Atoms and Isotopes Up and at them Atoms





- Slides: 18

Atoms and Isotopes “Up and at them”

Atoms n n An atom is composed of a central nucleus which consists of protons and neutrons, along with orbiting electrons that exist within ‘clouds’ or orbitals. These protons, neutrons, and electrons are commonly known as SUB-ATOMIC PARTICLES.

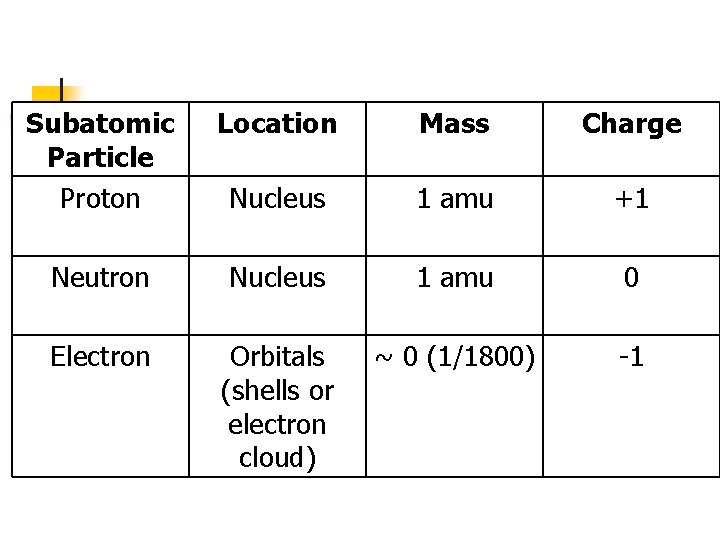
Subatomic Particle Proton Location Mass Charge Nucleus 1 amu +1 Neutron Nucleus 1 amu 0 Electron Orbitals (shells or electron cloud) ~ 0 (1/1800) -1

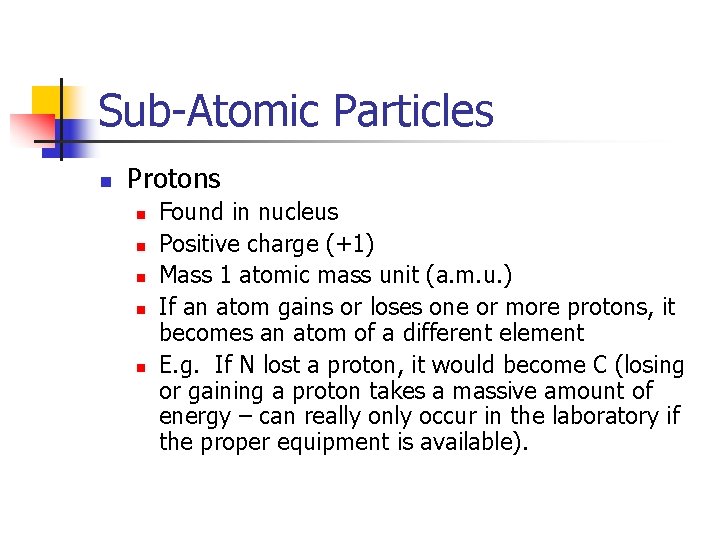
Sub-Atomic Particles n Protons n n n Found in nucleus Positive charge (+1) Mass 1 atomic mass unit (a. m. u. ) If an atom gains or loses one or more protons, it becomes an atom of a different element E. g. If N lost a proton, it would become C (losing or gaining a proton takes a massive amount of energy – can really only occur in the laboratory if the proper equipment is available).

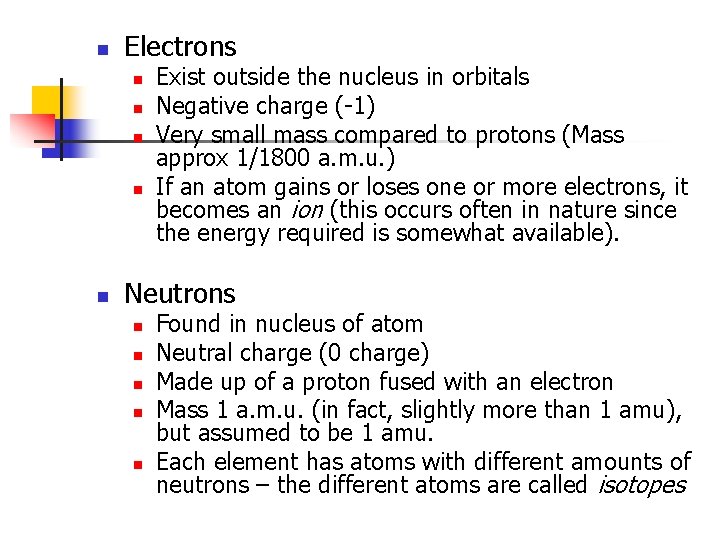
n Electrons n n n Exist outside the nucleus in orbitals Negative charge (-1) Very small mass compared to protons (Mass approx 1/1800 a. m. u. ) If an atom gains or loses one or more electrons, it becomes an ion (this occurs often in nature since the energy required is somewhat available). Neutrons n n n Found in nucleus of atom Neutral charge (0 charge) Made up of a proton fused with an electron Mass 1 a. m. u. (in fact, slightly more than 1 amu), but assumed to be 1 amu. Each element has atoms with different amounts of neutrons – the different atoms are called isotopes

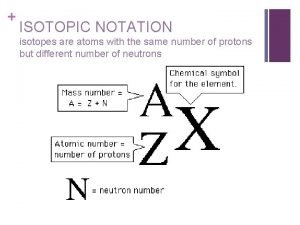
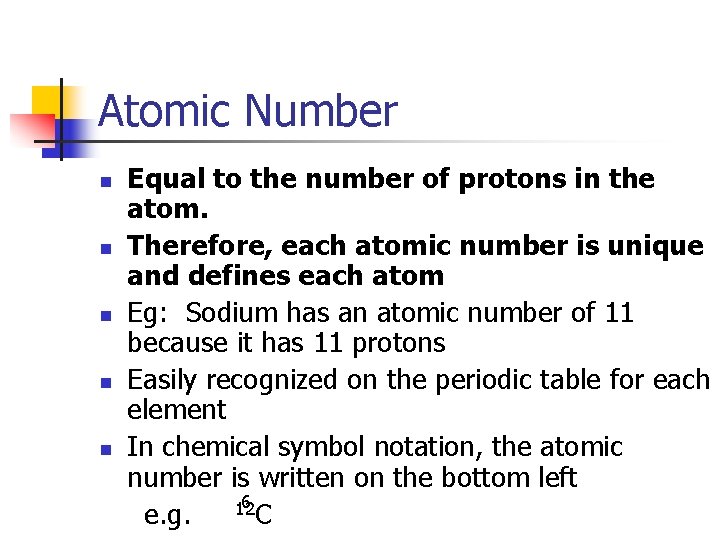
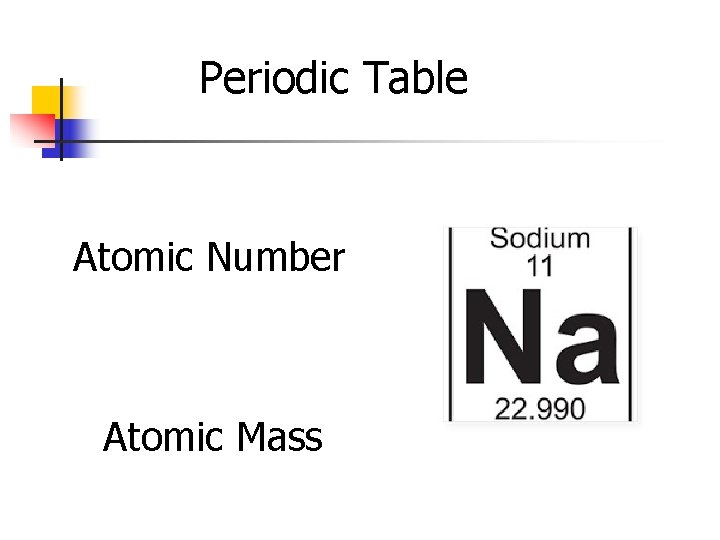
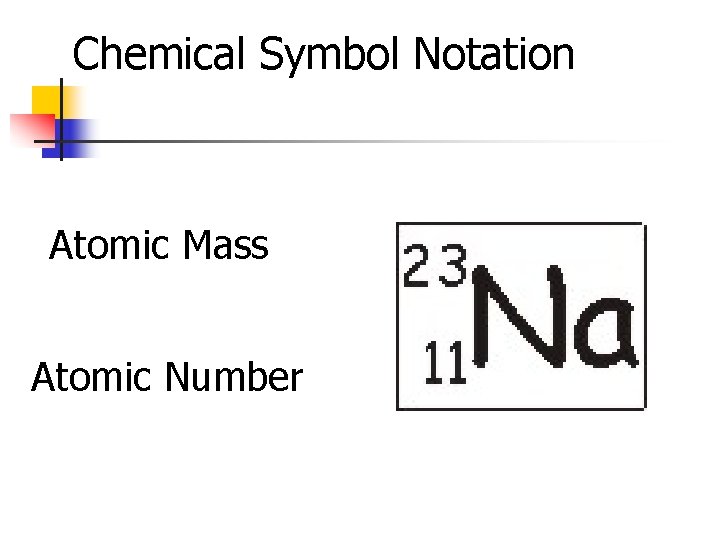
Atomic Number n n n Equal to the number of protons in the atom. Therefore, each atomic number is unique and defines each atom Eg: Sodium has an atomic number of 11 because it has 11 protons Easily recognized on the periodic table for each element In chemical symbol notation, the atomic number is written on the bottom left 6 12 e. g. C

Periodic Table Atomic Number Atomic Mass

Chemical Symbol Notation Atomic Mass Atomic Number

n The atomic number is found at the top of each square on the periodic table. Atomic number If you have a periodic table you can instantly find the number of protons that each atom possesses. It should also be noted that any element existing in its neutral state will also have the same number of electrons as its Atomic #.

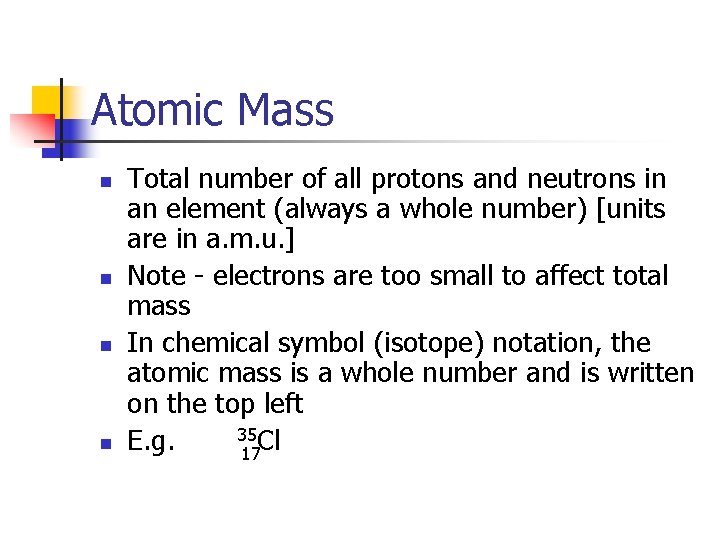
Atomic Mass n n Total number of all protons and neutrons in an element (always a whole number) [units are in a. m. u. ] Note - electrons are too small to affect total mass In chemical symbol (isotope) notation, the atomic mass is a whole number and is written on the top left 35 Cl E. g. 17

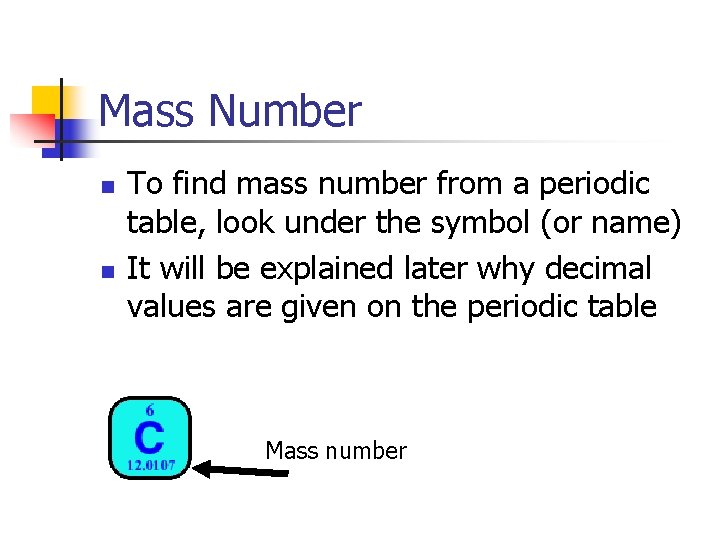
Mass Number n n To find mass number from a periodic table, look under the symbol (or name) It will be explained later why decimal values are given on the periodic table Mass number

Number of Neutrons n n Can be determined with the following formula: Atomic Mass = Protons + Neutrons

Number of Electrons n n Atoms have a neutral charge (uncharged). Since protons have a positive charge and electrons have a negative charge, the number of electrons must be equal to the number of protons in an atom.

Calculating Protons, Neutrons and Electrons n Find number of protons, neutrons, and electrons and write chemical symbol notation for each of the following Atomic Mass n n n Carbon-13 Sodium-23 Uranium-235

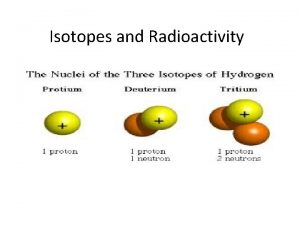
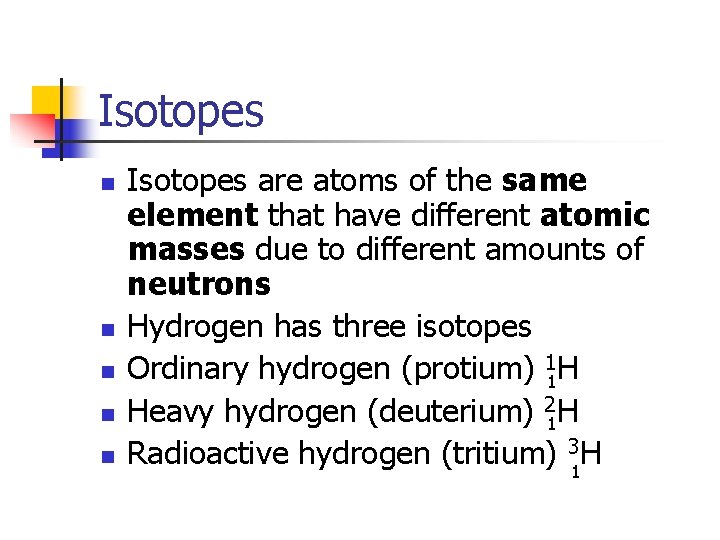
Isotopes n n n Isotopes are atoms of the same element that have different atomic masses due to different amounts of neutrons Hydrogen has three isotopes Ordinary hydrogen (protium) 11 H Heavy hydrogen (deuterium) 21 H Radioactive hydrogen (tritium) 31 H

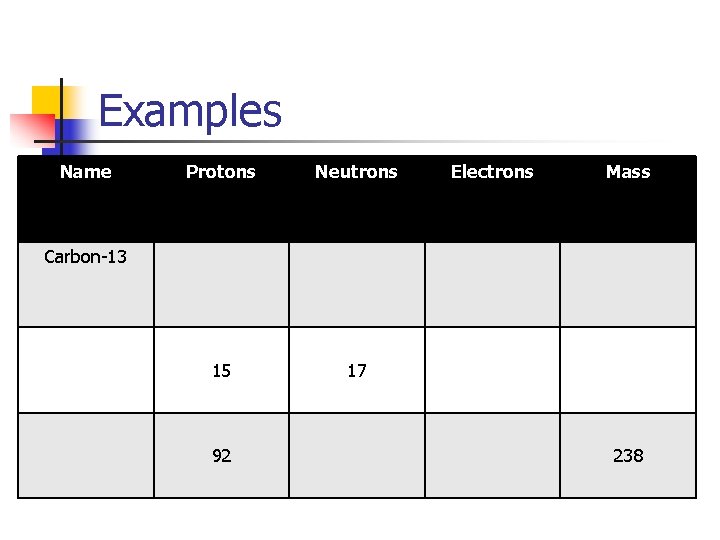
Examples Name Protons Neutrons 15 17 Electrons Mass Carbon-13 92 238

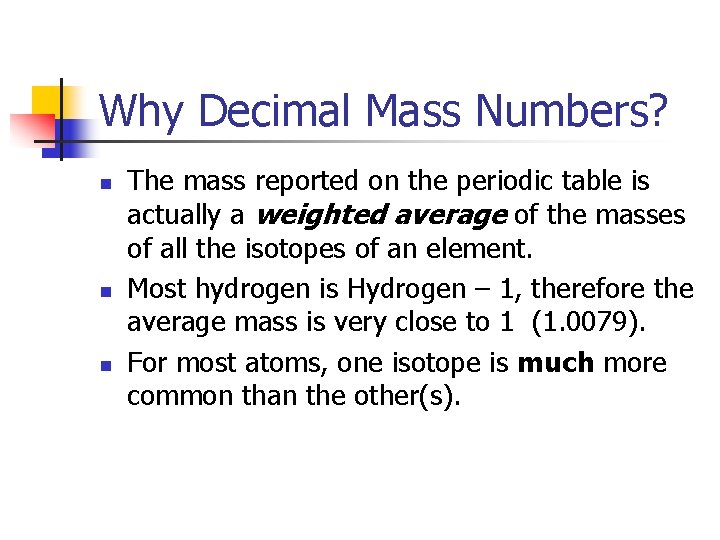
Why Decimal Mass Numbers? n n n The mass reported on the periodic table is actually a weighted average of the masses of all the isotopes of an element. Most hydrogen is Hydrogen – 1, therefore the average mass is very close to 1 (1. 0079). For most atoms, one isotope is much more common than the other(s).

Homework n ATOMIC STRUCTURE WORKSHEET n ONLY PART I
 Atoms and their isotopes pogil
Atoms and their isotopes pogil At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity If you can t beat them join them
If you can t beat them join them What is this called
What is this called Fertile isotopes
Fertile isotopes How to determine percent abundance
How to determine percent abundance Abundance calculation chemistry
Abundance calculation chemistry Atomic isotopes
Atomic isotopes Uses of radioactive isotopes
Uses of radioactive isotopes Rb orbital diagram
Rb orbital diagram Uses of radioactive isotopes in agriculture
Uses of radioactive isotopes in agriculture Argon hyphen notation
Argon hyphen notation Atomic number mass
Atomic number mass Subatomic heavyweights isotopes lesson 13
Subatomic heavyweights isotopes lesson 13 Isotopes
Isotopes What is isotopes
What is isotopes Oxoacids of nitrogen
Oxoacids of nitrogen Isotopic notation
Isotopic notation Hydrogen isotopes
Hydrogen isotopes