Chemical Kinetics Factors Affecting Reaction Rate Collision Theory
- Slides: 12

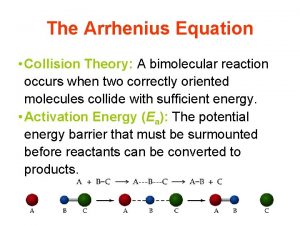
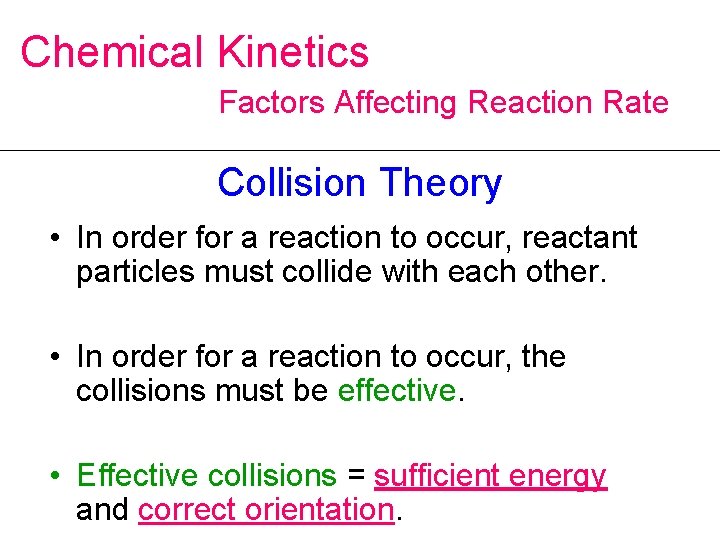
Chemical Kinetics Factors Affecting Reaction Rate Collision Theory • In order for a reaction to occur, reactant particles must collide with each other. • In order for a reaction to occur, the collisions must be effective. • Effective collisions = sufficient energy and correct orientation.

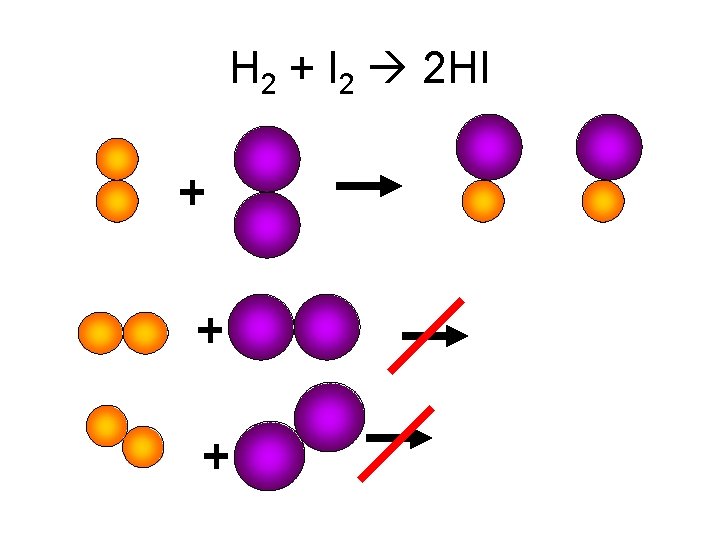
H 2 + I 2 2 HI

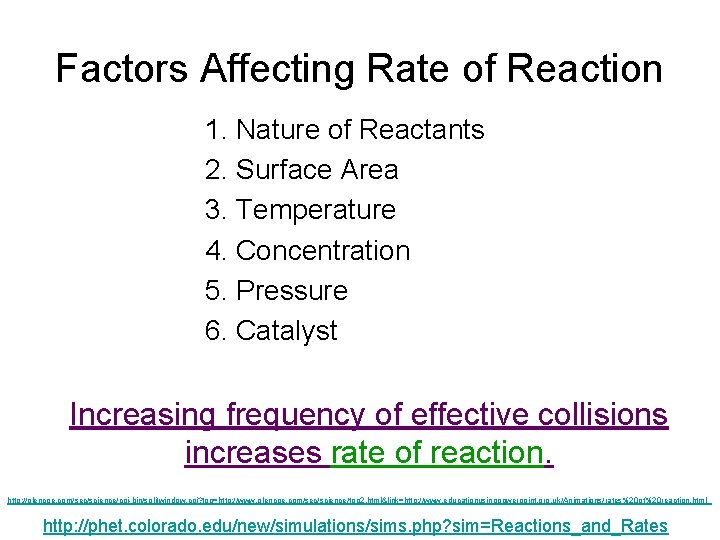
Factors Affecting Rate of Reaction 1. Nature of Reactants 2. Surface Area 3. Temperature 4. Concentration 5. Pressure 6. Catalyst Increasing frequency of effective collisions increases rate of reaction. http: //glencoe. com/sec/science/cgi-bin/splitwindow. cgi? top=http: //www. glencoe. com/sec/science/top 2. html&link=http: //www. educationusingpowerpoint. org. uk/Animations/rates%20 of%20 reaction. html http: //phet. colorado. edu/new/simulations/sims. php? sim=Reactions_and_Rates

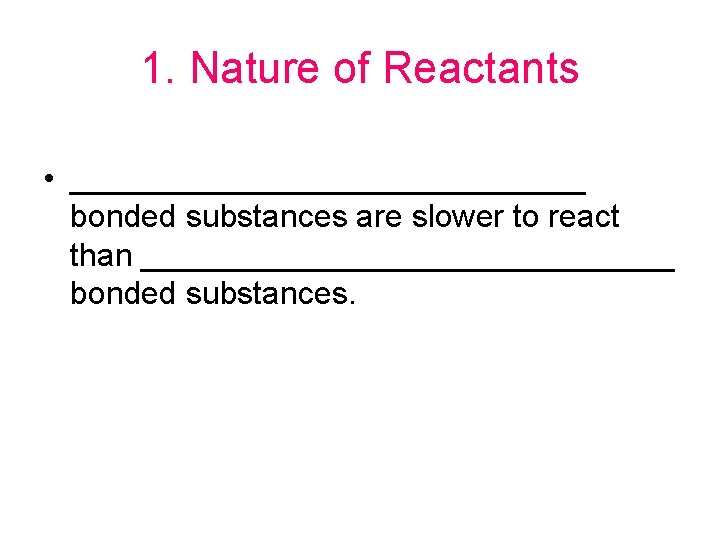
1. Nature of Reactants • _______________ bonded substances are slower to react than _______________ bonded substances.

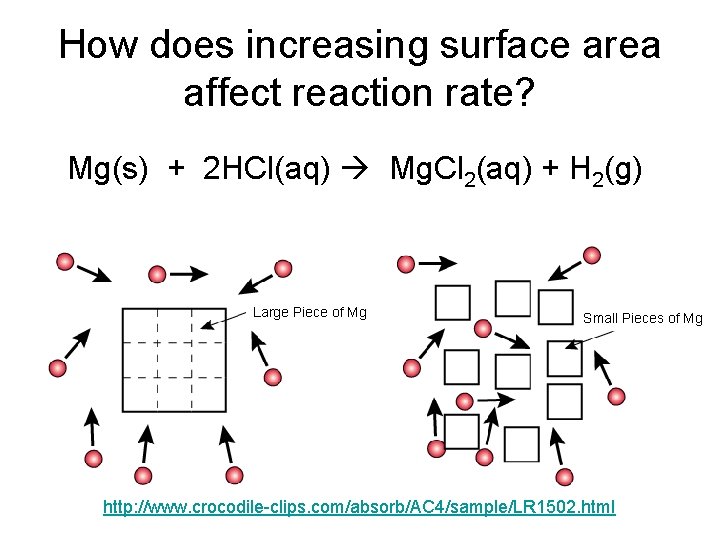
How does increasing surface area affect reaction rate? Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) Large Piece of Mg Small Pieces of Mg http: //www. crocodile-clips. com/absorb/AC 4/sample/LR 1502. html

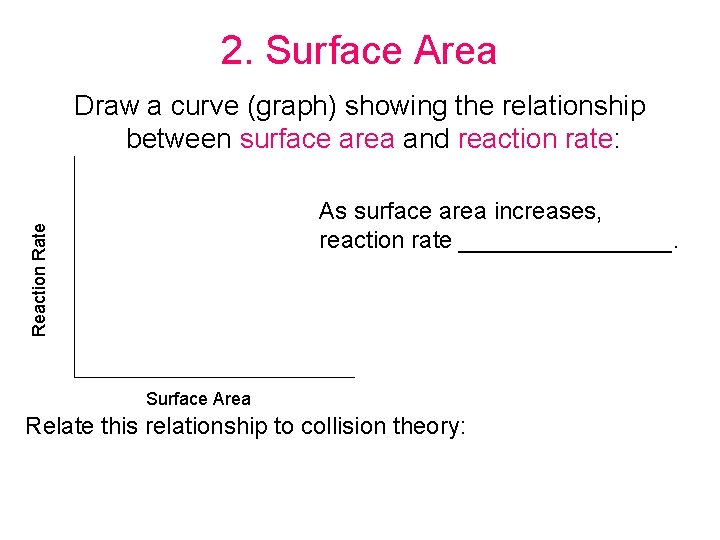
2. Surface Area Draw a curve (graph) showing the relationship between surface area and reaction rate: Reaction Rate As surface area increases, reaction rate ________. Surface Area Relate this relationship to collision theory:

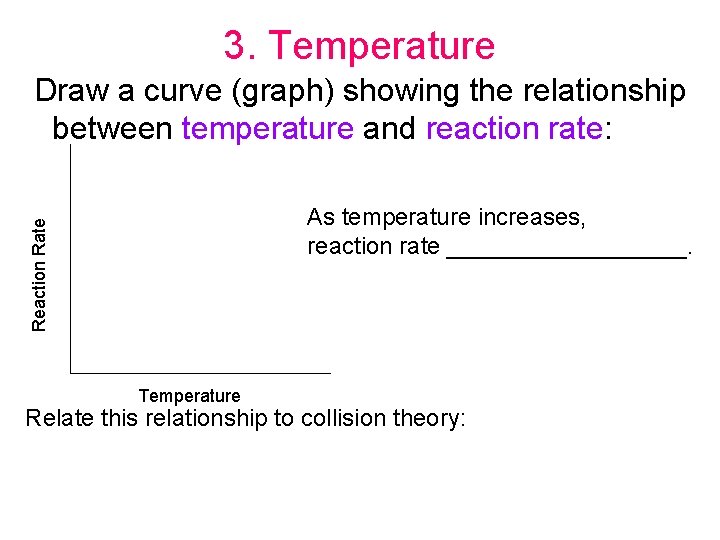
3. Temperature Draw a curve (graph) showing the relationship between temperature and reaction rate: Reaction Rate As temperature increases, reaction rate _________. Temperature Relate this relationship to collision theory:

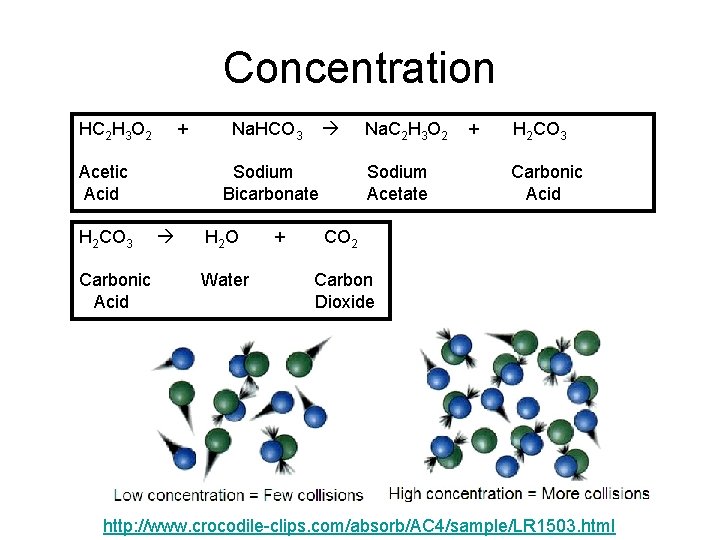
Concentration HC 2 H 3 O 2 + Acetic Acid H 2 CO 3 Carbonic Acid Na. HCO 3 Sodium Bicarbonate H 2 O Water + Na. C 2 H 3 O 2 Sodium Acetate + H 2 CO 3 Carbonic Acid CO 2 Carbon Dioxide http: //www. crocodile-clips. com/absorb/AC 4/sample/LR 1503. html

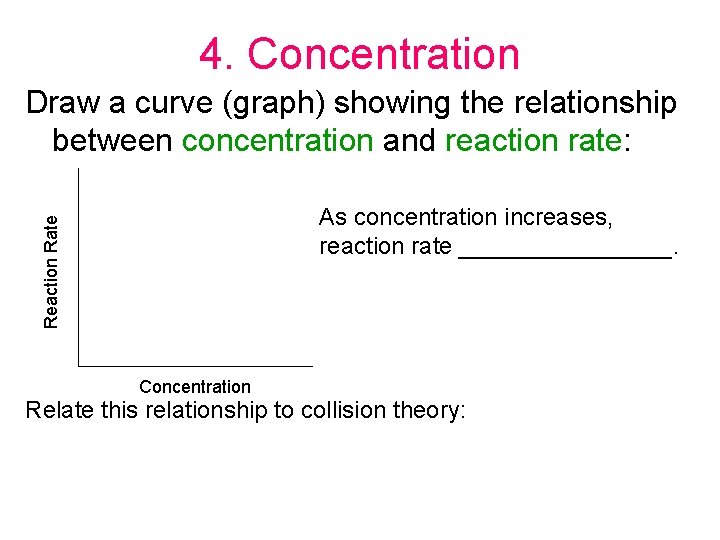
4. Concentration Draw a curve (graph) showing the relationship between concentration and reaction rate: Reaction Rate As concentration increases, reaction rate ________. Concentration Relate this relationship to collision theory:

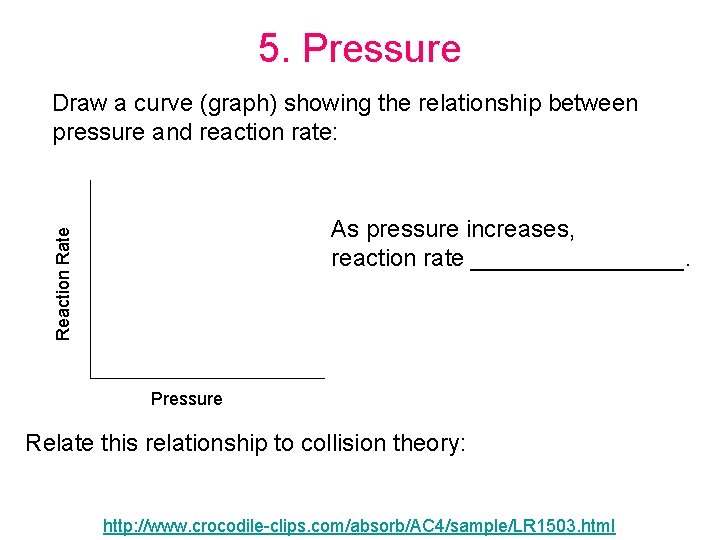
5. Pressure Draw a curve (graph) showing the relationship between pressure and reaction rate: Reaction Rate As pressure increases, reaction rate ________. Pressure Relate this relationship to collision theory: http: //www. crocodile-clips. com/absorb/AC 4/sample/LR 1503. html

6. Catalyst: The addition of a catalyst ______ reaction rate. Definition of a catalyst: http: //www. eepybird. com/dcm 1. html

Homework • Collision Theory: Orange Book: Pg. 110, Q 1 -10 *Pg. 121, Q 1, 2, 4, 5 *Pg. 123, Q 18, 19, 21, 22, 23 * Must do. Other HW questions are optional.
 The lower the activation energy the faster the reaction
The lower the activation energy the faster the reaction Collision theory of kinetics
Collision theory of kinetics Steric factor in arrhenius equation
Steric factor in arrhenius equation How to calculate initial rate of reaction
How to calculate initial rate of reaction Surface area affecting rate of reaction
Surface area affecting rate of reaction Elimination reaction conditions
Elimination reaction conditions Factors affecting evaporation
Factors affecting evaporation Solute potential definition biology
Solute potential definition biology Factors effecting gfr
Factors effecting gfr Factors that affect the rate constant
Factors that affect the rate constant Section 17.2 factors affecting chemical equilibrium
Section 17.2 factors affecting chemical equilibrium Larmor frequency formula
Larmor frequency formula Geminal and vicinal coupling constants
Geminal and vicinal coupling constants