Kinetics Reaction Rates Rate Law Collision Theory and





![The Initial Rate Method – Data Collection • Experiment [NH 4 NCO] M Rate The Initial Rate Method – Data Collection • Experiment [NH 4 NCO] M Rate](https://slidetodoc.com/presentation_image_h2/8ee9592bd78d78e1ab2930364c0864fc/image-18.jpg)

- Slides: 41

Kinetics Reaction Rates, Rate Law, Collision Theory and Activation Energy (PLN 710)

PLN 7 • Important Concepts: – Reactions can occur at different rates – Factors that help determine the reaction rate – Reaction characteristics: • Mechanism of reaction (PLN 11) • Rate of Reaction • Rate Law (PLN 8)

Basic Kinetics • Reaction Rate – Speed that reactants disappear and products form – How fast reactants become/form products

Examples: •

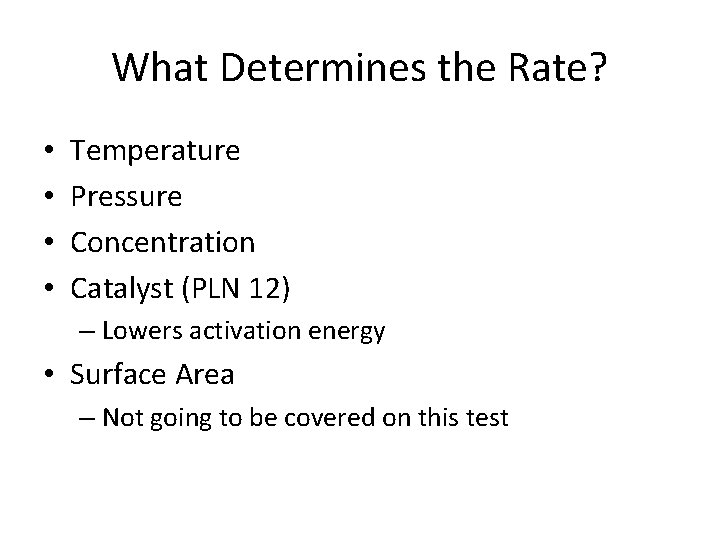
What Determines the Rate? • • Temperature Pressure Concentration Catalyst (PLN 12) – Lowers activation energy • Surface Area – Not going to be covered on this test

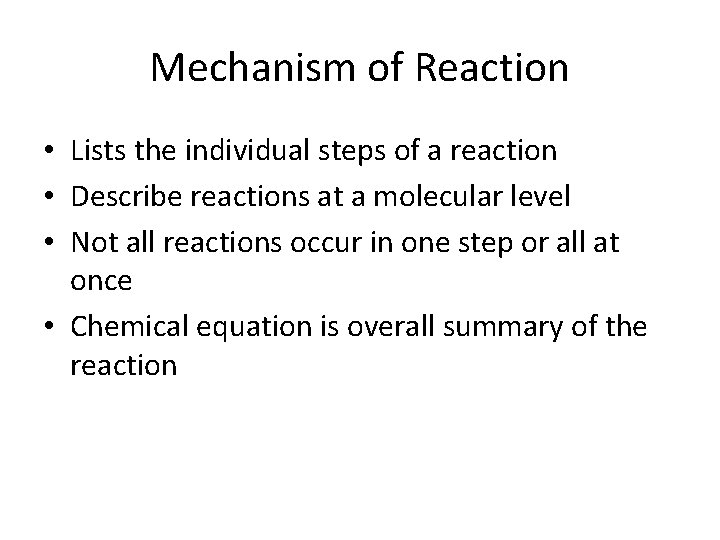
Mechanism of Reaction • Lists the individual steps of a reaction • Describe reactions at a molecular level • Not all reactions occur in one step or all at once • Chemical equation is overall summary of the reaction

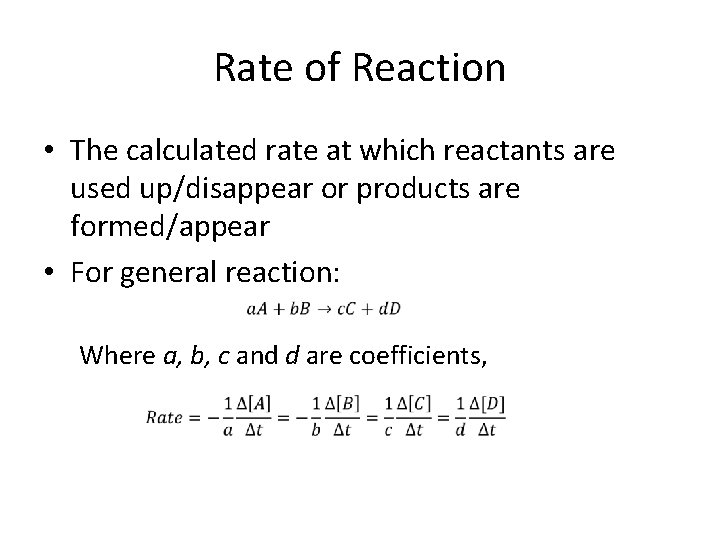
Rate of Reaction • The calculated rate at which reactants are used up/disappear or products are formed/appear • For general reaction: Where a, b, c and d are coefficients,

Rate Law •

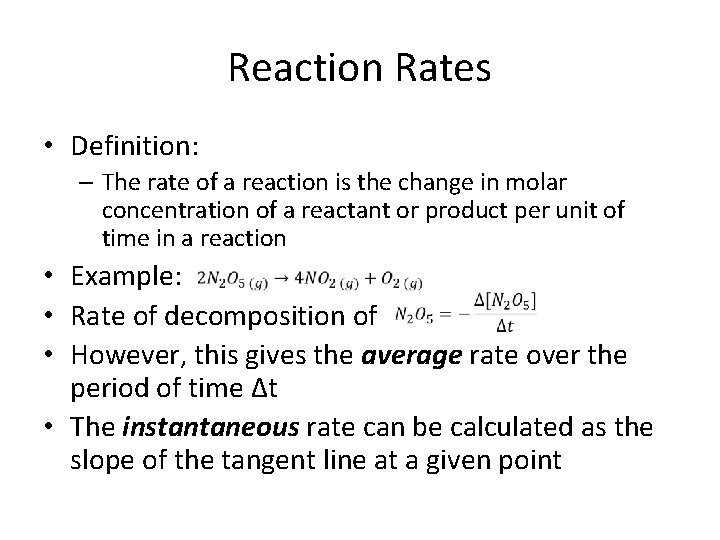
Reaction Rates • Definition: – The rate of a reaction is the change in molar concentration of a reactant or product per unit of time in a reaction • Example: • Rate of decomposition of • However, this gives the average rate over the period of time Δt • The instantaneous rate can be calculated as the slope of the tangent line at a given point

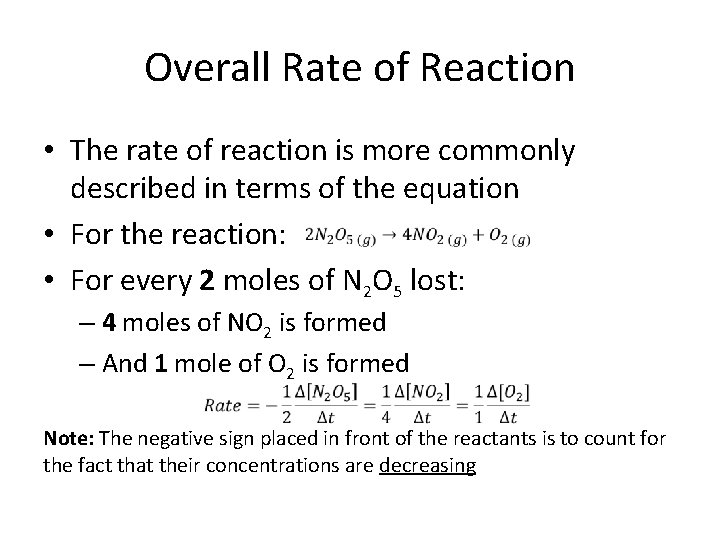
Overall Rate of Reaction • The rate of reaction is more commonly described in terms of the equation • For the reaction: • For every 2 moles of N 2 O 5 lost: – 4 moles of NO 2 is formed – And 1 mole of O 2 is formed Note: The negative sign placed in front of the reactants is to count for the fact that their concentrations are decreasing

PLN 8 • Important Concepts: – Rate Laws – Rate Constant (k) – Order of Reaction – Initial Rate Method

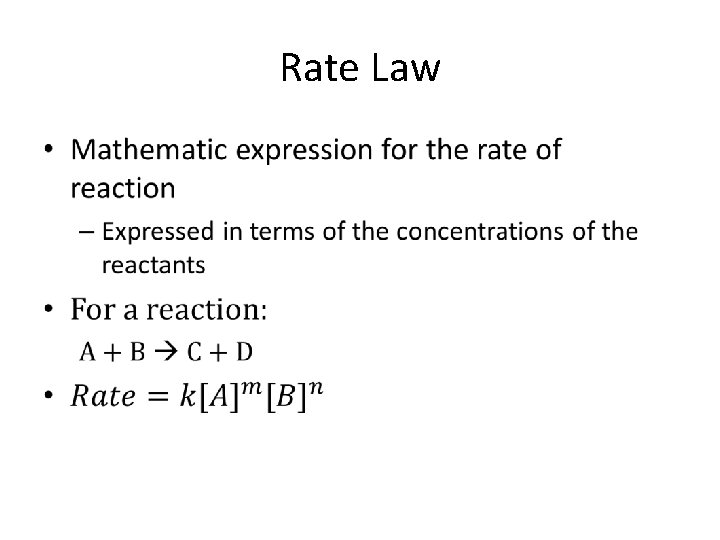
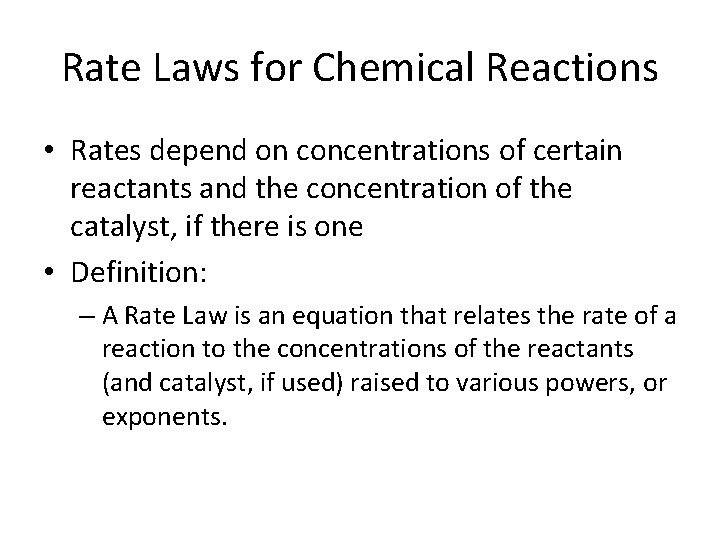
Rate Laws for Chemical Reactions • Rates depend on concentrations of certain reactants and the concentration of the catalyst, if there is one • Definition: – A Rate Law is an equation that relates the rate of a reaction to the concentrations of the reactants (and catalyst, if used) raised to various powers, or exponents.

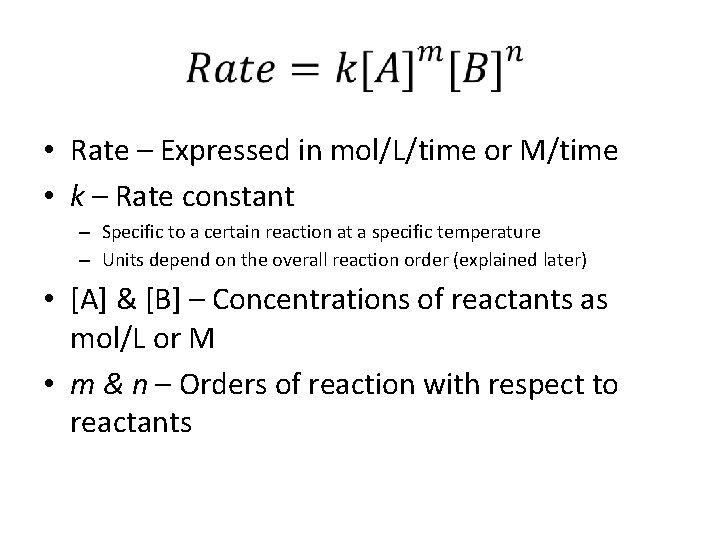
• Rate – Expressed in mol/L/time or M/time • k – Rate constant – Specific to a certain reaction at a specific temperature – Units depend on the overall reaction order (explained later) • [A] & [B] – Concentrations of reactants as mol/L or M • m & n – Orders of reaction with respect to reactants

k • The reaction constant, k, is called the rate constant and is dependent on the particular reaction as well as the specific temperature at which the reaction takes place • The units of k depend on the order of reaction

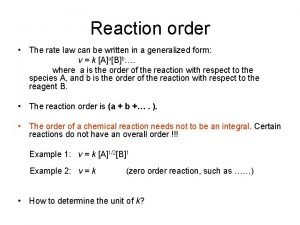
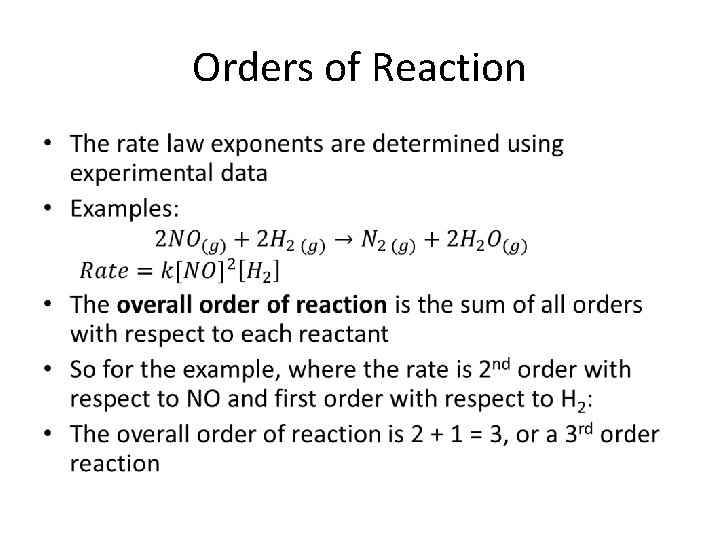
Orders of Reaction •

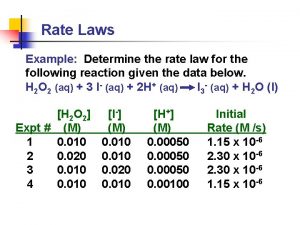
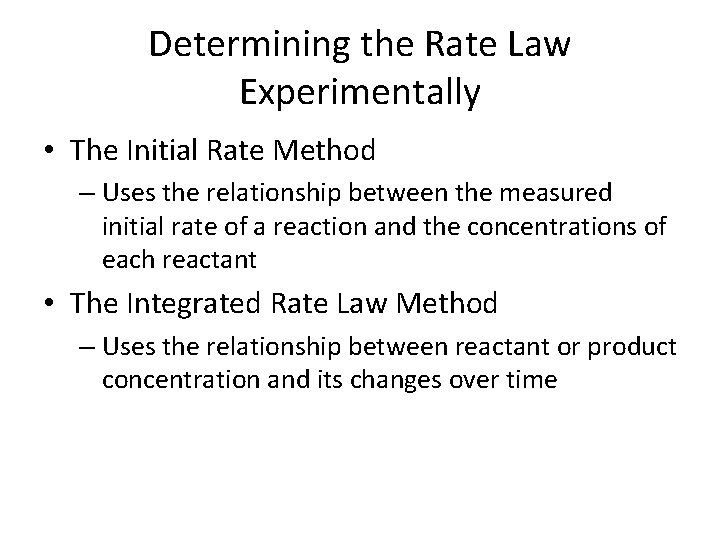
Determining the Rate Law Experimentally • The Initial Rate Method – Uses the relationship between the measured initial rate of a reaction and the concentrations of each reactant • The Integrated Rate Law Method – Uses the relationship between reactant or product concentration and its changes over time

The Initial Rate Method •
![The Initial Rate Method Data Collection Experiment NH 4 NCO M Rate The Initial Rate Method – Data Collection • Experiment [NH 4 NCO] M Rate](https://slidetodoc.com/presentation_image_h2/8ee9592bd78d78e1ab2930364c0864fc/image-18.jpg)
The Initial Rate Method – Data Collection • Experiment [NH 4 NCO] M Rate of loss of NH 4 NCO M/min 1 0. 14 2. 2 × 10 -4 2 0. 28 8. 8 × 10 -4

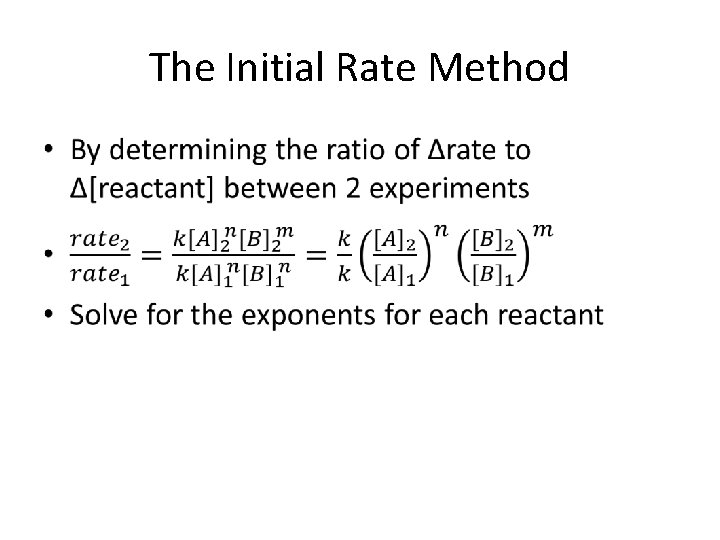
The Initial Rate Method – Calculations •

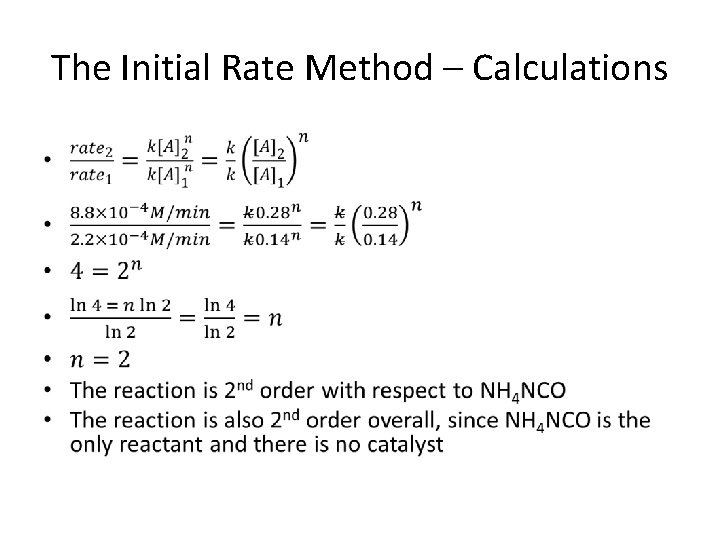
Initial Rate Method – Rate Law and k •

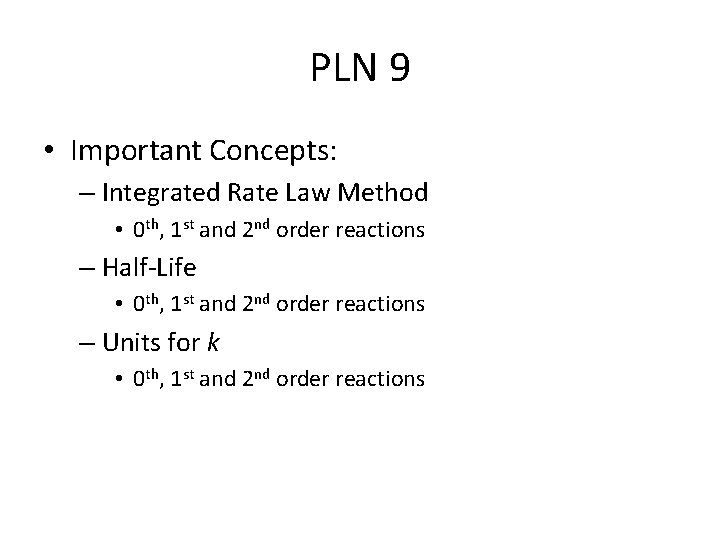
PLN 9 • Important Concepts: – Integrated Rate Law Method • 0 th, 1 st and 2 nd order reactions – Half-Life • 0 th, 1 st and 2 nd order reactions – Units for k • 0 th, 1 st and 2 nd order reactions

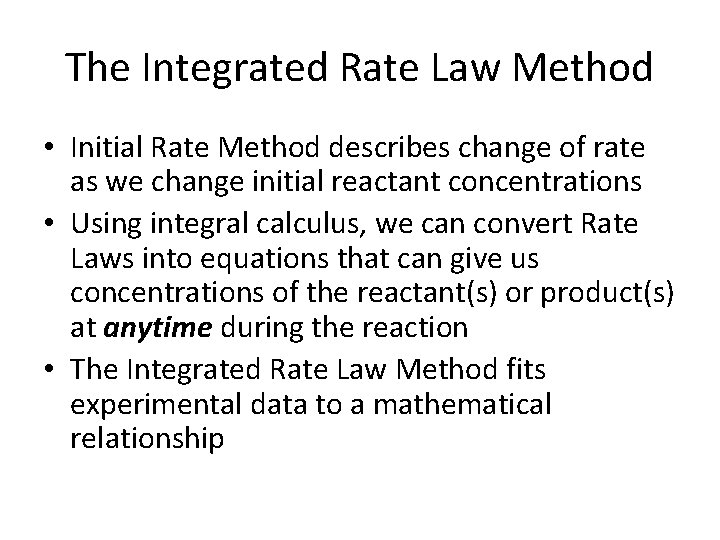
The Integrated Rate Law Method • Initial Rate Method describes change of rate as we change initial reactant concentrations • Using integral calculus, we can convert Rate Laws into equations that can give us concentrations of the reactant(s) or product(s) at anytime during the reaction • The Integrated Rate Law Method fits experimental data to a mathematical relationship

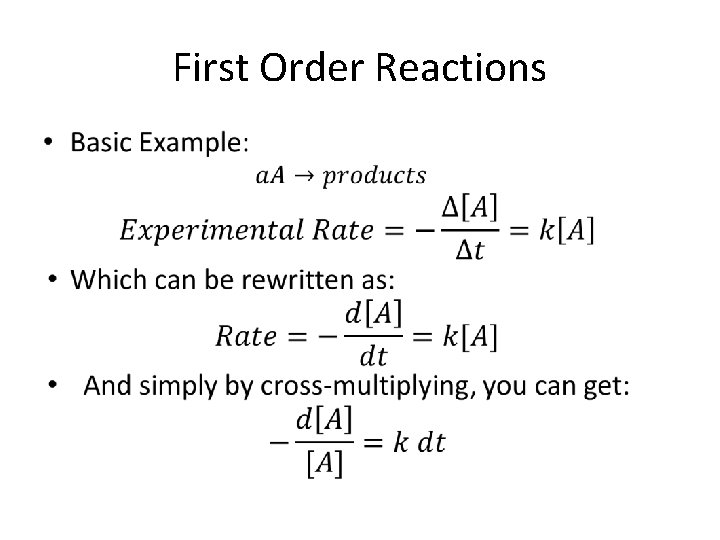
First Order Reactions •

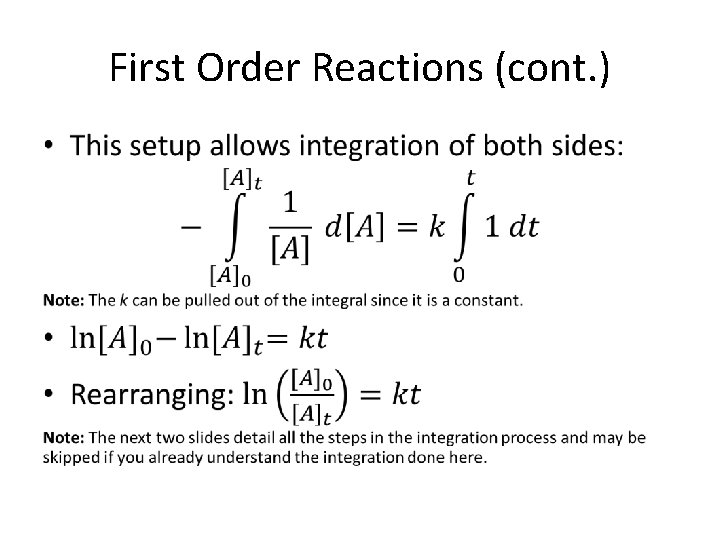
First Order Reactions (cont. ) •

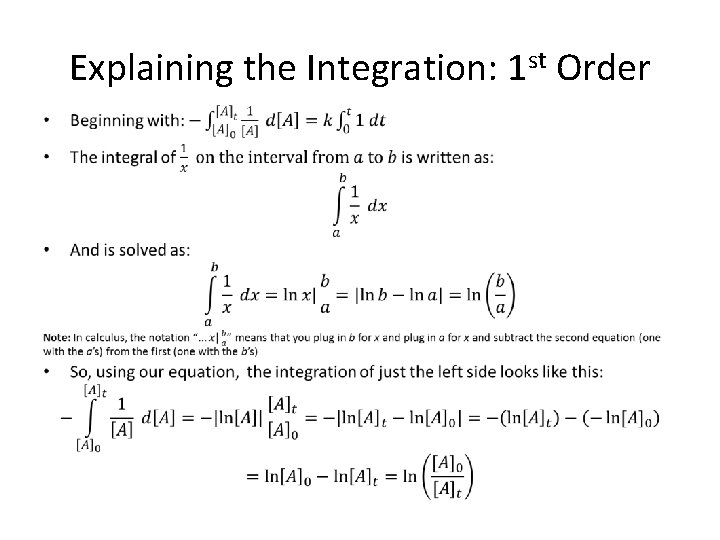
Explaining the Integration: 1 st Order •

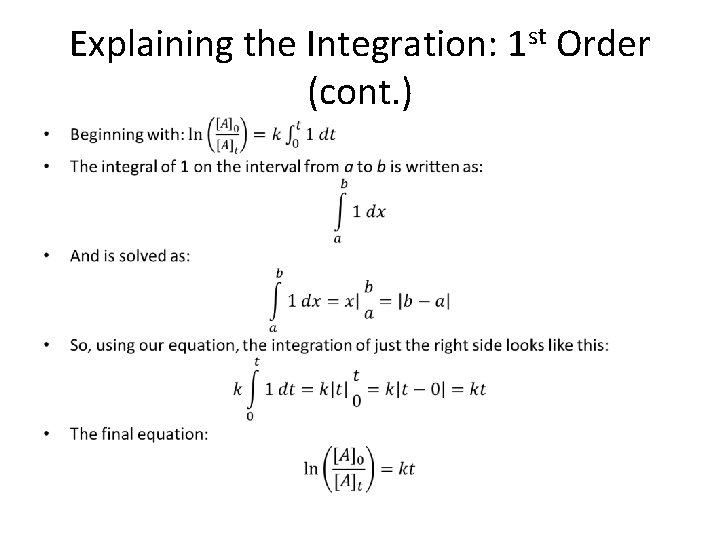
Explaining the Integration: 1 st Order (cont. ) •

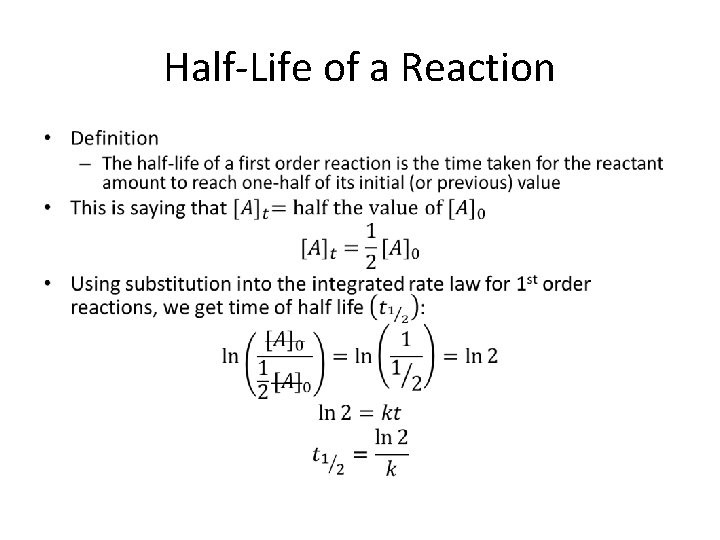
Half-Life of a Reaction •

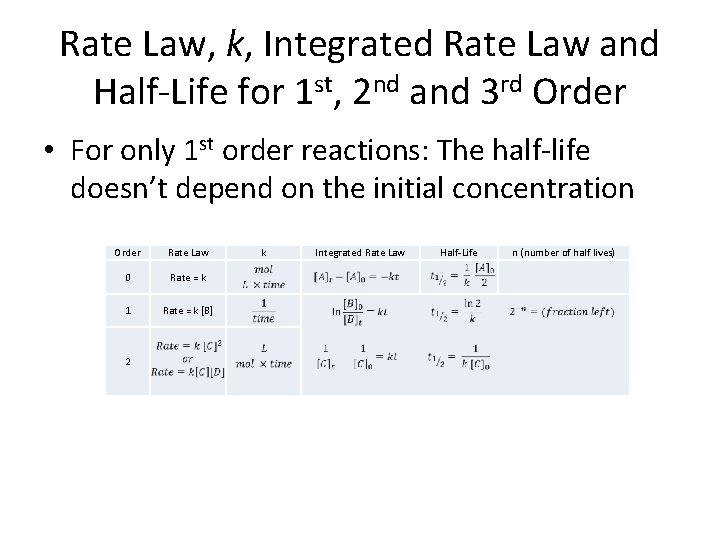
Rate Law, k, Integrated Rate Law and Half-Life for 1 st, 2 nd and 3 rd Order • For only 1 st order reactions: The half-life doesn’t depend on the initial concentration Order Rate Law 0 Rate = k 1 Rate = k [B] 2 k Integrated Rate Law Half-Life n (number of half lives)

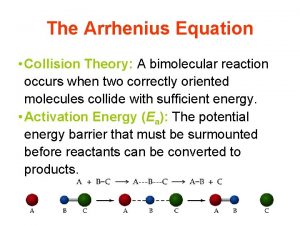
PLN 10 • Important Concepts: – Collision Theory • Pre-exponential constant (A) • f. KE – Importance of Correct Orientation – Arrhenius Equation – Activation Energy (EA) – Transition State Theory – Potential Energy Diagrams

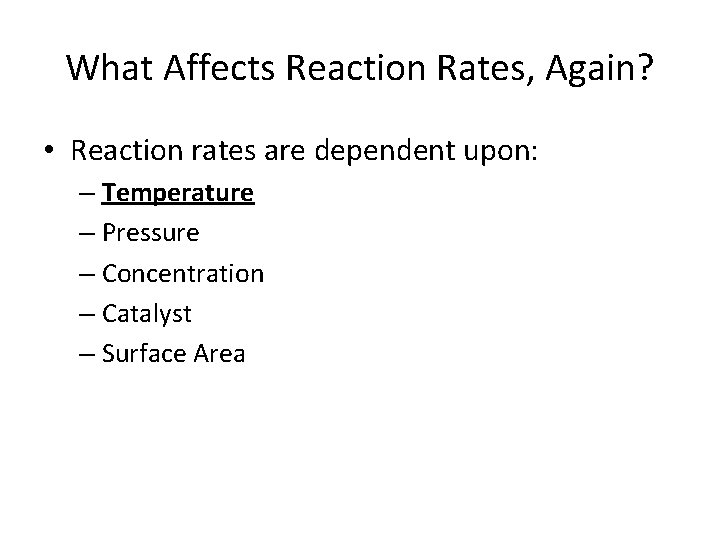
What Affects Reaction Rates, Again? • Reaction rates are dependent upon: – Temperature – Pressure – Concentration – Catalyst – Surface Area

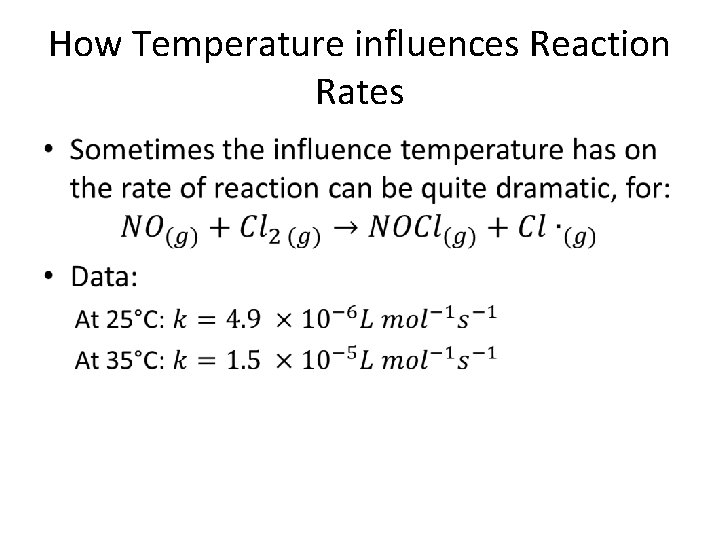
How Temperature influences Reaction Rates •

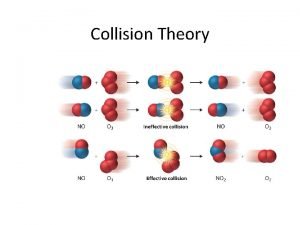
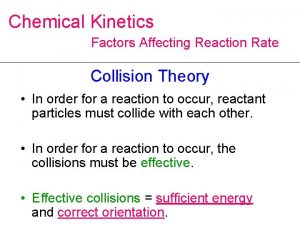
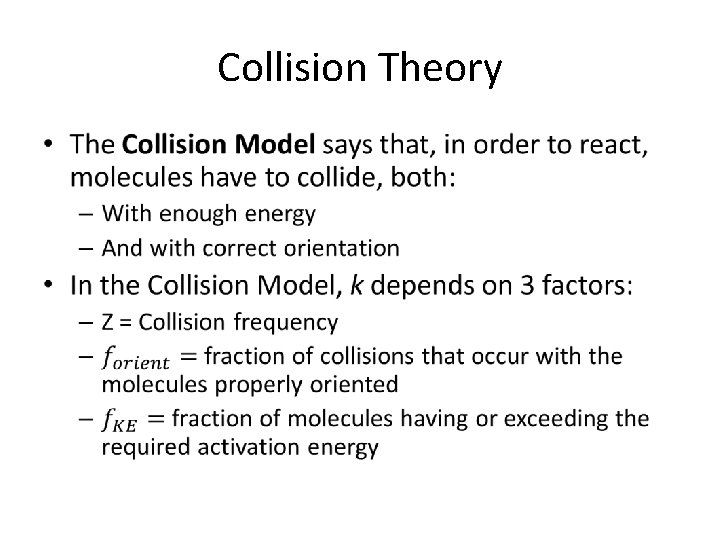
Collision Theory •

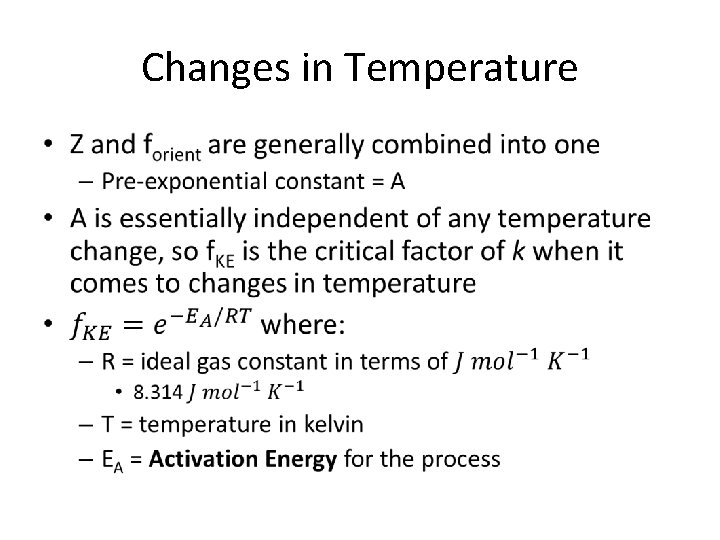
Changes in Temperature •

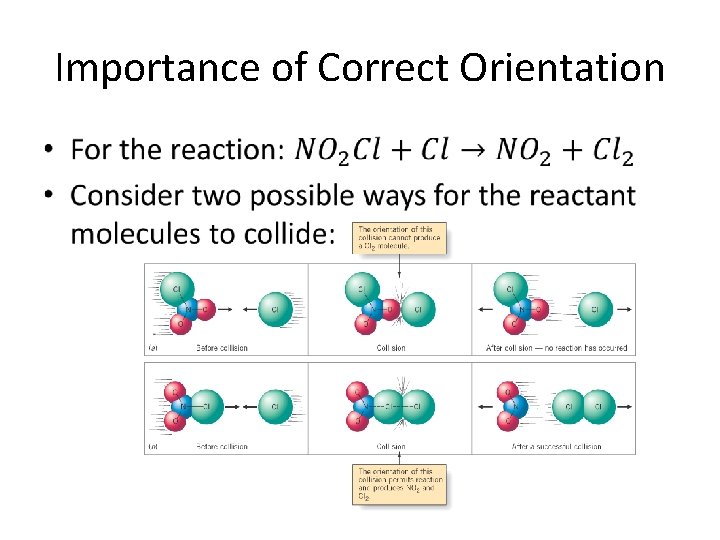
Importance of Correct Orientation •

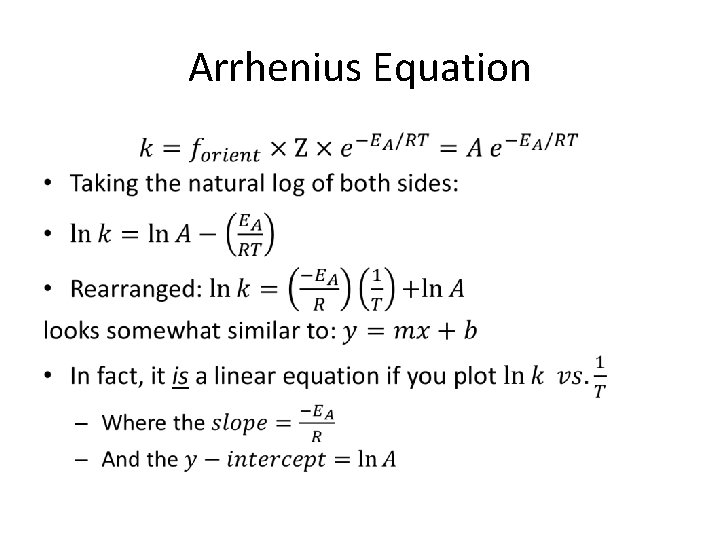
Arrhenius Equation •

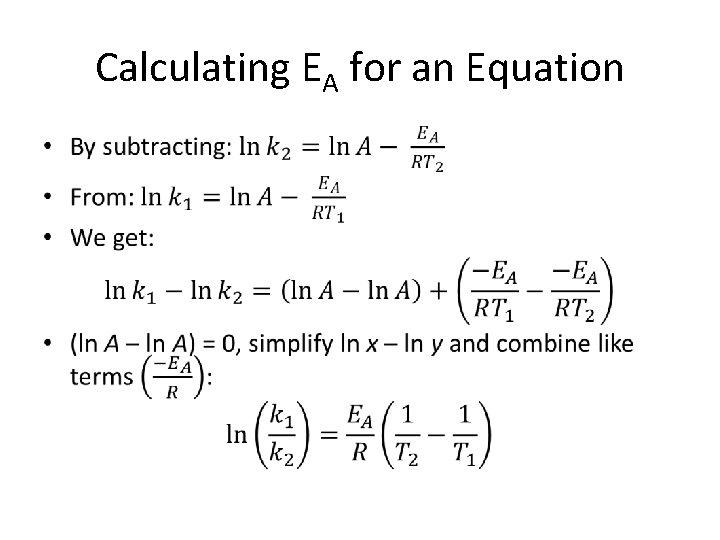
Calculating EA for an Equation •

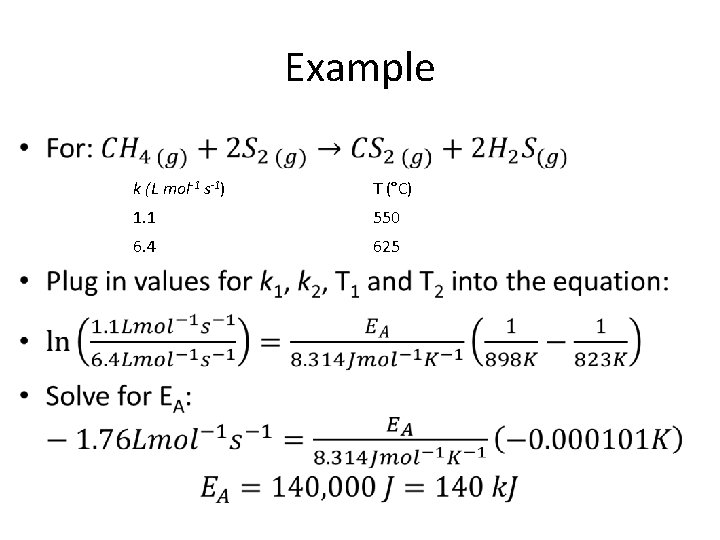
Example • k (L mol-1 s-1) T (°C) 1. 1 550 6. 4 625

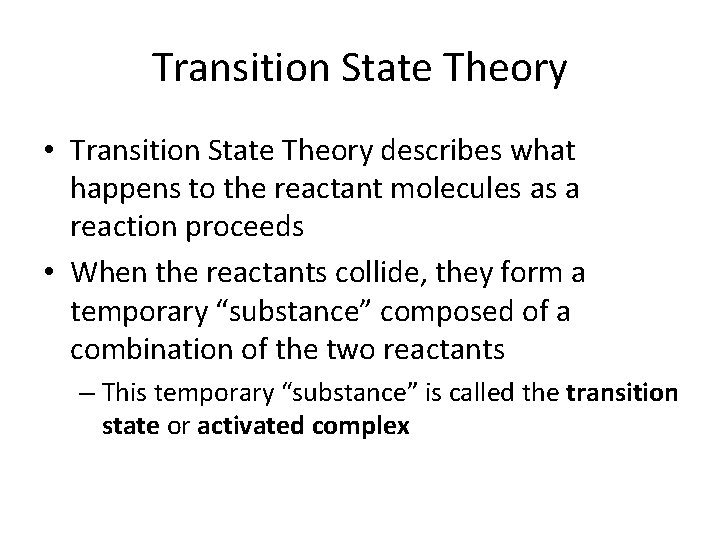
Transition State Theory • Transition State Theory describes what happens to the reactant molecules as a reaction proceeds • When the reactants collide, they form a temporary “substance” composed of a combination of the two reactants – This temporary “substance” is called the transition state or activated complex

Transition State •

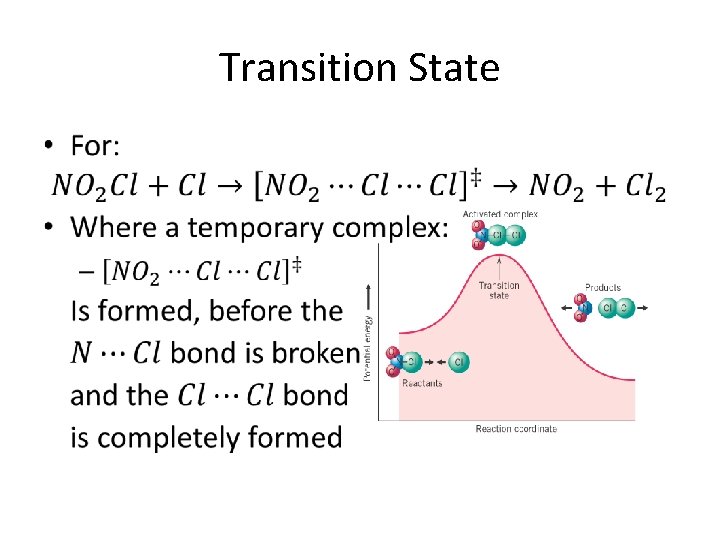
Transition States (cont. ) •

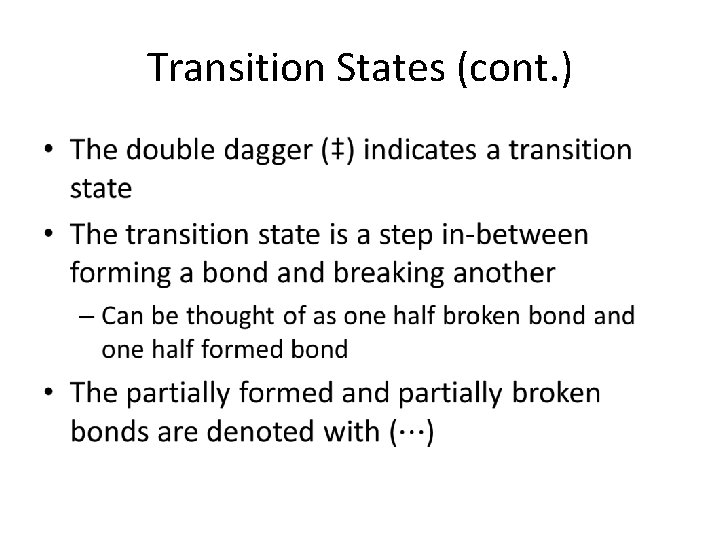
Potential Energy Diagrams • A graph of Potential Energy vs. the Reaction Coordinate – The reaction coordinate is essentially the progress of the reaction Exothermic Reaction Endothermic Reaction
 Collision theory of kinetics
Collision theory of kinetics M<1
M<1 A rate is a ratio
A rate is a ratio Equivalent ratios guided notes
Equivalent ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Reaction rate equation
Reaction rate equation Kinetics reaction
Kinetics reaction First order rate law
First order rate law Molecularity of reaction
Molecularity of reaction How to find the rate law
How to find the rate law Laws jespersen
Laws jespersen Overall rate law of a reaction
Overall rate law of a reaction Rate=k a b
Rate=k a b Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Kinetics of particles newton's second law
Kinetics of particles newton's second law Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Steric factor
Steric factor Expressing reaction rates
Expressing reaction rates Rates of reaction quiz
Rates of reaction quiz Expressing reaction rates
Expressing reaction rates Reaction rates
Reaction rates Factors affecting column chromatography
Factors affecting column chromatography Collision theory diagrams
Collision theory diagrams States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Collision theory diagrams
Collision theory diagrams Collision theory class 12
Collision theory class 12 Collision theory
Collision theory Chemistry collision theory
Chemistry collision theory Systems in nature tend to undergo changes toward
Systems in nature tend to undergo changes toward Collision theory
Collision theory Collision theory
Collision theory Collision theory
Collision theory E1cb elimination reaction
E1cb elimination reaction Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law