Atoms Ions and Isotopes Quick Review Atoms are









- Slides: 13

Atoms, Ions, and Isotopes

Quick Review • Atoms are made up of three particles: • Protons • Neutrons • Electrons • Question: Which of the three particles identifies what element an atom is? • The PROTON! (very important)

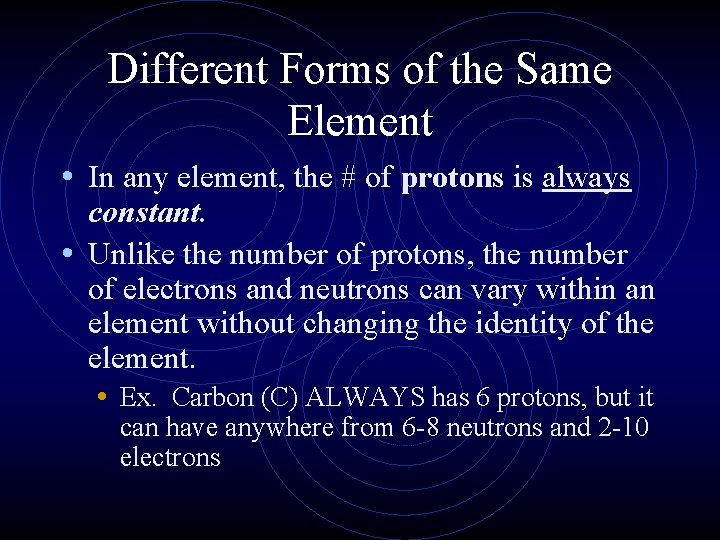
Different Forms of the Same Element • In any element, the # of protons is always constant. • Unlike the number of protons, the number of electrons and neutrons can vary within an element without changing the identity of the element. • Ex. Carbon (C) ALWAYS has 6 protons, but it can have anywhere from 6 -8 neutrons and 2 -10 electrons

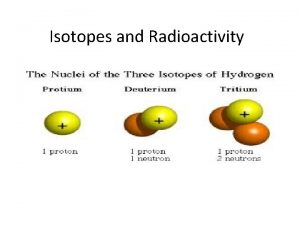
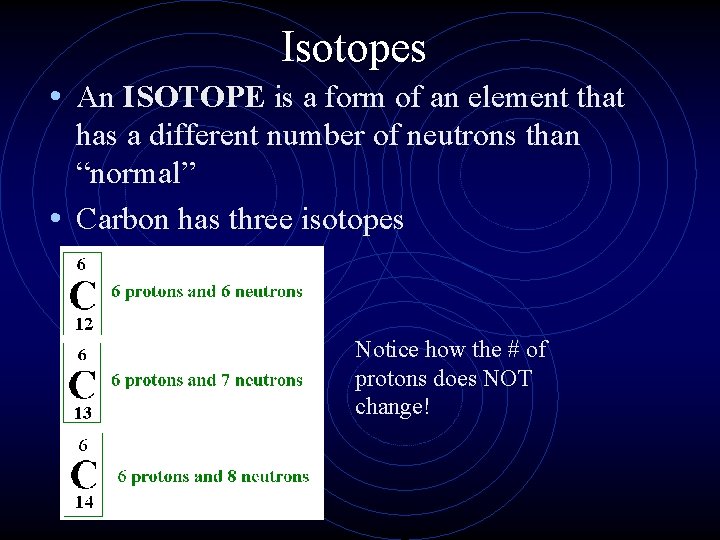
Isotopes • An ISOTOPE is a form of an element that has a different number of neutrons than “normal” • Carbon has three isotopes Notice how the # of protons does NOT change!

Other Isotopes • Most atoms have naturally occurring isotopes including: • Radon • Potassium • Uranium • When an element is “radioactive” it means it has an unstable number of neutrons (an unstable ISOTOPE)

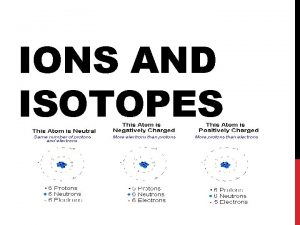
IONS • An atom usually has a neutral charge. That means it has the same number of protons as electrons • Remember, a proton has a positive charge and an electron has a negative charge • ION – an atom that has lost or gained one or more electrons and has become charged either positively or negatively

Positive Ions • When an atom LOSES electrons, it becomes more POSITIVE • Why? • If you are getting rid of negative particles (electrons) but your number of positive particles (protons) are staying the same. • In other words, you are subtracting negative numbers

Examples • What would the charge be if: • The neutral form of Gold (Au) lost 4 of its 79 electrons. It now has 79 protons and 75 electrons • The neutral form of Mg lost 2 of its 12 electrons. It now has 12 protons and 10 electrons.

Negative Ions • When an atom GAINS electrons it becomes more NEGATIVE • Why? • Electrons have a negative charge, so the more you have, the more negative you become

Representing Ions • Ions are represented by placing a “superscript” charge number next to the atomic symbol. • Ex. • O-2 = oxygen with a negative 2 charge • K+ = potassium with a positive 1 charge • N-3 = nitrogen with a negative 3 charge • And so on

Periodic Table • The elements, as they are found on the periodic table, are neutral atoms and their mass is an average of all isotopes • Remember the atomic mass is the average of ALL isotopes, but when we round it for calculating the number of neutrons, we always get the most abundant isotope.

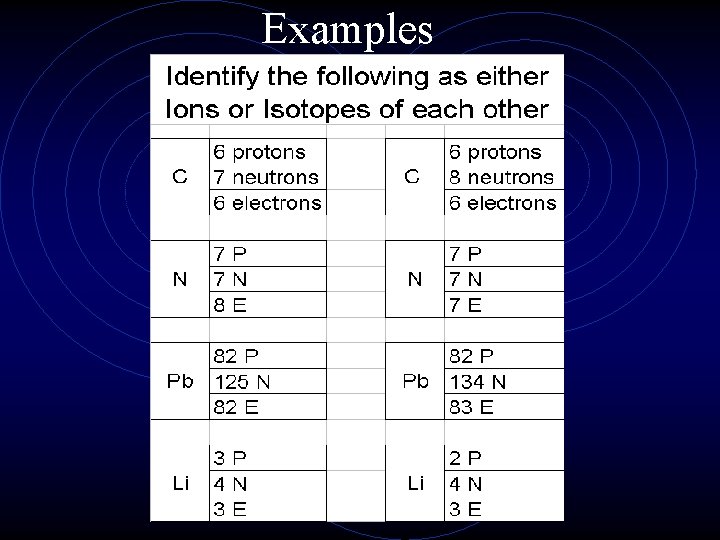
Examples

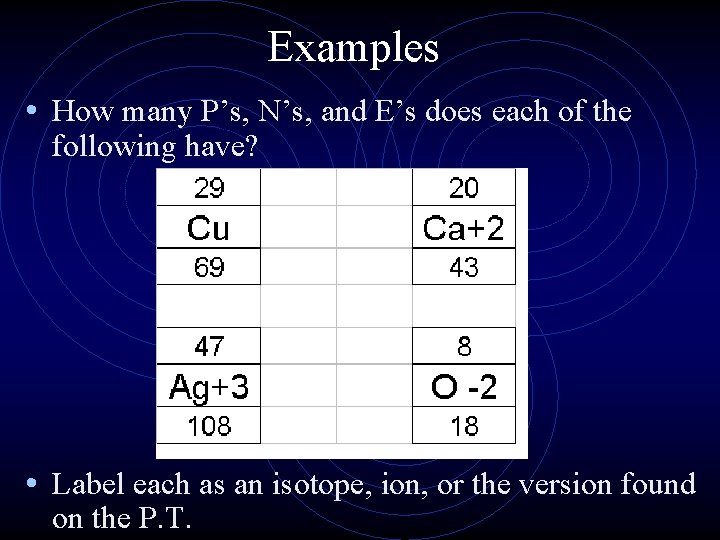
Examples • How many P’s, N’s, and E’s does each of the following have? • Label each as an isotope, ion, or the version found on the P. T.
 Insidan region jh
Insidan region jh Isotopes pogil
Isotopes pogil Positive ions and negative ions table
Positive ions and negative ions table Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Quick find algorithm
Quick find algorithm Quickchek menu
Quickchek menu Positive ions are atoms that have
Positive ions are atoms that have