Lipids Commonly known as fats and oils Contain




- Slides: 11

Lipids • Commonly known as fats and oils • Contain the elements carbon, hydrogen and oxygen • Generally, fats are lipids that are solid at room temperature • Non polar as electrons in the outer orbitals are more evenly distributed than in polar molecules. This means there are no positive or negative areas within the molecules and for this reason, lipids are insoluble in water. • Lipids are large complex molecules known as macromolecules

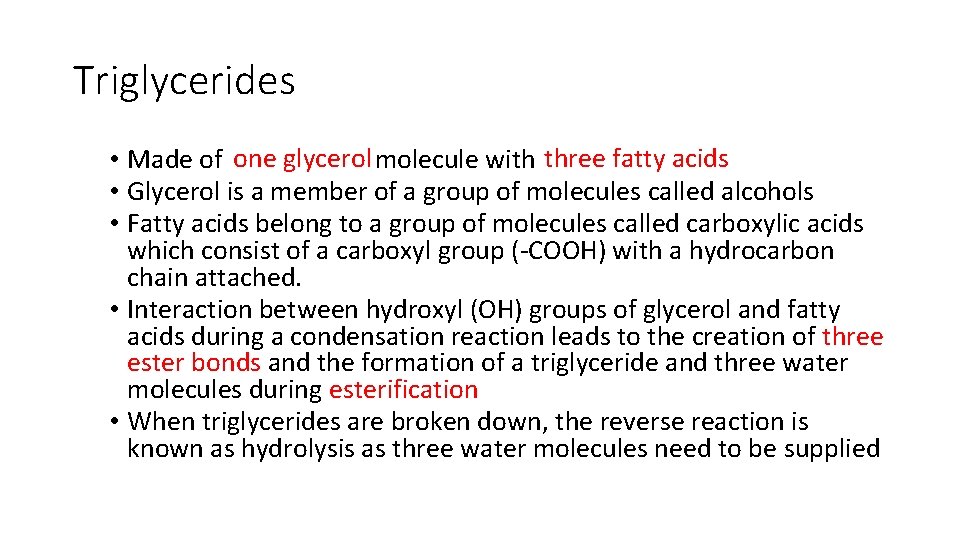
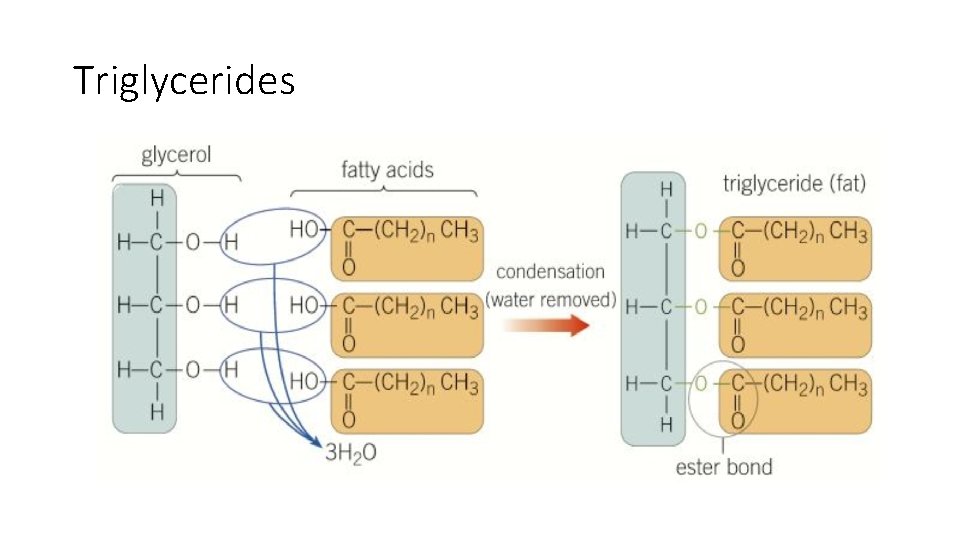
Triglycerides • Made of one glycerol molecule with three fatty acids • Glycerol is a member of a group of molecules called alcohols • Fatty acids belong to a group of molecules called carboxylic acids which consist of a carboxyl group (-COOH) with a hydrocarbon chain attached. • Interaction between hydroxyl (OH) groups of glycerol and fatty acids during a condensation reaction leads to the creation of three ester bonds and the formation of a triglyceride and three water molecules during esterification • When triglycerides are broken down, the reverse reaction is known as hydrolysis as three water molecules need to be supplied

Triglycerides

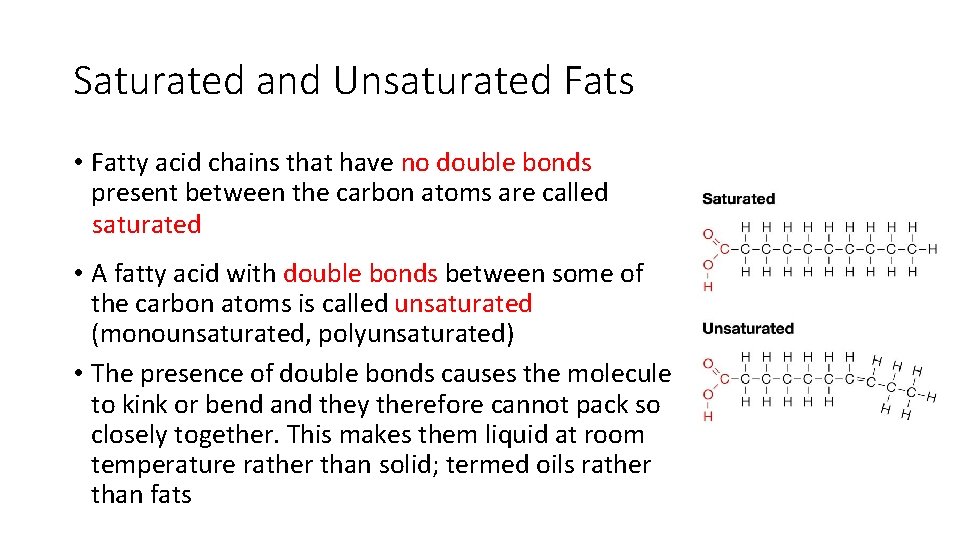
Saturated and Unsaturated Fats • Fatty acid chains that have no double bonds present between the carbon atoms are called saturated • A fatty acid with double bonds between some of the carbon atoms is called unsaturated (monounsaturated, polyunsaturated) • The presence of double bonds causes the molecule to kink or bend and they therefore cannot pack so closely together. This makes them liquid at room temperature rather than solid; termed oils rather than fats

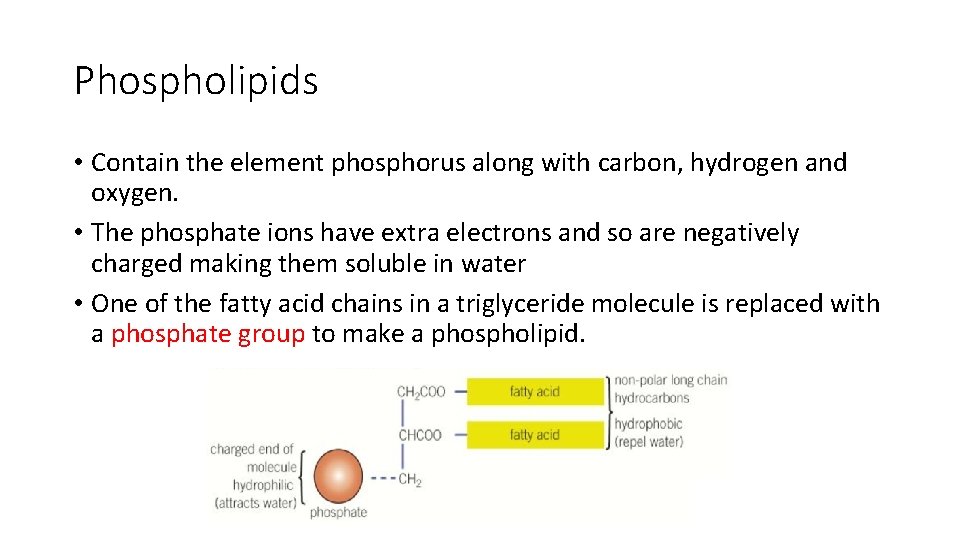
Phospholipids • Contain the element phosphorus along with carbon, hydrogen and oxygen. • The phosphate ions have extra electrons and so are negatively charged making them soluble in water • One of the fatty acid chains in a triglyceride molecule is replaced with a phosphate group to make a phospholipid.

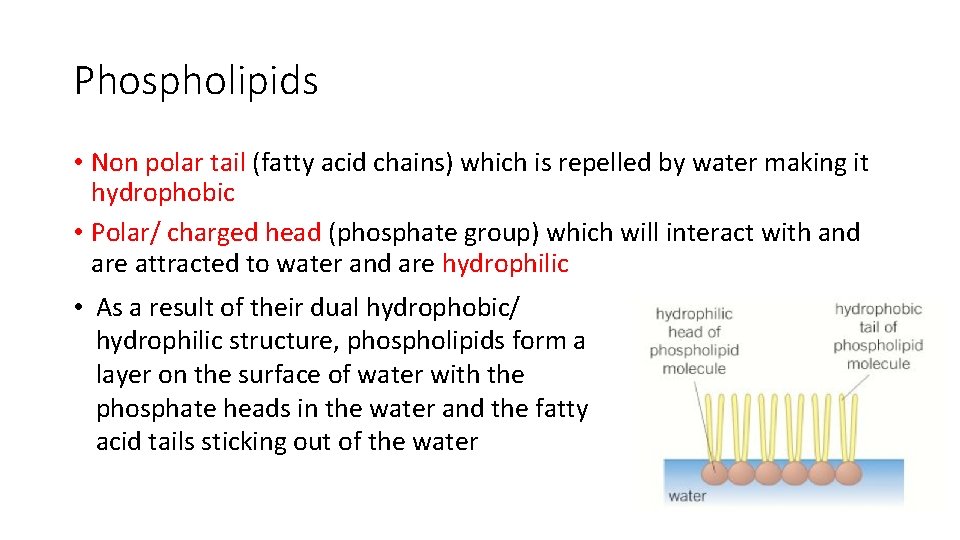
Phospholipids • Non polar tail (fatty acid chains) which is repelled by water making it hydrophobic • Polar/ charged head (phosphate group) which will interact with and are attracted to water and are hydrophilic • As a result of their dual hydrophobic/ hydrophilic structure, phospholipids form a layer on the surface of water with the phosphate heads in the water and the fatty acid tails sticking out of the water

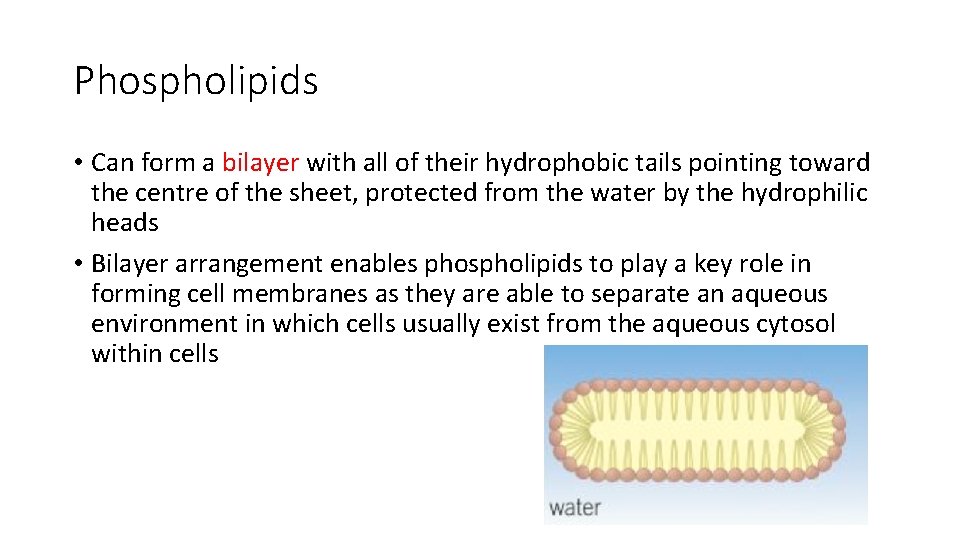
Phospholipids • Can form a bilayer with all of their hydrophobic tails pointing toward the centre of the sheet, protected from the water by the hydrophilic heads • Bilayer arrangement enables phospholipids to play a key role in forming cell membranes as they are able to separate an aqueous environment in which cells usually exist from the aqueous cytosol within cells

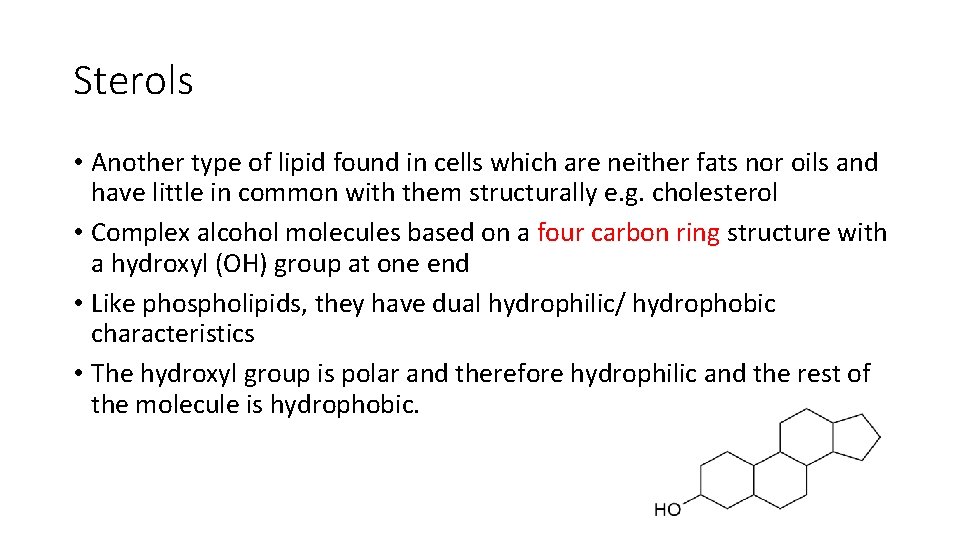
Sterols • Another type of lipid found in cells which are neither fats nor oils and have little in common with them structurally e. g. cholesterol • Complex alcohol molecules based on a four carbon ring structure with a hydroxyl (OH) group at one end • Like phospholipids, they have dual hydrophilic/ hydrophobic characteristics • The hydroxyl group is polar and therefore hydrophilic and the rest of the molecule is hydrophobic.

Roles of Lipids Due to non polar nature, they have many biological roles; ØMembrane formation and creation of hydrophobic barriers ØHormone production ØElectrical insulation necessary for impulse transmission ØWaterproofing e. g. on birds feathers and plant leaves Triglycerides in particularly are stored under the skin and have roles in long term energy storage; ØThermal insulation to reduce heat loss ØCushioning to protect vital organs e. g. heart and kidneys ØBuoyancy for aquatic animals

Identification of Lipids Emulsion test 1. The sample must first be mixed with ethanol 2. The resulting solution is mixed with water and shaken 3. If a white emulsion forms as a layer on top of the solution, this is a positive result, indicating the presence of a lipid 4. If the solution remains clear, the test is negative

 Are lipids energy storage
Are lipids energy storage Fats, oils, and waxes
Fats, oils, and waxes Lipids definition
Lipids definition Biomolecule
Biomolecule Fats spreads and oils
Fats spreads and oils Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Are fats and lipids the same thing
Are fats and lipids the same thing Fats and lipids
Fats and lipids Fats and lipids
Fats and lipids Spherical complexes of emulsified fats are known as
Spherical complexes of emulsified fats are known as Which lipid acts as a chemical messenger
Which lipid acts as a chemical messenger Types of optical storage
Types of optical storage