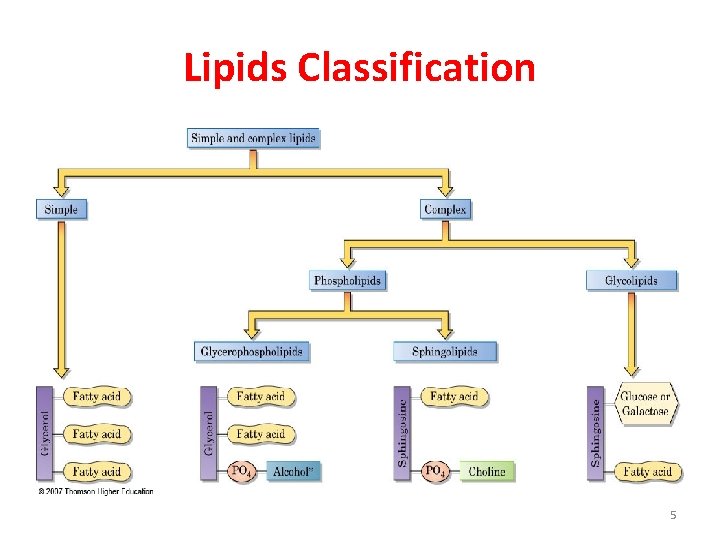
Lipids Classification 5

Fatty Acids 6

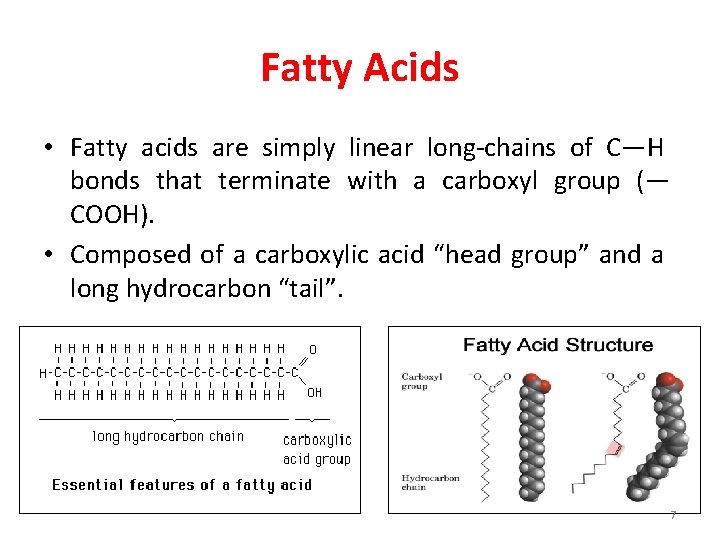
Fatty Acids • Fatty acids are simply linear long-chains of C—H bonds that terminate with a carboxyl group (— COOH). • Composed of a carboxylic acid “head group” and a long hydrocarbon “tail”. 7

Fatty Acids § Fatty acids are variable in length and can be classified as: • Short chain (4 -6 carbon atoms) fatty acids. • Medium chain (8 -12 carbon atoms) fatty acids. • Long chain (more than 12 carbon atoms) fatty acids. 8

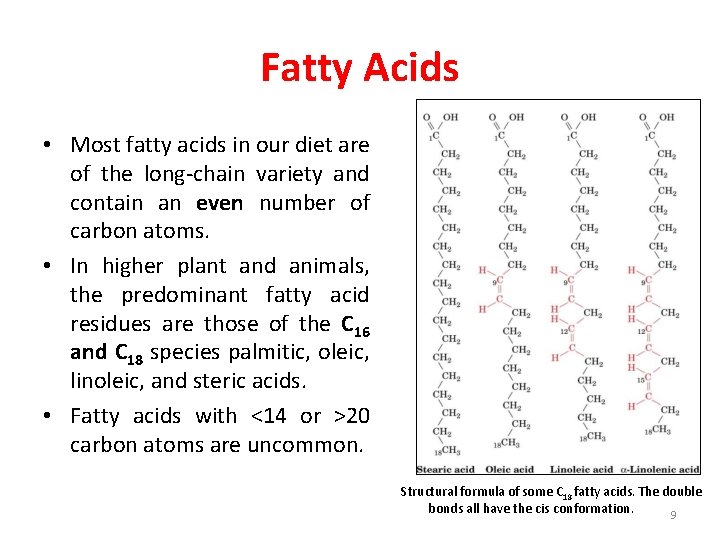
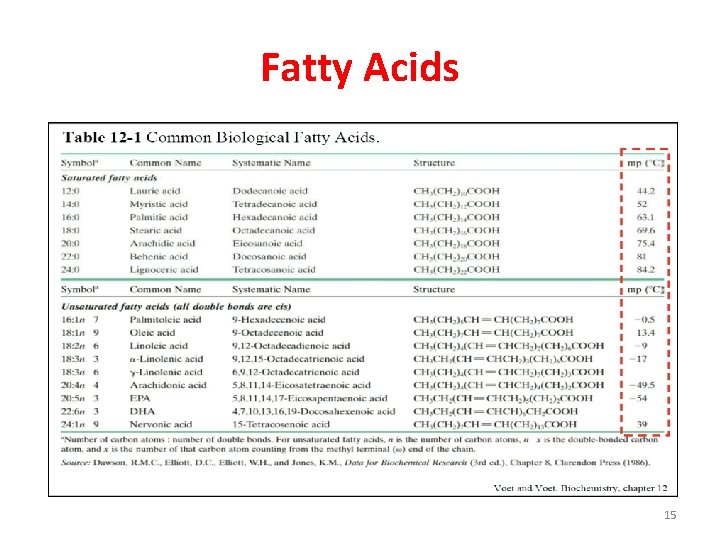
Fatty Acids • Most fatty acids in our diet are of the long-chain variety and contain an even number of carbon atoms. • In higher plant and animals, the predominant fatty acid residues are those of the C 16 and C 18 species palmitic, oleic, linoleic, and steric acids. • Fatty acids with <14 or >20 carbon atoms are uncommon. Structural formula of some C 18 fatty acids. The double bonds all have the cis conformation. 9

Fatty Acids • Not all of the carbon atoms on fatty acids are fully saturated or bonded with hydrogen atoms: some of them may instead form carbon=carbon (C=C) double bond. • Depending on the number of C=C double- bonds, fatty acids can be classified as: - Saturated (no double-bonds). - Unsaturated (contain double bonds). - Polyunsaturated (two or more double-bonds). • Over half of the fatty acid residues of plant and animal lipids are unsaturated and polyunsaturated. 10

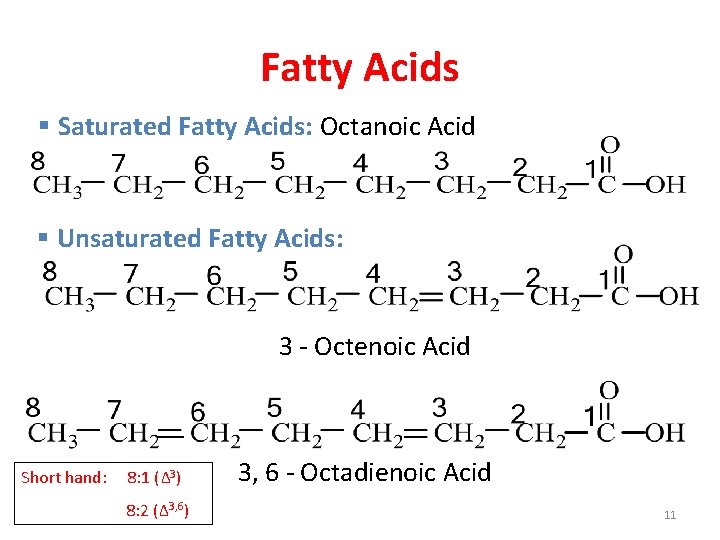
Fatty Acids § Saturated Fatty Acids: Octanoic Acid § Unsaturated Fatty Acids: 3 - Octenoic Acid Short hand: 8: 1 (Δ 3) 8: 2 (Δ 3, 6) 3, 6 - Octadienoic Acid 11

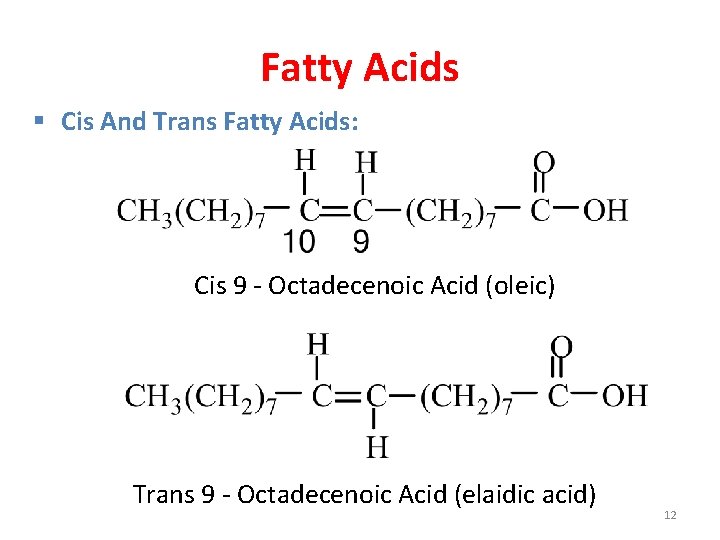
Fatty Acids § Cis And Trans Fatty Acids: Cis 9 - Octadecenoic Acid (oleic) Trans 9 - Octadecenoic Acid (elaidic acid) 12

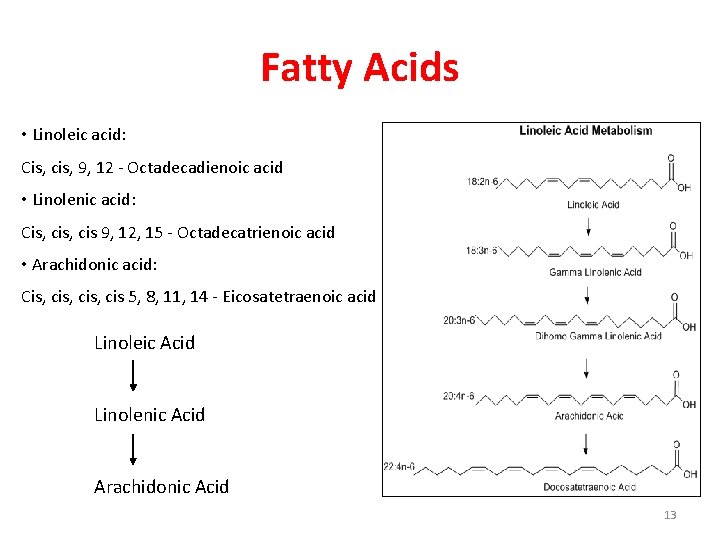
Fatty Acids • Linoleic acid: Cis, cis, 9, 12 - Octadecadienoic acid • Linolenic acid: Cis, cis 9, 12, 15 - Octadecatrienoic acid • Arachidonic acid: Cis, cis, cis 5, 8, 11, 14 - Eicosatetraenoic acid Linoleic Acid Linolenic Acid Arachidonic Acid 13

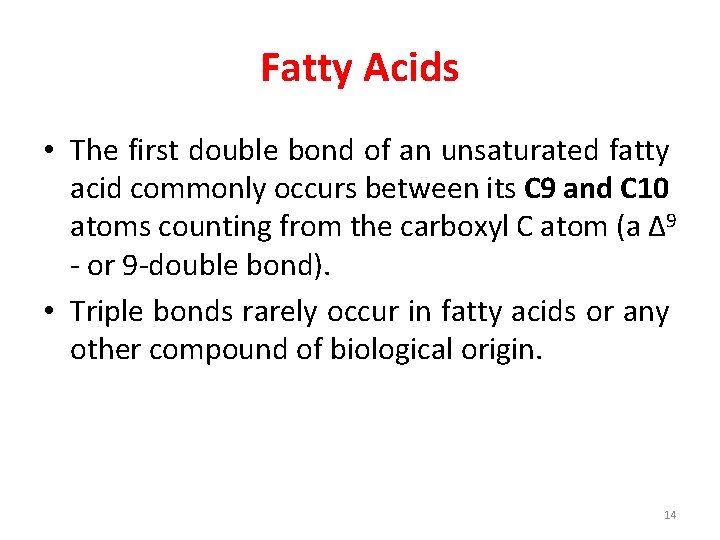
Fatty Acids • The first double bond of an unsaturated fatty acid commonly occurs between its C 9 and C 10 atoms counting from the carboxyl C atom (a Δ 9 - or 9 -double bond). • Triple bonds rarely occur in fatty acids or any other compound of biological origin. 14

Fatty Acids 15

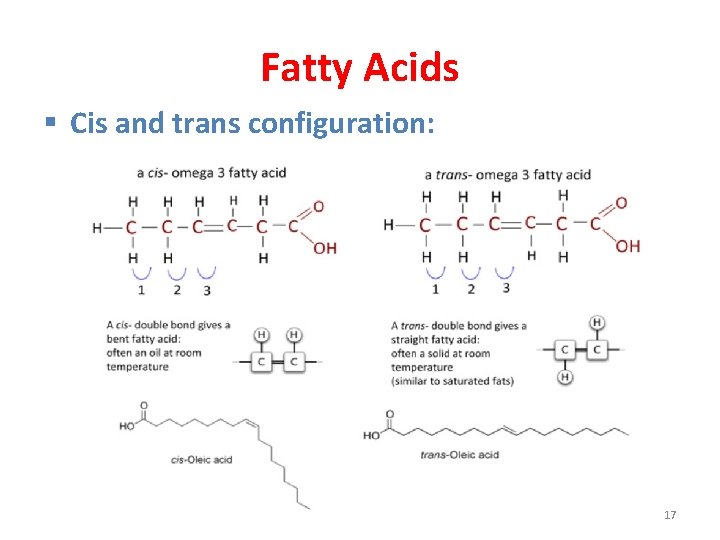
Fatty Acids § Cis and trans configuration: • The C=C double-bonds of unsaturated fatty acids are typically arranged in the cis form, with both hydrogen atoms on the same side of the C=C double-bonds, which causes a bend in their structure. • Fatty acid C=C double-bonds can also occur in the trans configuration, with both hydrogen atoms on opposite side of the C=C double-bond. 16

Fatty Acids § Cis and trans configuration: 17

Fatty Acids § Cis and trans configuration: • Fatty acid double bonds almost always have the cis configuration. • The trans fatty acids are not commonly in nature; however, they are present in our diet because the chemical hydrogenation treatment used in food processing for converting polyunsaturated plant oils into margarine introduces trans double bonds. 18

Fatty Acids • In plasma, only a relatively small amount of fatty acids exists in the free or unesterified form. • The majority of plasma fatty acids are found as a constituent of triglycerides or phospholipids. • Fatty acids are covalently attached to the glycerol backbone of triglycerides and phospholipids by an ester bond that forms between the carboxyl group (CO) on the fatty acid and the hydroxyl group (OH)on glycerol. 19

Simple Lipids: Triglycerides 20

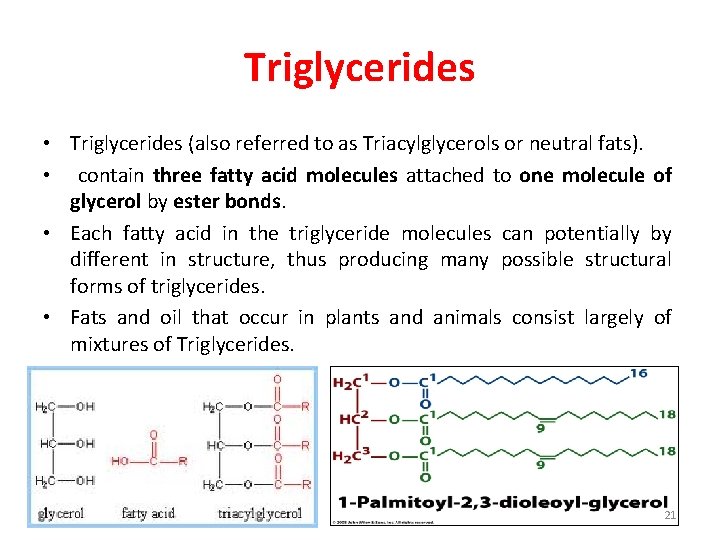
Triglycerides • Triglycerides (also referred to as Triacylglycerols or neutral fats). • contain three fatty acid molecules attached to one molecule of glycerol by ester bonds. • Each fatty acid in the triglyceride molecules can potentially by different in structure, thus producing many possible structural forms of triglycerides. • Fats and oil that occur in plants and animals consist largely of mixtures of Triglycerides. 21

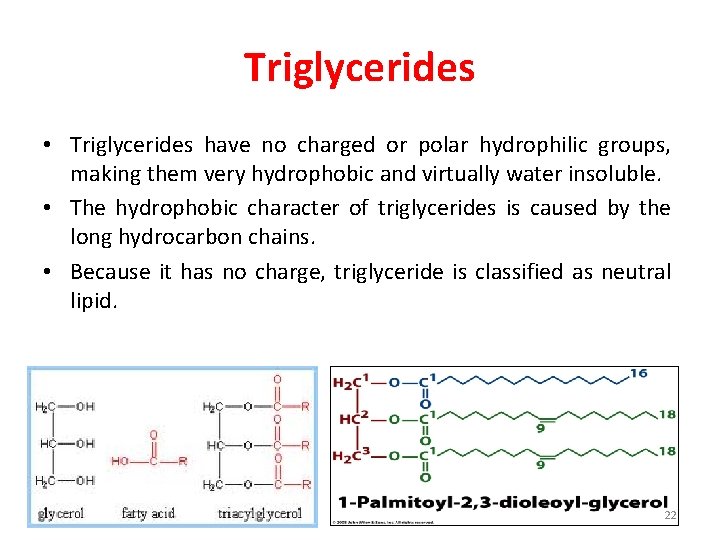
Triglycerides • Triglycerides have no charged or polar hydrophilic groups, making them very hydrophobic and virtually water insoluble. • The hydrophobic character of triglycerides is caused by the long hydrocarbon chains. • Because it has no charge, triglyceride is classified as neutral lipid. 22

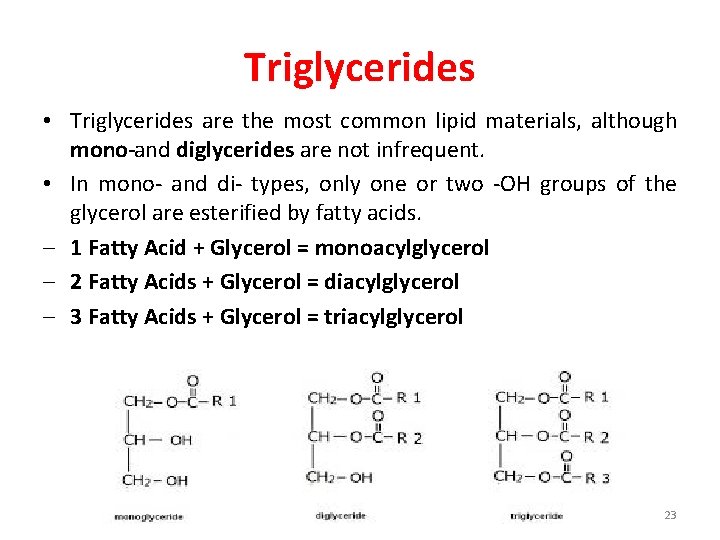
Triglycerides • Triglycerides are the most common lipid materials, although mono and diglycerides are not infrequent. • In mono- and di- types, only one or two -OH groups of the glycerol are esterified by fatty acids. - 1 Fatty Acid + Glycerol = monoacylglycerol - 2 Fatty Acids + Glycerol = diacylglycerol - 3 Fatty Acids + Glycerol = triacylglycerol 23

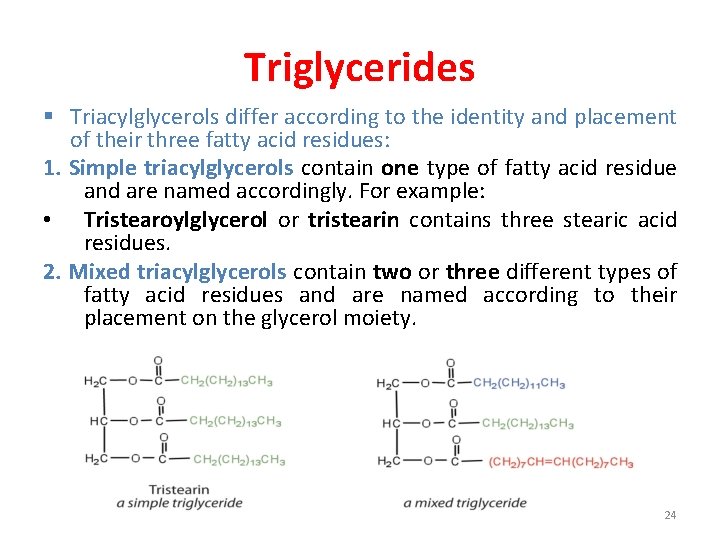
Triglycerides § Triacylglycerols differ according to the identity and placement of their three fatty acid residues: 1. Simple triacylglycerols contain one type of fatty acid residue and are named accordingly. For example: • Tristearoylglycerol or tristearin contains three stearic acid residues. 2. Mixed triacylglycerols contain two or three different types of fatty acid residues and are named according to their placement on the glycerol moiety. 24

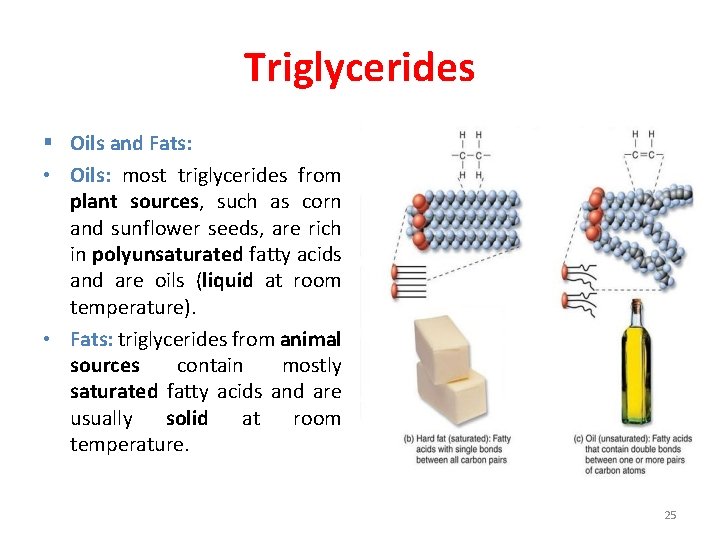
Triglycerides § Oils and Fats: • Oils: most triglycerides from plant sources, such as corn and sunflower seeds, are rich in polyunsaturated fatty acids and are oils (liquid at room temperature). • Fats: triglycerides from animal sources contain mostly saturated fatty acids and are usually solid at room temperature. 25

Pure Fats § Pure fats and oils are colorless, odorless, and tasteless. • This statement may seem surprising because we all know the tastes and colors of such fats and oils as butter, and olive oil. The tastes, odors, and colors are caused by small amounts of other substances dissolved in the fat or oil. 26

Triglycerides are efficient energy reserves § Fats are highly efficient form in which to store metabolic energy because: • Fats are less oxidized than are carbohydrates or proteins and hence yield significantly more energy on oxidation. • Fats, being non polar substances, are stored in anhydrous form, whereas glycogen bind about twice its weight of water. Fats therefore provide about six times the metabolic energy of an equal weight of hydrates glycogen. 27

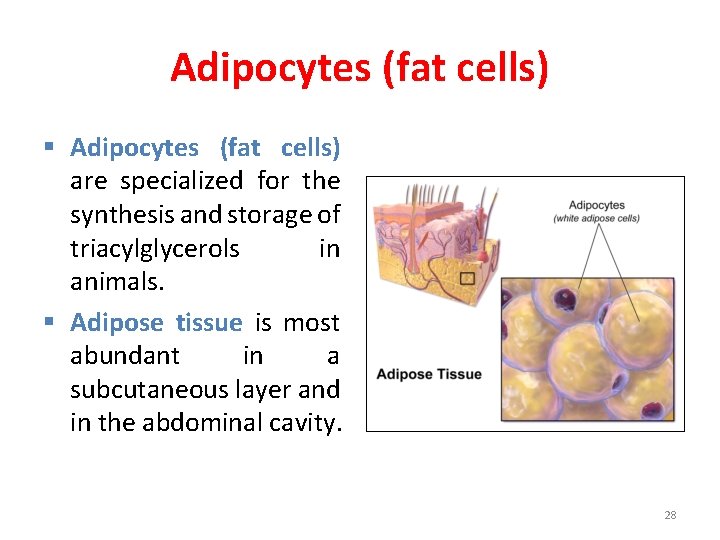
Adipocytes (fat cells) § Adipocytes (fat cells) are specialized for the synthesis and storage of triacylglycerols in animals. § Adipose tissue is most abundant in a subcutaneous layer and in the abdominal cavity. 28

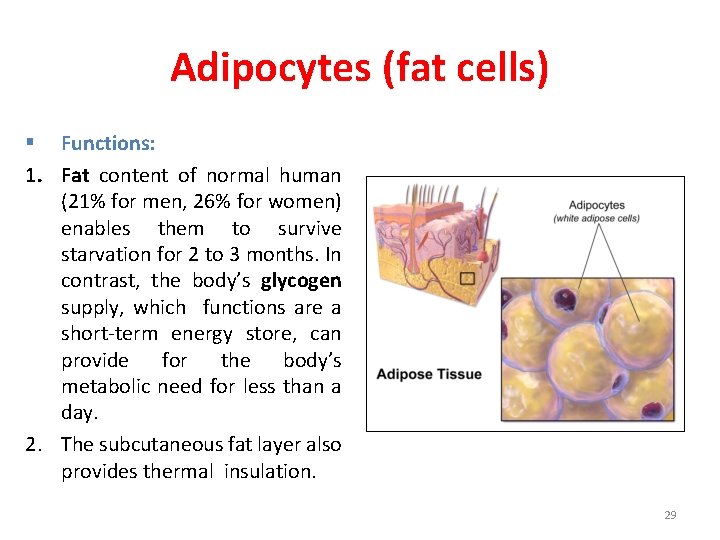
Adipocytes (fat cells) § Functions: 1. Fat content of normal human (21% for men, 26% for women) enables them to survive starvation for 2 to 3 months. In contrast, the body’s glycogen supply, which functions are a short-term energy store, can provide for the body’s metabolic need for less than a day. 2. The subcutaneous fat layer also provides thermal insulation. 29

Saponification (Soap making) • Saponification is a process by which triglycerides are reacted with sodium or potassium hydroxide to produce glycerol and a fatty acid salt, called 'soap'. • Lipids that contain fatty acid ester linkages can undergo hydrolysis. This reaction is catalyzed by a strong acid or base. Saponification is the alkaline hydrolysis of the fatty acid esters. 30
 Mikael ferm
Mikael ferm Spongocel
Spongocel Lipids vs fats
Lipids vs fats Saponifiable and non saponifiable lipids
Saponifiable and non saponifiable lipids Fats and lipids
Fats and lipids Spherical complexes of emulsified fats are known as
Spherical complexes of emulsified fats are known as Fats meaning
Fats meaning Section 8-1 carbohydrates fats and proteins answer key
Section 8-1 carbohydrates fats and proteins answer key Fats spreads and oils
Fats spreads and oils Rich in unsaturated fats
Rich in unsaturated fats Biological importance of fats
Biological importance of fats 3 functions of lipids
3 functions of lipids Type 2 diabetes diet sheet
Type 2 diabetes diet sheet Food pyramid carbohydrates fats proteins
Food pyramid carbohydrates fats proteins What's the worst type of fat
What's the worst type of fat The most concentrated source of energy is
The most concentrated source of energy is Hydrolysis vs dehydration
Hydrolysis vs dehydration Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Solid fats and added sugars
Solid fats and added sugars Quick breads definition
Quick breads definition Fats quiz brainpop answers
Fats quiz brainpop answers Lipids function
Lipids function Characteristics of fats
Characteristics of fats Biomolecule
Biomolecule Unsaturated fats
Unsaturated fats Are lipids long term energy storage
Are lipids long term energy storage Fats tes
Fats tes Absorption of fats
Absorption of fats Venn diagram saturated and unsaturated fats
Venn diagram saturated and unsaturated fats Sudan iv test
Sudan iv test Role of fats
Role of fats