Immunization Champions Handout Highlights from the 2016 ACIP




























































- Slides: 77

Immunization Champions Handout Highlights from the 2016 ACIP Adult Immunization Schedule Sandra Adamson Fryhofer, MD, MACP Practicing General Internist, Atlanta, GA Adjunct Associate Professor of Medicine, Emory University School of Medicine Past President, American College of Physicians ACP Liaison to ACIP

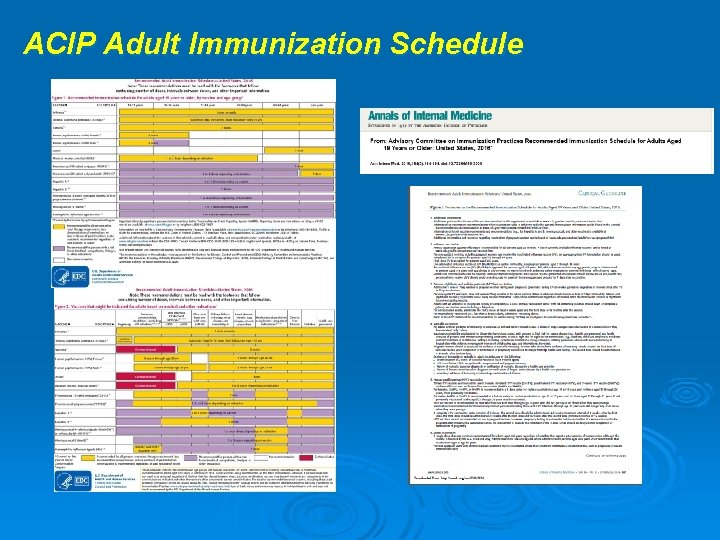
ACIP Adult Immunization Schedule

ACIP (Advisory Committee on Immunization Practices) Ø 15 voting members (appointed by DHH Secretary) l l Ø ACIP recs become official CDC policy-signed by the CDC director, accepted by HHS Secretary, published in MMWR October 2010, evidence-based process – GRADE l Ø 8 ex officio reps; 30 non-voting liaisons (Grading of Recommendations, Assessment, Development and Evaluation) ACIP recs: l Category A recommendation • For all persons in an age or risk factor based group l Category B recommendation • For individual clinical decision making

Vaccine Information Statements (VIS) http: //www. cdc. gov/vaccines/pubs/vis/default. htm

ACIP recs: coverage clout under new ACA plans Ø New health plans - required to cover new ACIP recommendations without cost-sharing in the next plan year that occurs one year after the date of the recommendation. ****Does not apply to Medicare **** Ø ACIP recommendations are not always consistent with FDA licensing.

FDA Licensing Process Ø IND: Investigational New Drug Application l Ø Includes protocol for human studies Preclinical licensure trials: l l l Phase 1 studies (small study) Phase 2 Studies (larger study- hundreds of patients) Phase 3 Studies (vaccine effectiveness and safety- thousands of patients) BLA: Biologics License Application Request Ø Accelerated Approval Pathway (Fast Track) Ø l l Based on early evidence of effectiveness May not satisfy comprehensive ACIP GRADE evidence assessment

The Big 5: Influenza FLU FACTS http: //www. cdc. gov/flu/about/qa/disease. htm On average each year: Ø Up to 20% of US get flu Ø More than 200, 000 hospitalized Ø Up to 49, 000 die

FLU: Everyone 6 months & older needs flu vaccine every year

Flu Vaccination Formulation: gets “MAKEOVER” each year Ø Trivalent vaccines will contain: l l Ø 2 A’s 1 B Quadrivalent vaccines: l l 2 A’s 2 B’s Ø Updated formulation usually announced in March

Look for 2016 -2017 update in MMWR

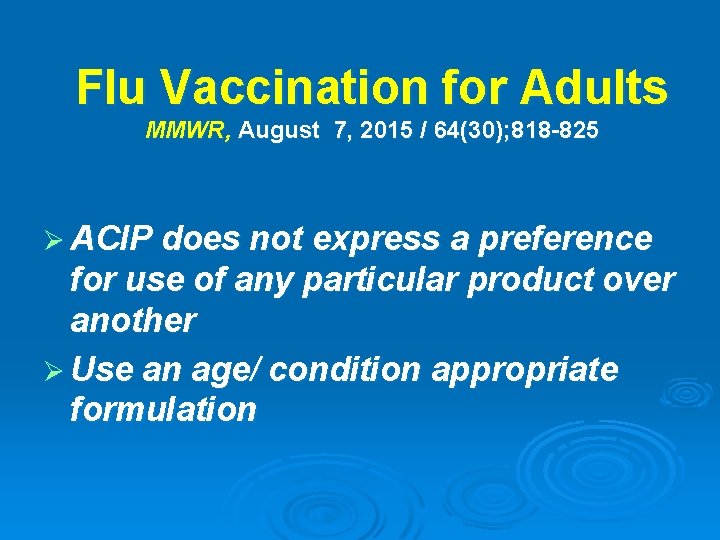
Flu Vaccination for Adults MMWR, August 7, 2015 / 64(30); 818 -825 Ø ACIP does not express a preference for use of any particular product over another Ø Use an age/ condition appropriate formulation

Flu Prevention: Lots of Choices Which flu vaccine to give? Ø Inactivated Flu Shot Ø Intradermal: 18 -64 Ø Needleless: Pharmajet- 18 -54 Ø High Dose: > 65 Ø Nasal Flu Vaccine: age 2 -49 & healthy (& not pregnant)

Efficacy of High-Dose versus Standard. Dose Influenza Vaccine in Older Adults NEJM 2014; 371: 635 -45 Ø 2 flu seasons / 26 centers (US & Canada) Ø 32, 000 adults, 65 & older l l 2011 -2012: low flu activity - good match between vaccine & circulating strains 2012 -2013: high flu activity - mismatch between vaccine & circulating strains Ø Results: High dose flu vaccine l l l Better antibody response, Less likely to get sick (lab confirmed flu) Relative efficacy 24. 2% over standard dose

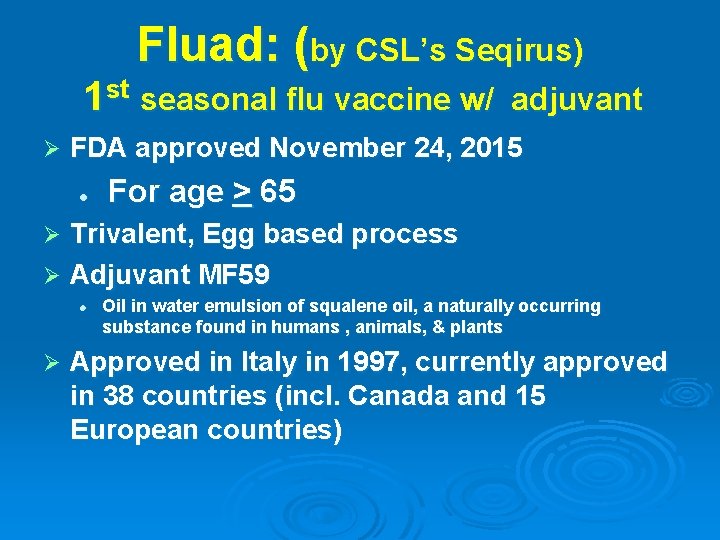
Fluad: (by CSL’s Seqirus) 1 st seasonal flu vaccine w/ Ø adjuvant FDA approved November 24, 2015 l For age > 65 Trivalent, Egg based process Ø Adjuvant MF 59 Ø l Ø Oil in water emulsion of squalene oil, a naturally occurring substance found in humans , animals, & plants Approved in Italy in 1997, currently approved in 38 countries (incl. Canada and 15 European countries)

Cell Culture based Inactivated Influenza Vaccine (cc. IIV 3, Flucelvax by Novartis) Trivalent Ø “Almost egg-free” Ø Ovalbumin no directly measured Ø Estimated to contain max of 5 x 10 (-8) mcg/0. 5 ml dose Ø ONLY for 18 and older Ø FDA approved November 20, 2012 Ø Almost egg free!

Recombinant Influenza Vaccine (RIV 3; Flublok) Ø Trivalent Ø Totally egg free Ø ONLY for 18 and older Ø FDA approved January 16, 2013 Ø (RIV 4 under development) Totally Egg - free!

MMWR, August 7, 2015 / 64(30); 818 -825

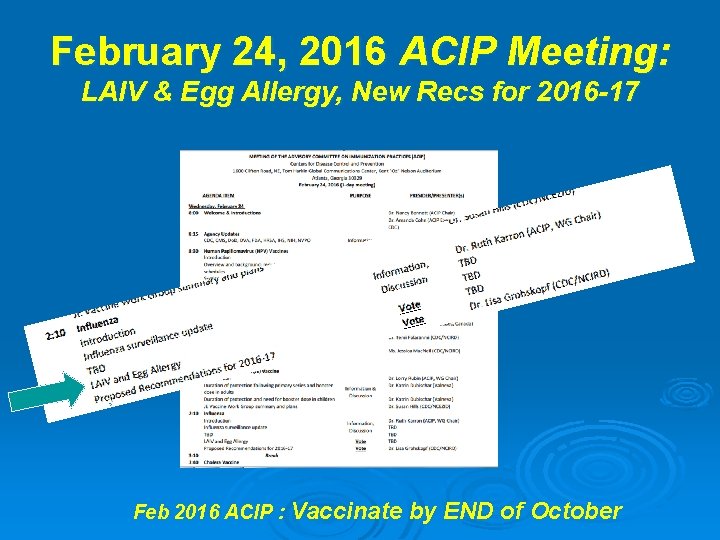
February 24, 2016 ACIP Meeting: LAIV & Egg Allergy, New Recs for 2016 -17 Feb 2016 ACIP : Vaccinate by END of October

Mild (hives only) Egg Allergy Ø Can get the inactivated flu shot (IIV). Ø l That’s what’s been studied. l They can’t get the nasal flu vaccine. Person giving the vaccine should l Be familiar with manifestations of “egg allergy” Ø Observe patient for at least 30 minutes (should observe 15 minutes after all vaccines) Ø May receive IIV, cc. IIV, RIV 3, AND LAIV (Feb 2016 ACIP)!

Recombinant Influenza Vaccine (RIV 3; Flublok) Trivalent Ø For 18 and older Ø Ø Totally egg free Ø Can be given to age appropriate with egg allergy of any severity Totally Egg - free!

More Severe Egg Allergy Ø Angioedema, respiratory distress, lightheadedness, or recurrent emesis Ø Have required epinephrine in past Ø May receive totally egg free. RIV 3 (Flublok) if age 18 or older Ø NEW Feb 2016 ACIP: may receive IIV or LAIV l “IN MEDICAL SETTING IN WHICH HEALTHCARE PROVIDER with experience in recognizing & managing severe allergic conditions IS IMMEDIATELY AVAILAVLE”

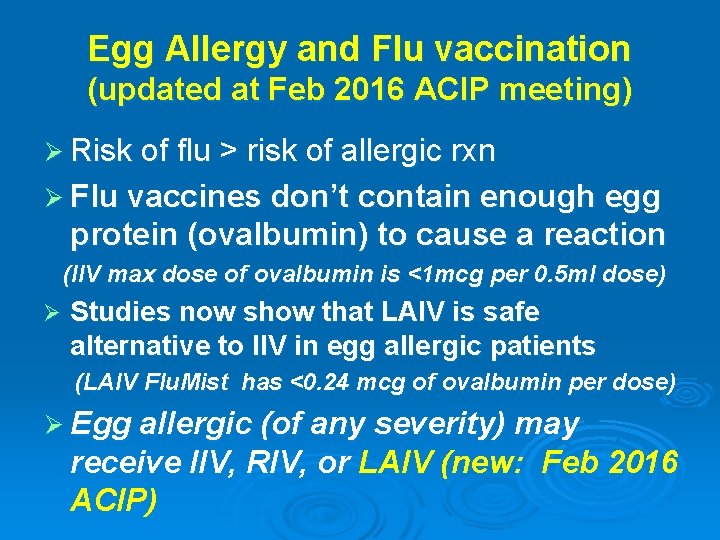
Egg Allergy and Flu vaccination (updated at Feb 2016 ACIP meeting) Ø Risk of flu > risk of allergic rxn Ø Flu vaccines don’t contain enough egg protein (ovalbumin) to cause a reaction (IIV max dose of ovalbumin is <1 mcg per 0. 5 ml dose) Ø Studies now show that LAIV is safe alternative to IIV in egg allergic patients (LAIV Flu. Mist has <0. 24 mcg of ovalbumin per dose) Ø Egg allergic (of any severity) may receive IIV, RIV, or LAIV (new: Feb 2016 ACIP)


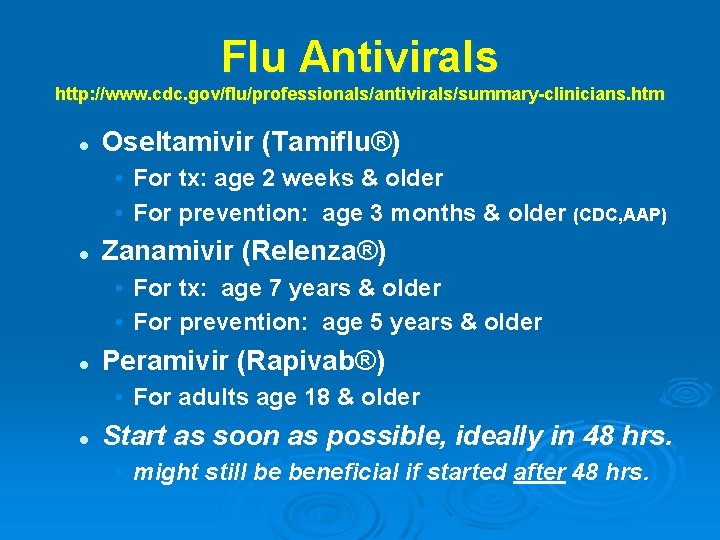
Flu Antivirals http: //www. cdc. gov/flu/professionals/antivirals/summary-clinicians. htm l Oseltamivir (Tamiflu®) • For tx: age 2 weeks & older • For prevention: age 3 months & older (CDC, AAP) l Zanamivir (Relenza®) • For tx: age 7 years & older • For prevention: age 5 years & older l Peramivir (Rapivab®) • For adults age 18 & older l Start as soon as possible, ideally in 48 hrs. • might still be beneficial if started after 48 hrs.

Tdap: A Family Affair (tetanus/diphtheria/pertussis) Ø Ø Pertussis = whooping cough Last 10 years - surge in pertussis related deaths in infants Cocoon in a circle of familial protection Household members are to blame for up to 83% of transmission l Past: moms; now: siblings

February 2012 ACIP Tdap for Adults: Universal Recs Ø Adults age 65 + had higher rates of hospitalization than those age 19 -64 Ø Expand Tdap booster to ALL adults age 65 and older –not just those with close contact with infants Ø Universal Tdap booster for ALL adults!

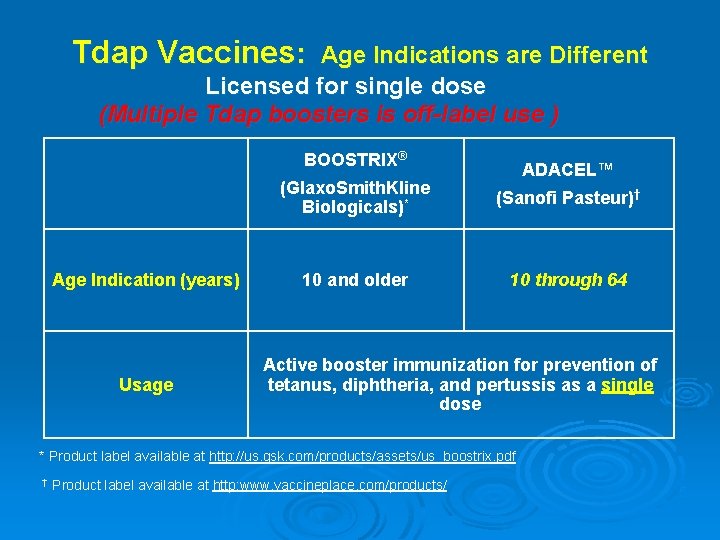
Tdap Vaccines: Age Indications are Different Licensed for single dose (Multiple Tdap boosters is off-label use ) BOOSTRIX® Age Indication (years) Usage ADACEL™ (Glaxo. Smith. Kline Biologicals)* (Sanofi Pasteur)† 10 and older 10 through 64 Active booster immunization for prevention of tetanus, diphtheria, and pertussis as a single dose * Product label available at http: //us. gsk. com/products/assets/us_boostrix. pdf † Product label available at http: www. vaccineplace. com/products/ 5

ACIP Guidance Statement MMWR, June 29, 2012 / 61(25); 468 -470 http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 6125 a 4. htm Ø Vaccinating with either product is preferable to a missing a vaccination opportunity Ø Currently, only a single booster dose of Tdap is recommended (except for pregnancy) Ø October 2012: Pregnant women need Tdap in EACH pregnancy (Multiple Tdap boosters is off label use )

HPV Related Cancers Ø Females: l Cervical, vulvar, and vaginal cancer Ø Males: l Penile cancer Ø Both l males and females: Anal cancer & oropharyngeal cancer

HPV Vaccines Ø 2 v HPV vaccine: l l l 16, 18 Bivalent Vaccine FDA approved only for females in 2009 Brand name: Cevarix (Glaxo. Smith. Kline) Ø 4 v HPV vaccine: 6, 11, 16, 18 l l l Quadrivalent Vaccine FDA approved in 2006: males & females Brand name: Gardasil (Merck ) Ø 9 v HPV (NEW Nine Valent) vaccine 6, 11, 16, 18 plus 31, 33, 45, 52, 58 l l FDA approved in Dec. 2014 for males & females Brand name: Gardasil 9 (Merck )

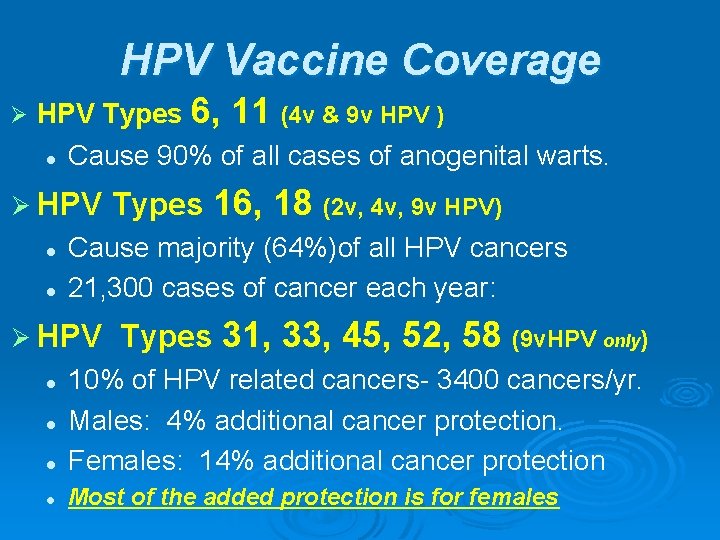
HPV Vaccine Coverage Ø HPV Types 6, l Cause 90% of all cases of anogenital warts. Ø HPV l l 11 (4 v & 9 v HPV ) Types 16, 18 (2 v, 4 v, 9 v HPV) Cause majority (64%)of all HPV cancers 21, 300 cases of cancer each year: Ø HPV Types 31, 33, 45, 52, 58 (9 v. HPV only) l 10% of HPV related cancers- 3400 cancers/yr. Males: 4% additional cancer protection. Females: 14% additional cancer protection l Most of the added protection is for females l l

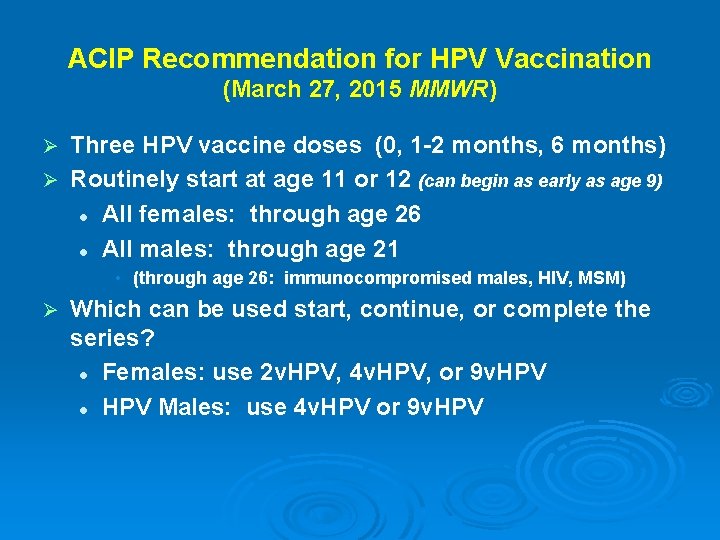
ACIP Recommendation for HPV Vaccination (March 27, 2015 MMWR) Three HPV vaccine doses (0, 1 -2 months, 6 months) Ø Routinely start at age 11 or 12 (can begin as early as age 9) l All females: through age 26 l All males: through age 21 Ø • (through age 26: immunocompromised males, HIV, MSM) Ø Which can be used start, continue, or complete the series? l Females: use 2 v. HPV, 4 v. HPV, or 9 v. HPV l HPV Males: use 4 v. HPV or 9 v. HPV

Guidance on ACIP Website: Additional HPV 9 Vaccine Doses Completing HPV vaccination http: //www. cdc. gov/vaccines/who/teens/downloads/9 v. HPV-guidance. pdf There is NO ACIP recommendation for routine additional 9 v. HPV vaccination for anyone that’s already completed 4 v. HPV or 2 v. HPV vaccination series. Ø No serious safety concerns giving 9 v. HPV after 3 dose 4 v. HPV series (but more injection site reactions) Ø Additional HPV 9 v protection: mostly limited to females (cervical cancers & pre-cancers) Ø

HPV Vaccine FAQ Ø Can you get HPV infection from getting the vaccine? l No, the vaccine does not contain any viral DNA so there is no way to become infected with the virus by getting the vaccine. Ø Do you have to do pregnancy test before giving vaccine? l No, but neither vaccine should be given to women who are pregnant or are planning to get pregnant soon.

HPV Vaccine FAQ Ø Can you get HPV vaccine while nursing? l Yes, ACIP says lactating women can receive HPV vaccine. Ø Can the HPV vaccine be used to treat abnormal pap smear? l NO. They are not meant to be a treatment for HPV infection or HPV related disease.

HPV vaccine FAQ Ø Do you still have to get cervical cancer screening if you get HPV vaccine? l Yes. HPV Vaccines are prophylactic vaccines. They work best if given before exposure to HPV virus. They are not meant to be a treatment for HPV infection or HPV related disease. Women must still get regular cervical cancer screening. (Begin at age 21)

The Big 5: Hepatitis B Hep B Facts Ø Chronic Infection l l l 800, 000 to 1. 4 million people suffer from it 3000 cases of acute Hepatitis B each year Can lead to liver cancer

The Big 5: Hepatitis B Ø Transmitted by exposure to infected blood or body fluid Ø Who needs a Hep B vaccine ? Ø Hepatitis B series (3 dose) : l l l All health care workers All diabetics < 60; > 1 sex partner over last 6 months MSM And more…Check the schedule!

The Big 5: Shingles Facts Ø Ø Ø If had chickenpox…at risk for shingles More that 90% of all adults in the United States infected with varicella zoster virus One million cases/yr. Lifetime risk: 30% Risk increases with age (starting at age 50)

Key HZ Symptom: Pain Ø Pain prior to rash onset: 84% of cases § Starts as abnormal skin sensation, itching or tingling § Precedes rash by 1 -5 days but occasionally weeks or more § Diagnostic dilemmas & work-ups (e. g. , cardiac, gallbladder) Ø Pain once rash develops: 89% of cases

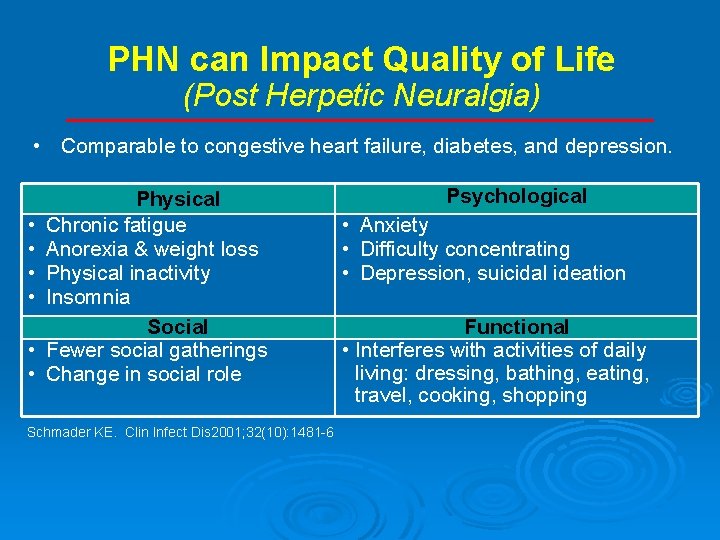
PHN can Impact Quality of Life (Post Herpetic Neuralgia) • Comparable to congestive heart failure, diabetes, and depression. • • • Physical Chronic fatigue Anorexia & weight loss Physical inactivity Insomnia Social Fewer social gatherings Change in social role Schmader KE. Clin Infect Dis 2001; 32(10): 1481 -6 Psychological • Anxiety • Difficulty concentrating • Depression, suicidal ideation Functional • Interferes with activities of daily living: dressing, bathing, eating, travel, cooking, shopping

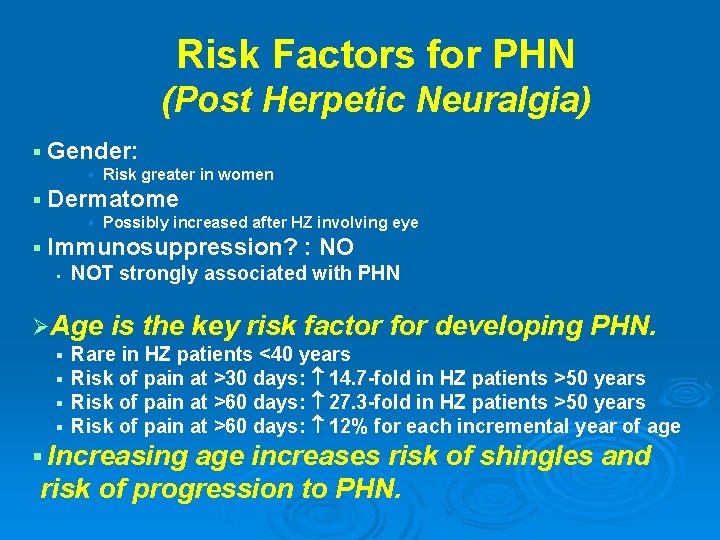
Risk Factors for PHN (Post Herpetic Neuralgia) § Gender: § § Dermatome § § Risk greater in women Possibly increased after HZ involving eye Immunosuppression? : NO § NOT strongly associated with PHN ØAge § § is the key risk factor for developing PHN. Rare in HZ patients <40 years Risk of pain at >30 days: 14. 7 -fold in HZ patients >50 years Risk of pain at >60 days: 27. 3 -fold in HZ patients >50 years Risk of pain at >60 days: 12% for each incremental year of age § Increasing age increases risk of shingles and risk of progression to PHN.

Live Attenuated Virus Vaccine

Shingles Vaccine (varicella zoster vaccine) (brand name Zostavax , by Merck) ØThe Shingles Prevention Study NEJM June 2, 2005 Ø 38, 500+ patients 60 and older ØVaccine Effectiveness: • Reduced incidence shingles by 51% • Reduced incidence of PHN by 66. 5% l Risk of PHN (post herpetic neuralgia) increases after age 50.

Shingles vaccine: Who should get it? For prevention of shingles May 2006 - FDA approved: Age 60 & older l October 2006: ACIP recommends dose at age 60+ Ø March 2011 - FDA approved- expanded to 50 -59 l Works even better in this younger age group Ø • Reduced shingles risk by nearly 70% (69. 8%) Vaccine supply problems: l June 2011: ACIP still recommends start at age 60+ Ø Vaccine supply stabilized l Concerns that those vaccinated at younger age (50 -60) might not be protected at older ages when the risk of severe disease is higher Ø ACIP says: Start vaccinating at age 60+ Ø

Shingles Vaccine: ACIP Recommendations Ø You don’t have to check varicella history or titers before administering HZV l l l Just about everyone ≥ 60 has serologic evidence of prior varicella, even if they do not recall having the illness No evidence that giving Shingles vaccine to someone without prior varicella raises safety concerns Determining varicella history: a major and unnecessary barrier to vaccination

Varicella-Zoster: How does Shingles vaccine differ from the Chickenpox vaccine? Ø Chickenpox vaccine - Varivax l FDA approved in March 1995 Ø Shingles vaccine - Zostavax l FDA approved May 2006 Ø Both are made from Oka Merck strain of live attenuated varicella zoster virus. Ø Shingles vaccine (Zostavax) is about 10 times stronger.

Chickenpox vaccine (Varivax) 2 doses, at least 4 weeks apart Generally: If born in U. S. before 1980, you are considered to be “immune” Ø This does not apply to health-care personnel and pregnant women Ø l l Ø Birth before 1980 should not be considered evidence of immunity Check the footnotes on the adult schedule for further details. Target new mothers and women of child bearing potential

Shingles (Herpes Zoster) Vaccine : Storage & Handling Ø Must be stored frozen l l Ø Need dedicated freezer only: combined fridge/freezer models may not work l Ø Must be stored at freezer temperature (≤ 5º F) HZV is the ONLY freezer-requiring vaccine for adults Can reduce fridge temps below freezing and ruin refrigerated vaccines Unused HZV must be discarded 30 min after reconstitution

Who Should NOT Get it? (Shingles Vaccine) Ø Live attenuated virus vaccine Ø It should NOT be given to l l l People with immune system problems Women who are or may be pregnant Anyone allergic to vaccine components including gelatin, neomycin Ø Contraindicated in those with immune system problems including patients on high dose steroids (20 mg or higher daily)

HEADS UP: Investigational Subunit Adjuvanted Shingles Vaccine (HZ/su) NEJM 2015; 372: 2149 -50 Ø Randomized, placebo-controlled phase 3 study l l Ø More than 15, 000 patients, age 50 and older Conducted in 18 countries Study indicated vaccine efficacy of 97. 2% More injection site & systemic reactions as compared to placebo Ø Duration of protection? So far- mean follow up 3. 2 yrs Ø Trial underway to compare with currently available live attenuated herpes zoster vaccine l

The Big 5: Pneumococcal Vaccine Facts about Streptococcus pneumonia (aka the pneumococcus) Kills 4000 in US each year (mostly adults) Ø Leading cause of serious illness: Ø bacteremia, meningitis, pneumonia Ø Source: MMWR Oct 12, 2012 , 816 -819

Two Pneumococcal Vaccines: FDA Approved for Adults Ø Pneumococcal Polysaccharide vaccine (PPSV 23 - Pneumovax 23 by Merck) l Ø Licensed for routine use in adults 50 & older and age 2– 49 with certain risk factors Pneumococcal Conjugate vaccine (PCV 13 Prevnar by Pfizer) l FDA approved for use in adults age 50 and older in December 2011 NOTE: Prevnar 13 is NOT FDA approved for age 18 to 49!

Invasive Pneumococcal Disease: Risk is increased in immunocompromised adults MMWR Oct 12, 2012 , 816 -819. Ø **Risk in immunocompromised -20 x than for those without high risk conditions** • June 2012: ACIP recommended routine PCV 13 conjugate for immunocompromised adults: (off label use - not FDA approved for adults < 50) This recommendation applies to l Immunocompromised , asplenic l “High risk” immunocompetent (CSF leaks / cochlear implants) Ø **Risk of invasive disease in older adults is 10 times higher than in younger adults **

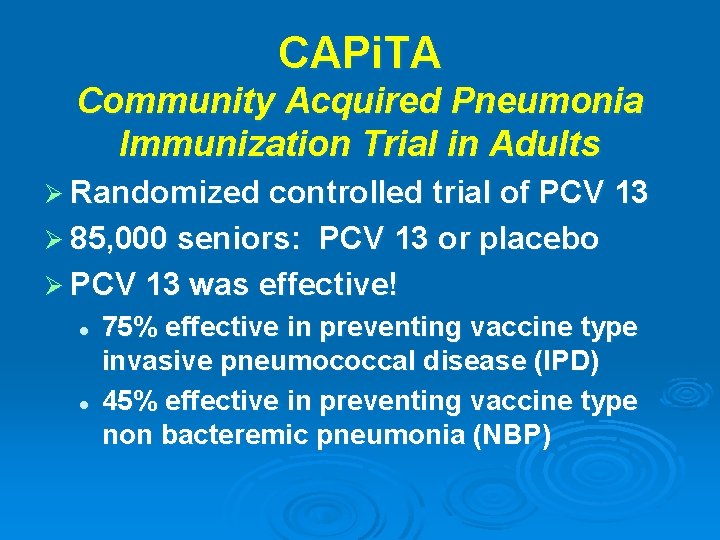
CAPi. TA Community Acquired Pneumonia Immunization Trial in Adults Ø Randomized controlled trial of PCV 13 Ø 85, 000 seniors: PCV 13 or placebo Ø PCV 13 was effective! l l 75% effective in preventing vaccine type invasive pneumococcal disease (IPD) 45% effective in preventing vaccine type non bacteremic pneumonia (NBP)

Emergency ACIP Meeting on August 13, 2014 Ø Purpose: vote on routine use for PCV 13 for all seniors Ø VOTE: 13 to 2 - in favor of Routine PCV 13 vaccination for all age 65 /+ (in addition to PPSV 23) Ø Based on strong quality evidence

Pneumococcal Vaccination: “Ground” RULES Ø PCV 13 & PPSV 23 should not be given at same visit. Ø If • need both, best to give PCV 13 first. Only single dose PCV 13 is recommended for adults. Ø Only one PPSV 23 dose at / after age 65

Pneumococcal Vaccination: “Intervals” -- depends on condition Ø PCV 13 l l l then PPSV 23 (best!)******* Adults (all ages, 19 & +): Immunocompromised or HIGH RISK Immunocompetent (CSF leaks, cochlear implants) • Interval - 8 weeks Healthy, 65 & older: interval - at least 1 year Ø If PPSV 23 l Ø is given before PCV 13 Interval - at least a year When needs revaccinate: PPSV 23 then PPSV 23) l Interval – 5 years

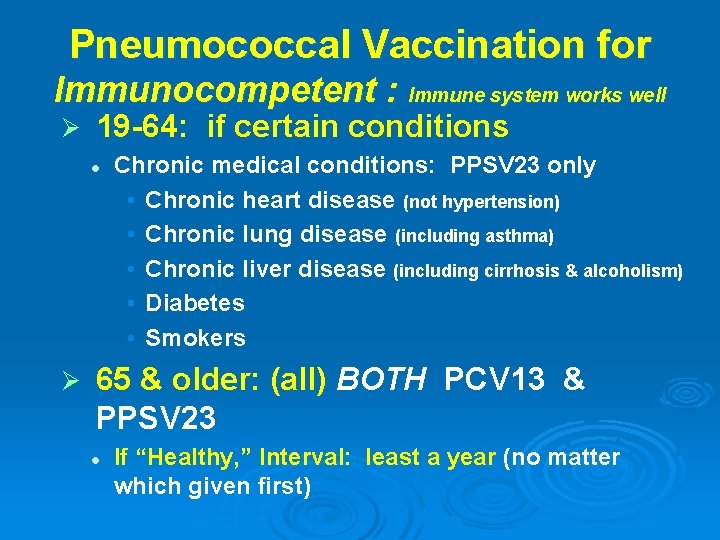
Pneumococcal Vaccination for Immunocompetent : Immune system works well Ø 19 -64: if certain conditions l Ø Chronic medical conditions: PPSV 23 only • Chronic heart disease (not hypertension) • Chronic lung disease (including asthma) • Chronic liver disease (including cirrhosis & alcoholism) • Diabetes • Smokers 65 & older: (all) BOTH PCV 13 & PPSV 23 l If “Healthy, ” Interval: least a year (no matter which given first)

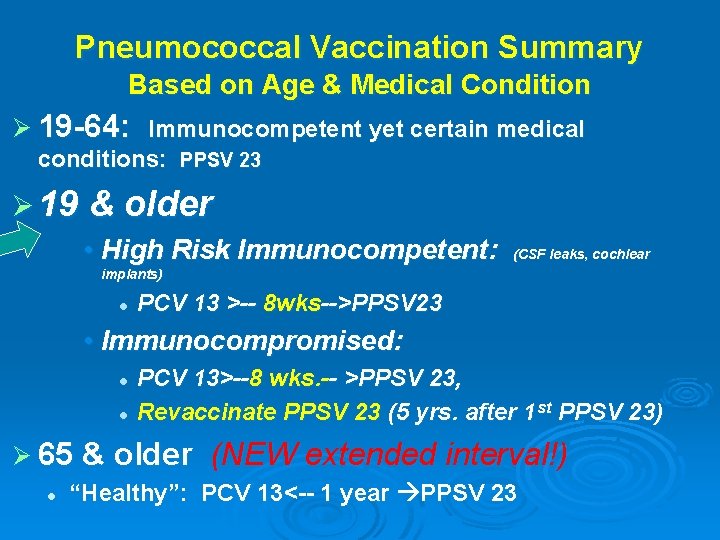
Pneumococcal Vaccination Summary Based on Age & Medical Condition Ø 19 -64: Immunocompetent yet certain medical conditions: PPSV 23 Ø 19 & older • High Risk Immunocompetent: (CSF leaks, cochlear implants) l PCV 13 >-- 8 wks-->PPSV 23 • Immunocompromised: l l PCV 13>--8 wks. -- >PPSV 23, Revaccinate PPSV 23 (5 yrs. after 1 st PPSV 23) Ø 65 & older l (NEW extended interval!) “Healthy”: PCV 13<-- 1 year PPSV 23

Pneumococcal Vaccination for Immunocompromised Ø Immunocompromising l conditions: Congenital or acquired immunodeficiency , HIV • (B cell, T cell, complement deficiencies, phagocytic disorders except chronic granulomatous disease) l l Ø Chronic renal failure/ nephrotic syndrome Leukemia, lymphoma, Hodgkin’s, generalized malignancy, multiple myeloma Solid organ transplant Iatrogenic immunosuppression( long term steroids or radiation therapy) Asplenia (functional or anatomic, incl. sickle cell) Need PCV 13 then PPSV 23: Interval 8 wks. Ø Also need PPSV 23 revaccinate, 5 years after last Ø PPSV 23

When Previously PPSV 23 Vaccinated Patients Reach Age 65: l One time PPSV 23 revaccination: • If more than five years have passed since the last vaccination & patient < 65 at time of primary vaccination.

Pneumococcal Vaccination ACIP Recs & Medicare Part B coverage: “Some” HARMONY Source: http: //www. medicare. gov/coverage/pneumococcal-shots. html Medicare Part B (Medical Insurance) Ø Covers a pneumococcal shot to prevent pneumococcal infections Ø Also covers a different second shot 11 months after the first shot. ACIP Recs (June 2015): (“healthy”) 65 & older Ø PCV 13………at least a year…. . PPSV 23 Ø PPSV 23…… at least a year…. PCV 13

Medicare Coverage Donut Hole for 2 nd Pneumococcal Vaccine for Immunocompromised & High Risk Immunocompetent (ACIP rec interval = 8 weeks) Medicare will only cover if 11 months apart

Meningococcal Disease FACTS Each year: estimated 1, 400 -2, 800 cases occur in the United States. Ø 10%-14% of cases are fatal. Of patients who recover : Ø 11%-19% have permanent hearing loss, mental retardation, loss of limbs, or other serious sequelae. Ø Five main serogroups of Neisseria meningitidis : A, B, C, Y, and W Ø Types B, C, Y are major causes of disease in US. Ø

Meningococcal vaccine: Adult Immunization Schedule 2012 Ø “First-year college students up through age 21 years who are living in residence halls should be vaccinated if they have not received a dose on or after their 16 th birthday. ” Ø Previously Licensed Meningococcal Vaccines cover A, C, W-135, Y l l Polysaccharide vaccine: Menomune [MPSV 4] Conjugate vaccines: Menactra [Men. ACWY-D] Menveo [Men. ACWY]) --None of them cover serotype B--

Meningococcal B Facts Men B causes half of all cases of meningococcal disease in those age 17 -22 Ø Each year, about 55 -65 young people age 1624 get sick with Men B Ø Strikes quickly, unforgiving, often deadly. Ø Men B NOT limited to college campuses l About 30 -60% of cases occur in young people NOT in college Ø

Men B Vaccines (FDA approved in 2014) Ø Men. B-FHbp: brand name Trumenba (Pfizer) l Ø Men. B-4 C l Ø Three-dose series (1, 2, 6 months) brand name Bexsero (Novartis) Two-dose series (0, 1 months) Both are FDA approved only for those age 10 -25.

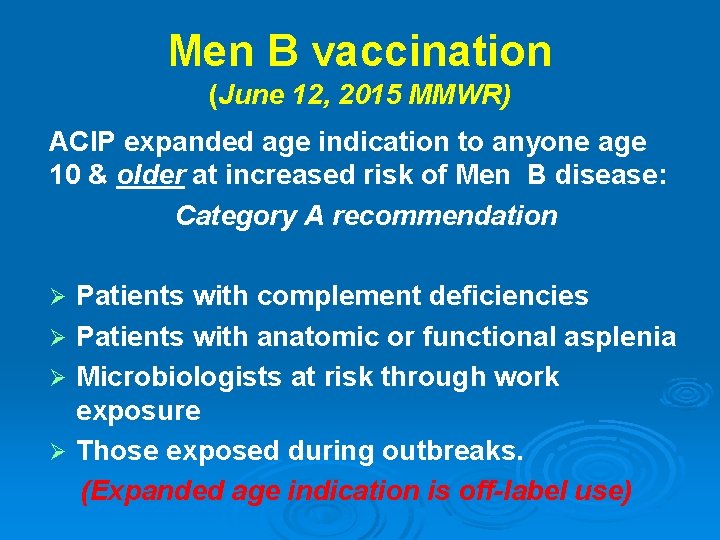
Men B vaccination (June 12, 2015 MMWR) ACIP expanded age indication to anyone age 10 & older at increased risk of Men B disease: Category A recommendation Patients with complement deficiencies Ø Patients with anatomic or functional asplenia Ø Microbiologists at risk through work exposure Ø Those exposed during outbreaks. (Expanded age indication is off-label use) Ø

NEW ACIP Recommendation for Men B Vaccination (June 24, 2015) Category B recommendation Ø “May” be administered to age 16 -23 l Preferred vaccination age range: 16 -18 Ø Either l l vaccine product may be used The same product should be used for all doses in the series. No Men B boosters (after initial series) recommended at this time

Measles is very contagious! Ø If one person has it, 90% of the people close to that person - who are not immune - will also become infected. Measles Death: July 2, 2015

MMR Vaccine ØMeasles ØMumps ØRubella Ø If unsure if you’re immune: l l Get antibody titers checked or Get the shot!

Big 5 Itinerary: ACIP Adult Immunization Schedule

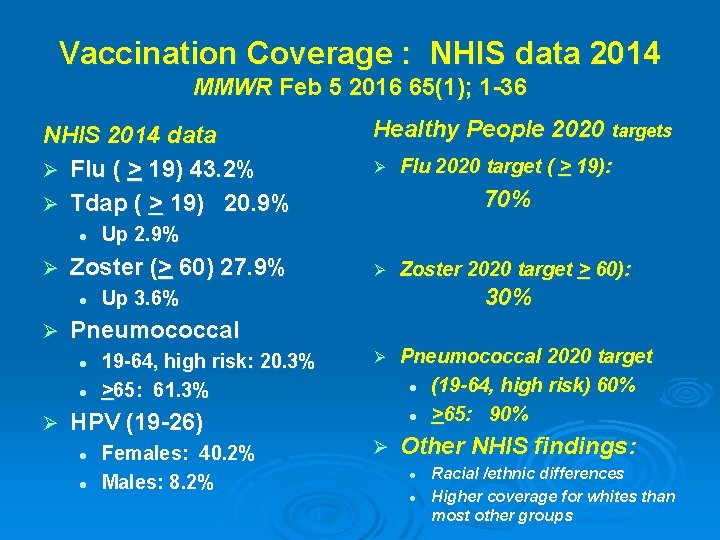
Vaccination Coverage : NHIS data 2014 MMWR Feb 5 2016 65(1); 1 -36 NHIS 2014 data Ø Flu ( > 19) 43. 2% Ø Tdap ( > 19) 20. 9% l Ø Ø Flu 2020 target ( > 19): 70% Ø Zoster 2020 target > 60): 30% Up 3. 6% Pneumococcal l l Ø Ø Up 2. 9% Zoster (> 60) 27. 9% l Healthy People 2020 targets 19 -64, high risk: 20. 3% >65: 61. 3% Ø Pneumococcal 2020 target l (19 -64, high risk) 60% l >65: 90% Ø Other NHIS findings: HPV (19 -26) l l Females: 40. 2% Males: 8. 2% l l Racial /ethnic differences Higher coverage for whites than most other groups

Remember “Vaccines are not just for kids…… Adults need them, too. ”

ICD-10 Codes for Immunizations Z 23

? ? Questions ? ? THE END