ISOTOPES WHAT IS AN ISOTOPE Atoms of the























- Slides: 28

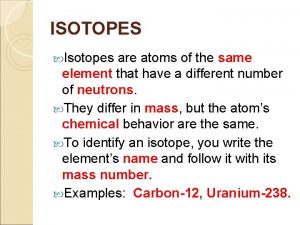
ISOTOPES

WHAT IS AN ISOTOPE? � Atoms of the same element with different numbers of neutrons. � What has changed if there is a different number of neutrons. HINT: look at your periodic tables. Something must have changed.

WHAT IS AN ISOTOPE � If you have a different number of neutrons, that means your atomic mass has changed. � Remember that atomic mass = neutrons + protons � THE NUMBER OF PROTONS NEVER CHANGES. � Why doesn’t this number ever change?

WHAT IS AN ISOTOPE? � If you change the number of protons you now have a different element. � EX: If you have an atom of Ne (Neon) with 11 protons now. . You actually would have an atom of Na (Sodium)

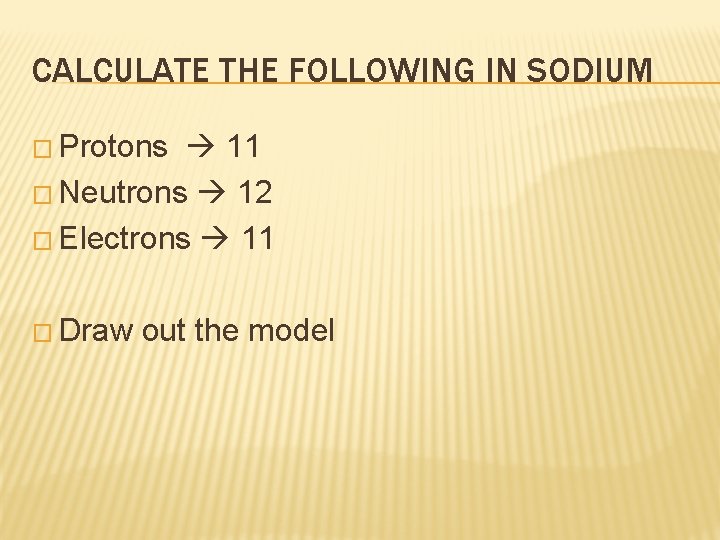
CALCULATE THE FOLLOWING IN SODIUM � Protons � Neutrons � Electrons

CALCULATE THE FOLLOWING IN SODIUM � Protons 11 � Neutrons 12 � Electrons 11 � Draw out the model

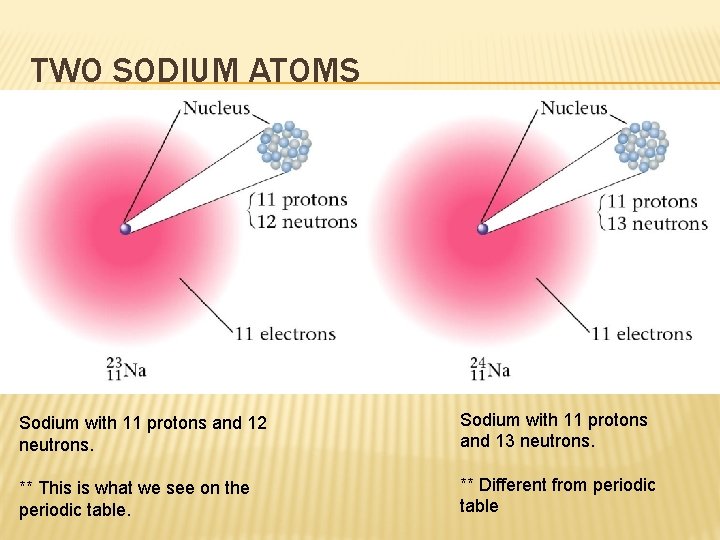
TWO SODIUM ATOMS Sodium with 11 protons and 12 neutrons. Sodium with 11 protons and 13 neutrons. ** This is what we see on the periodic table. ** Different from periodic table

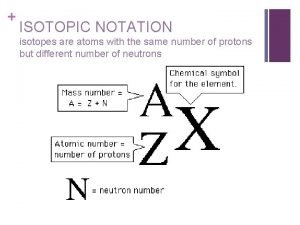
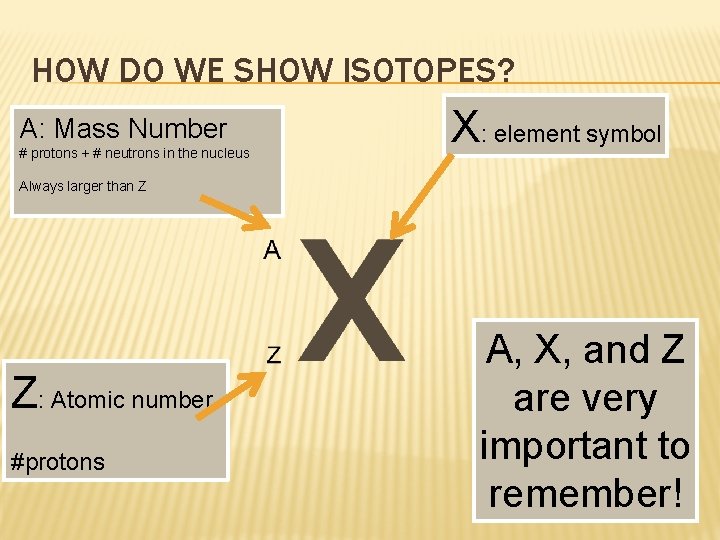
HOW DO WE SHOW ISOTOPES? A: Mass Number # protons + # neutrons in the nucleus X: element symbol Always larger than Z Z: Atomic number #protons A, X, and Z are very important to remember!

TRY IT IN YOUR NOTES � What is the element? � How many protons are in the element? � What is the elements atomic mass? N 23 11

TRY IT IN YOUR NOTES � What is the element? � Sodium N 23 � How many protons are in the element? � 11 � What is the elements atomic mass? � 23 11

TRY IT IN YOUR NOTES � What is the element? � How many protons are in the element? � What is the elements atomic mass? Cl 33 17

TRY IT IN YOUR NOTES � What is the element? � � How many protons are in the element? � � Chlorine 17 What is the elements atomic mass? � 33 Cl 33 17

TRY IT IN YOUR NOTES � An atom of Nitrogen � 7 protons � Mass of 14 number

TRY IT IN YOUR NOTES � An atom of Nitrogen N 14 � 7 protons � Atomic 14 mass of 7

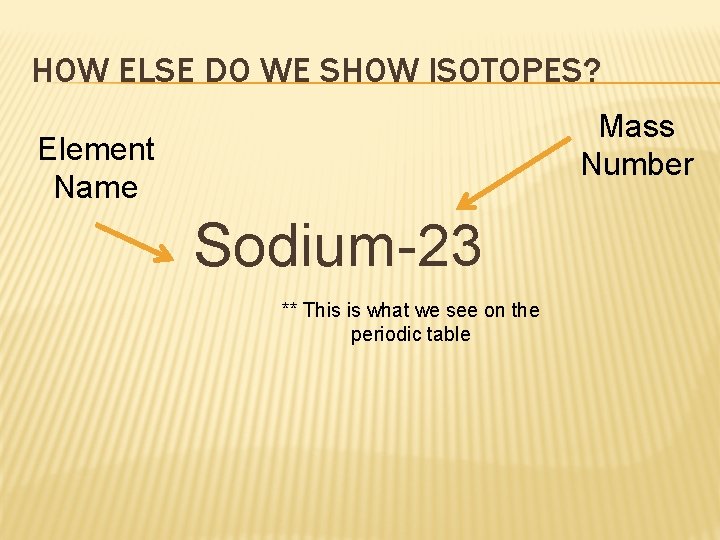
HOW ELSE DO WE SHOW ISOTOPES? Mass Number Element Name Sodium-23 ** This is what we see on the periodic table

Questions, Comments, Concerns?

� Please take out your whiteboards.

� Write the isotope symbol for the chemical element that has 6 protons and 8 neutrons (write the symbol both ways)

� Write the isotope symbol for the chemical element that has 6 protons and 8 neutrons (write the symbol both ways) Carbon – 14 14 6 C

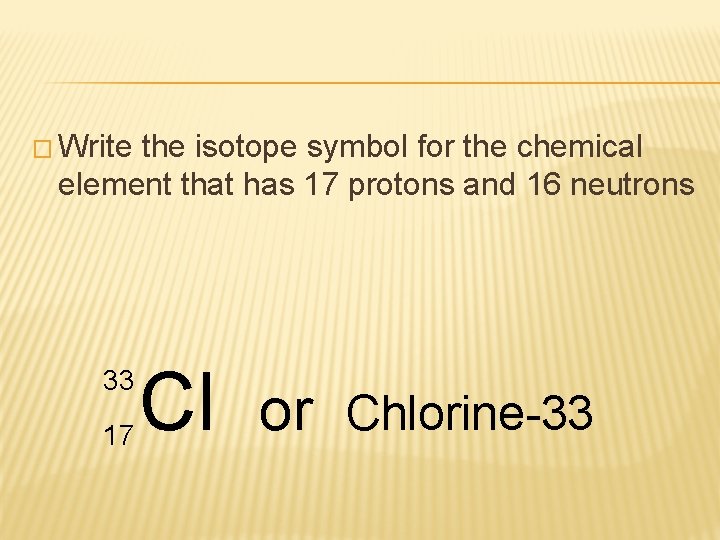
� Write the isotope symbol for the chemical element that has 17 protons and 16 neutrons

� Write the isotope symbol for the chemical element that has 17 protons and 16 neutrons 33 17 Cl or Chlorine-33

� How many protons are in the isotope 54 Cr? (Chromium-54)

� How many protons are in the isotope 54 Cr? (Chromium-54) � 24 just like CHANGE all Chromium Protons NEVER

TRUE/FALSE � Isotopes of an element always have the same number of neutrons. � A. False � B. True

TRUE/FALSE � Isotopes of an element always have the same number of neutrons. � A. False

TRUE/FALSE � Isotopes of an element always have the same number of protons. � A. False � B. True

TRUE/FALSE � Isotopes of an element always have the same number of protons. � B. True

�Z =9 � A = 29 � Draw the symbol. � How many protons? � How many neutrons?
 Isotopes pogil
Isotopes pogil Atomic structure essential questions
Atomic structure essential questions Given the atomic notation of 64/30 zn
Given the atomic notation of 64/30 zn Regents periodic table
Regents periodic table Isotopes properties
Isotopes properties Formula for calculating average atomic mass
Formula for calculating average atomic mass Isotopic notation example
Isotopic notation example Orbital diagram for rubidium
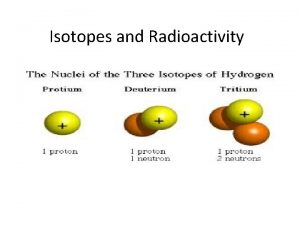
Orbital diagram for rubidium Hydrogen isotopes
Hydrogen isotopes Isotopes
Isotopes Lesson 13 subatomic heavyweights isotopes
Lesson 13 subatomic heavyweights isotopes Northwest medical isotopes
Northwest medical isotopes Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Hydrogen isotopes
Hydrogen isotopes Four fundamental forces of nature
Four fundamental forces of nature Stable vs unstable isotope
Stable vs unstable isotope Number of proton
Number of proton Isotopes properties
Isotopes properties A x z atom
A x z atom Atomic isotopes
Atomic isotopes Isotopes
Isotopes Hyphen notation of the three isotopes of hydrogen
Hyphen notation of the three isotopes of hydrogen Fertile isotopes
Fertile isotopes Isotopes examples
Isotopes examples Hydrogen isotopes
Hydrogen isotopes Isotopes examples
Isotopes examples Applications of radioisotopes
Applications of radioisotopes Isotopes radioactifs
Isotopes radioactifs Isotopes of an element have a different number of
Isotopes of an element have a different number of