Immunization Recommendation Updates SEMIC Immunization Conference April 27












![HPV Coverage in Minnesota 90 80 [VALUE]% 70 60 [VALUE]% 50 40 30 20 HPV Coverage in Minnesota 90 80 [VALUE]% 70 60 [VALUE]% 50 40 30 20](https://slidetodoc.com/presentation_image/d6e1b82e5714f50d6a156a4586c25ae3/image-25.jpg)

- Slides: 26

Immunization Recommendation Updates SEMIC Immunization Conference April 27, 2017

Disclosures • No conflicts of interest • Will discuss off-label use Tdap Meningococcal B vaccine

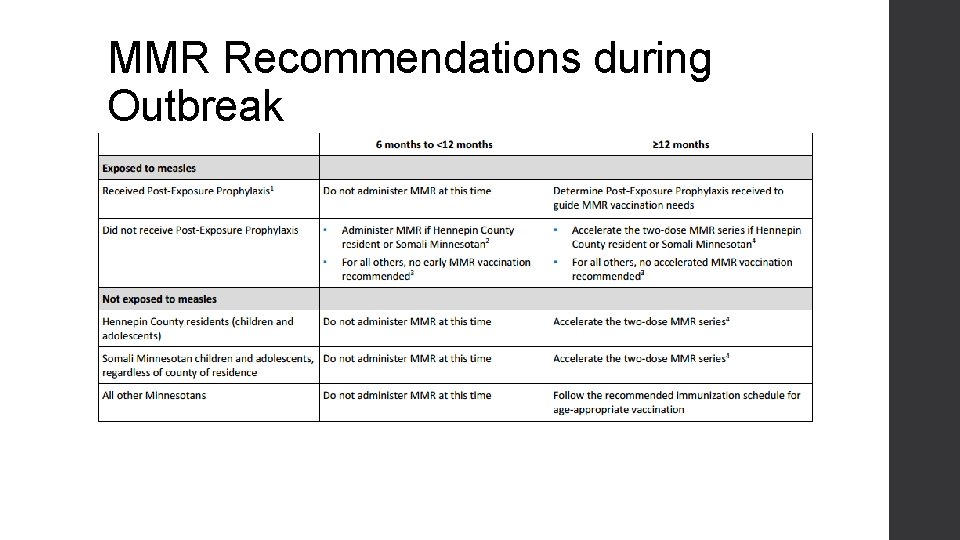
Measles 2017 • Case count: 26 25/26 had 0 MMR Between ages 0 -5 25 cases in Hennepin, 1 in Stearns • Index case unknown • High number of children exposed In emergency departments and urgent care In childcare settings • Hennepin Co. and MDH Exposure notification and exclusion recommendations Case investigation Community outreach

MMR Recommendations during Outbreak

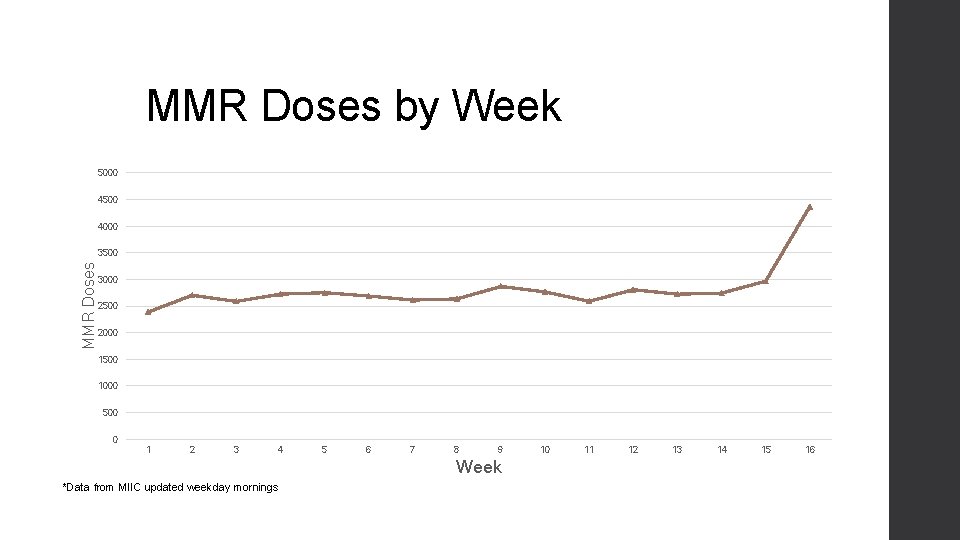
MMR Doses by Week 5000 4500 4000 MMR Doses 3500 3000 2500 2000 1500 1000 500 0 1 2 3 4 5 6 7 8 9 Week *Data from MIIC updated weekday mornings 10 11 12 13 14 15 16

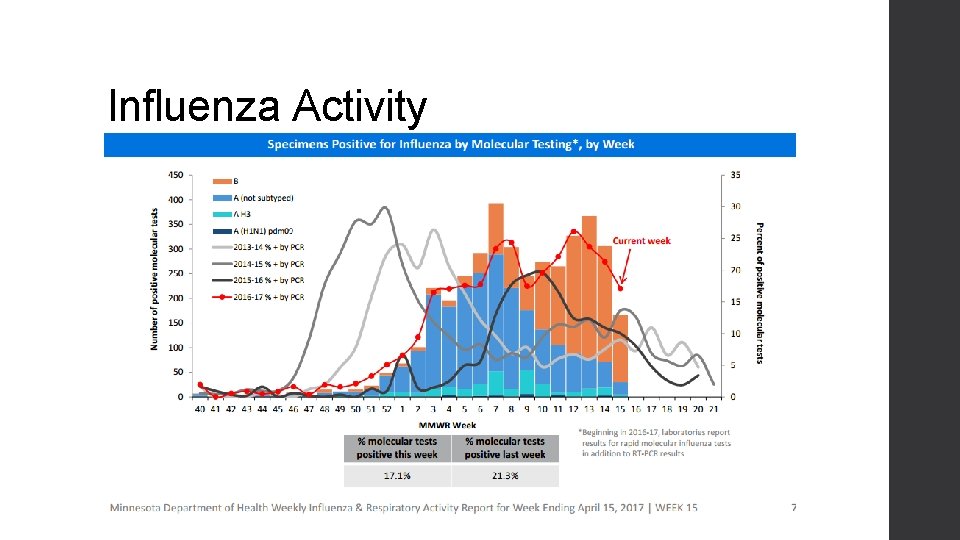
Influenza Activity

Influenza Mid-season Vaccine Effectiveness (VE) Overall: 48% (CI 37 -57%) Influenza A H 3 N 2 43% (CI 29 -54%) VE Influenza B 73% (54 -84%) VE • VE varies by age group • ACIP and CDC continue to explore why VE is lower for H 3 N 2 comparatively MMWR 2017 (RR-6); 66: 167– 171.

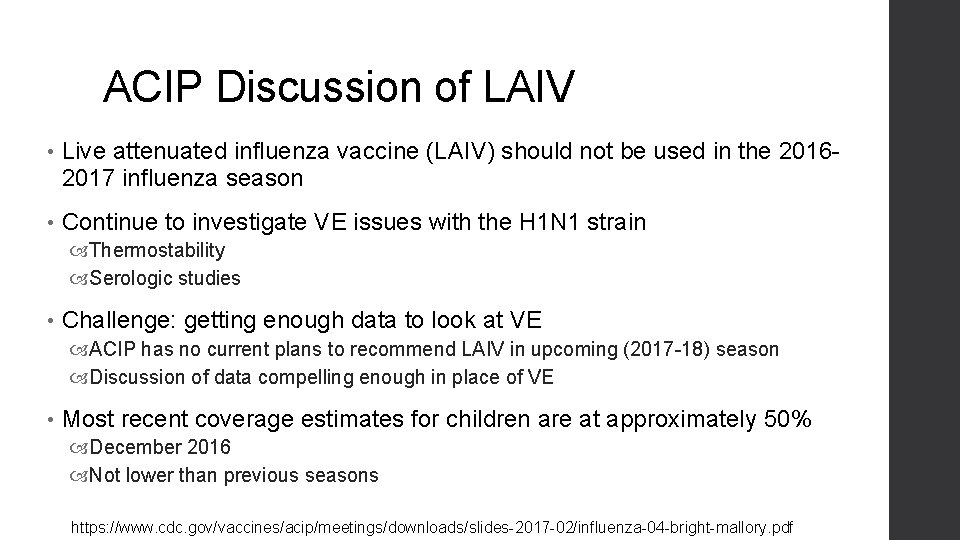
ACIP Discussion of LAIV • Live attenuated influenza vaccine (LAIV) should not be used in the 20162017 influenza season • Continue to investigate VE issues with the H 1 N 1 strain Thermostability Serologic studies • Challenge: getting enough data to look at VE ACIP has no current plans to recommend LAIV in upcoming (2017 -18) season Discussion of data compelling enough in place of VE • Most recent coverage estimates for children are at approximately 50% December 2016 Not lower than previous seasons https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2017 -02/influenza-04 -bright-mallory. pdf

Egg allergy and Influenza Vaccine • Residual egg protein in flu vaccine is not likely to induce an allergic reaction, even in severely allergic people The amount of egg protein is actually undetectable (less than 0. 5 µg) and it is thought that at least 130 µg is needed for a reaction to occur • Anaphylaxis can occur with any vaccine, to any component • Data is reassuring and compelling • 2700 published studies involving more than 4100 allergic subjects, including known anaphylaxis to egg ingestion Received influenza vaccination without serious reactions, including respiratory distress or hypotension Minor reactions such as hives, mild wheezing, but seen equally among non-egg allergic controls MMWR 2016; 65(RR-5): 29 -30

Vaccinating Egg Allergic Patients Risk of anaphylaxis due to egg allergy small compared to risk of hospitalization and death due to influenza • People experiencing reactions to egg of any severity (hives to angioedema) may receive any licensed and recommended influenza vaccine (i. e. IIV, RIV) that is otherwise appropriate for the recipient’s age and health status. • Vaccine administration should be supervised by a health care provider who is able to recognize and manage severe allergic conditions. • A previous severe allergic reaction to influenza vaccine, regardless of the component suspected of being responsible for the reaction, is a contraindication to future receipt of the vaccine. MMWR 2016; 65(RR-5): 29 -30

Hepatitis B Strengthening the safety net: • Monovalent Hepatitis B vaccine should be administered within 24 h of birth for medically stable infants weighing ≥ 2000 g born to HBs. Agnegative mothers. • Preterm infants weighing <2000 g born to HBs. Ag-negative mothers should receive the first dose of vaccine 1 month after birth of at hospital discharge. • A new statement will be published to incorporate recommendations for specific populations and permissive language will be removed. https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2016 -10/hepatitis-02 -schillie-october-2016. pdf

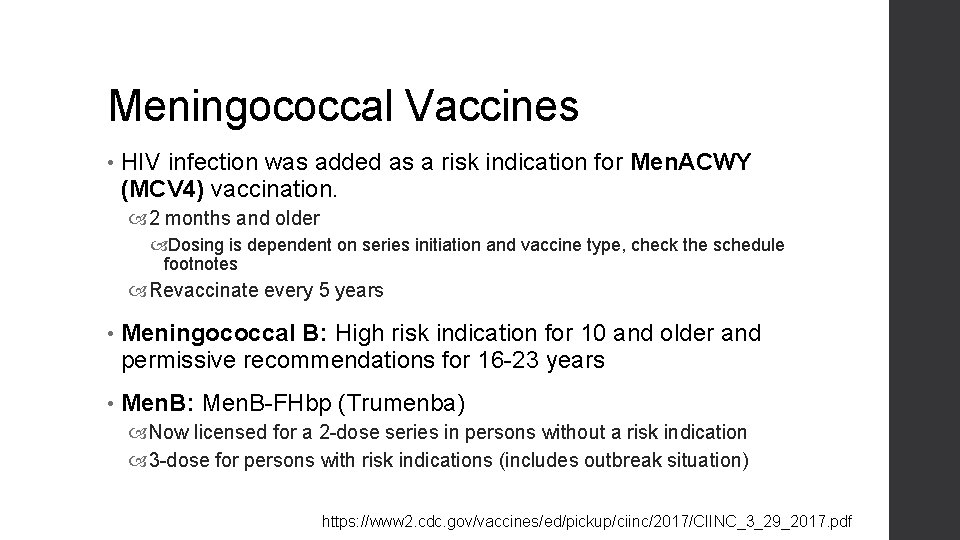
Meningococcal Vaccines • HIV infection was added as a risk indication for Men. ACWY (MCV 4) vaccination. 2 months and older Dosing is dependent on series initiation and vaccine type, check the schedule footnotes Revaccinate every 5 years • Meningococcal B: High risk indication for 10 and older and permissive recommendations for 16 -23 years • Men. B: Men. B-FHbp (Trumenba) Now licensed for a 2 -dose series in persons without a risk indication 3 -dose for persons with risk indications (includes outbreak situation) https: //www 2. cdc. gov/vaccines/ed/pickup/ciinc/2017/CIINC_3_29_2017. pdf

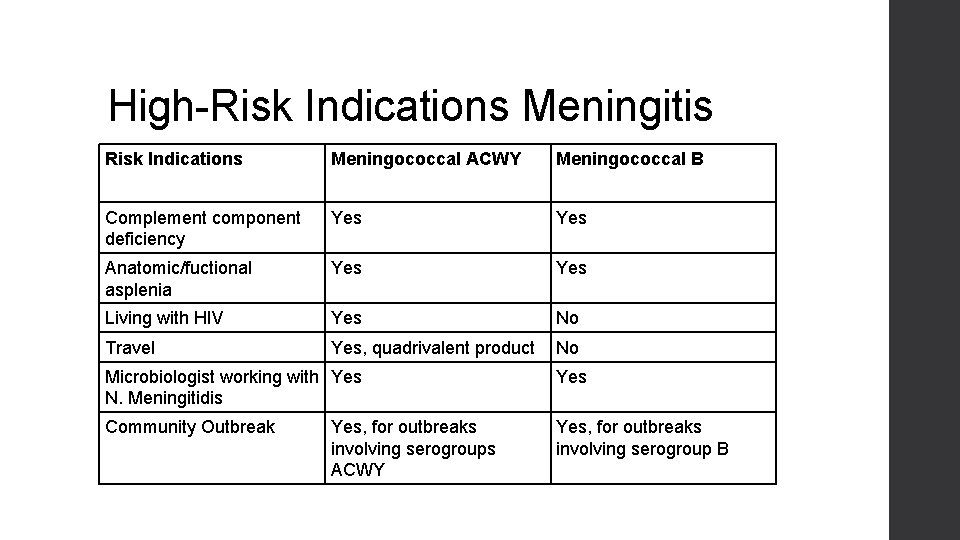
High-Risk Indications Meningitis Risk Indications Meningococcal ACWY Meningococcal B Complement component deficiency Yes Anatomic/fuctional asplenia Yes Living with HIV Yes No Travel Yes, quadrivalent product No Microbiologist working with Yes N. Meningitidis Yes Community Outbreak Yes, for outbreaks involving serogroup B Yes, for outbreaks involving serogroups ACWY


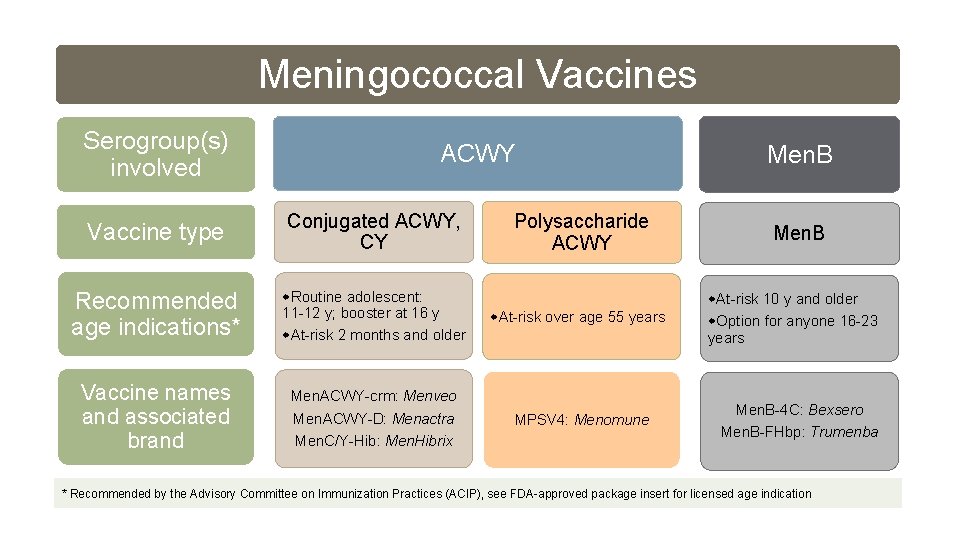
Meningococcal Vaccines Serogroup(s) involved ACWY Vaccine type Conjugated ACWY, CY Recommended age indications* Routine adolescent: 11 -12 y; booster at 16 y At-risk 2 months and older At-risk over age 55 years Vaccine names and associated brand Men. ACWY-crm: Menveo Men. ACWY-D: Menactra Men. C/Y-Hib: Men. Hibrix MPSV 4: Menomune Polysaccharide ACWY Men. B At-risk 10 y and older Option for anyone 16 -23 years Men. B-4 C: Bexsero Men. B-FHbp: Trumenba * Recommended by the Advisory Committee on Immunization Practices (ACIP), see FDA-approved package insert for licensed age indication

Tetanus, Diphtheria and Pertussis (Tdap) • For persons aged 7 through 10 years who receive a dose of Tdap as part of the catch-up series, an adolescent Tdap vaccine dose may be given at age 11 through 12 years In line with guidance of children for which Tdap is inadvertently administered https: //www 2. cdc. gov/vaccines/ed/pickup/ciinc/2017/CIINC_3_29_2017. pdf

Tdap Vaccination in Pregnancy • Maternal vaccination strategy is very promising! Data presented to ACIP show 78 -91% efficacy Large study recently published: 90%+ in first 2 months of life (Baxter et al, 2017) • Tdap should be given in the earlier part of the 27 -36 weeks gestation period for vaccination of pregnant women. Evidence suggests that this timeframe allows for greater maternal pertussis antibody transfer. Data presented that show higher serological biomarkers in infant cord-blood May suggest longer exposure may be more important than peak transfer • Safety data continue to be reassuring, including for repeated doses https: //www 2. cdc. gov/vaccines/ed/pickup/ciinc/2017/CIINC_3_29_2017. pdf and https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2016 -10/pertussis-02 -liang. pdf

Polio • MMWRs published 1/13/17 and 2/17/17 provide additional guidance regarding assessment of poliovirus vaccination status and vaccination of children who have received poliovirus vaccine outside the U. S. If both OPV and IPV were administered as part of a series, the total number of doses needed to complete the series is the same as that recommended for the U. S. IPV schedule. A minimum interval of 4 weeks should separate doses in the series, with the final dose administered on or after the fourth birthday and at least 6 months after the previous dose. If only OPV was administered, and all doses were given before age 4 years, 1 dose of IPV should be given at 4 years or older and at least 6 months after the last OPV dose. Only trivalent OPV (t. OPV) counts toward the U. S. vaccination requirements https: //www 2. cdc. gov/vaccines/ed/pickup/ciinc/2017/CIINC_3_29_2017. pdf

Human Papilloma Virus (HPV) Recommendation for 2 Doses • First recommended for girls 11 -12 years in 2006 • Routinely recommended for boys 11 -12 years in 2011 • 9 valent vaccine licensed in 2015 • Move from 3 to 2 doses: Immunogenicity Post-hoc analysis of efficacy trials Post licensure effectiveness Health economic models Duration of protection

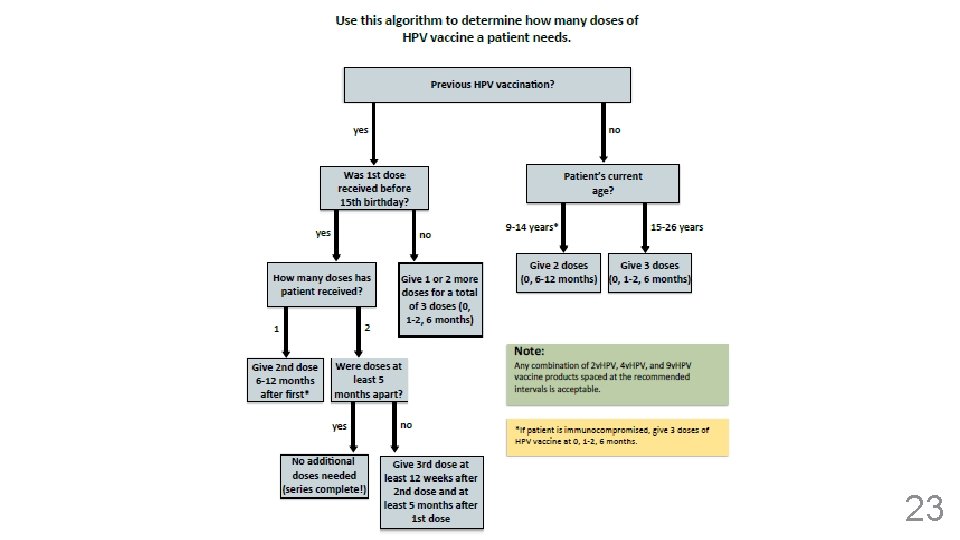
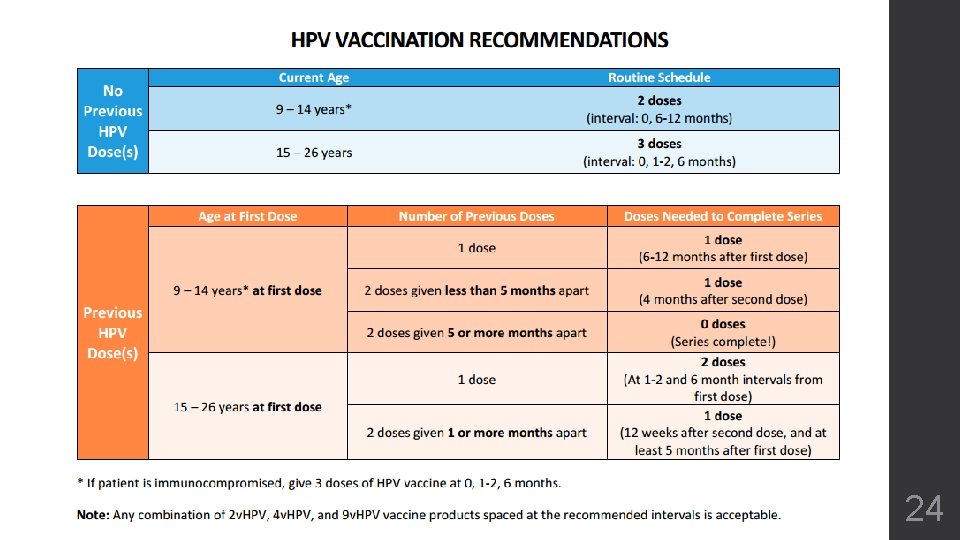
HPV Vaccine: Basics 9 v. HPV vaccine was approved for a 2 -dose series (0, 6 months). 2 doses now recommended for: - Healthy adolescents beginning the series before 15 th birthday - Adolescents who have begun series but not completed three doses: - Series was started before age 15 and doses were at least 5 months apart 3 doses recommended for: - Adolescents/young adults beginning the series after 15 th birthday - Adolescents with an immunocompromising condition Any combination of 2 v. HPV, 4 v. HPV, or 9 v. HPV is acceptable for both the 2 -dose and 3 -dose series. 20

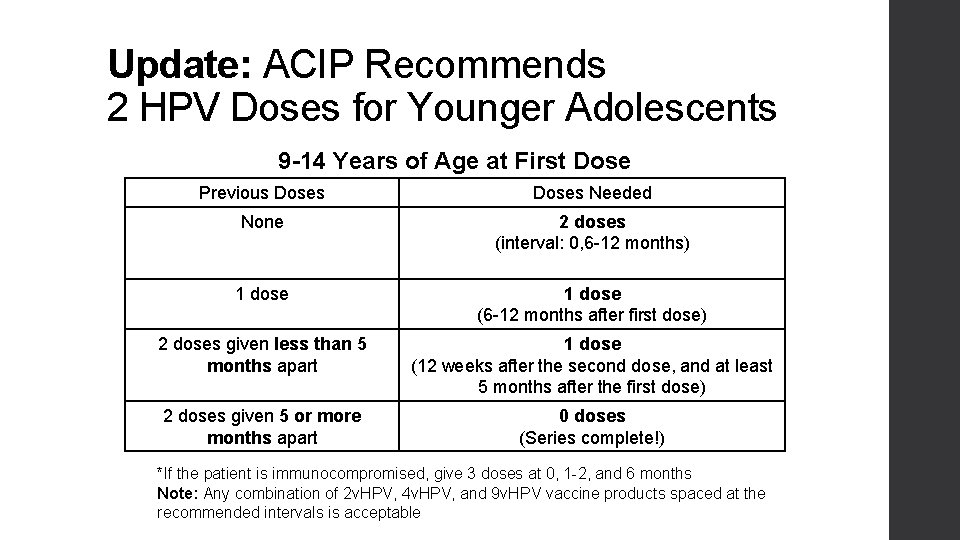
Update: ACIP Recommends 2 HPV Doses for Younger Adolescents 9 -14 Years of Age at First Dose Previous Doses Needed None 2 doses (interval: 0, 6 -12 months) 1 dose (6 -12 months after first dose) 2 doses given less than 5 months apart 1 dose (12 weeks after the second dose, and at least 5 months after the first dose) 2 doses given 5 or more months apart 0 doses (Series complete!) *If the patient is immunocompromised, give 3 doses at 0, 1 -2, and 6 months Note: Any combination of 2 v. HPV, 4 v. HPV, and 9 v. HPV vaccine products spaced at the recommended intervals is acceptable

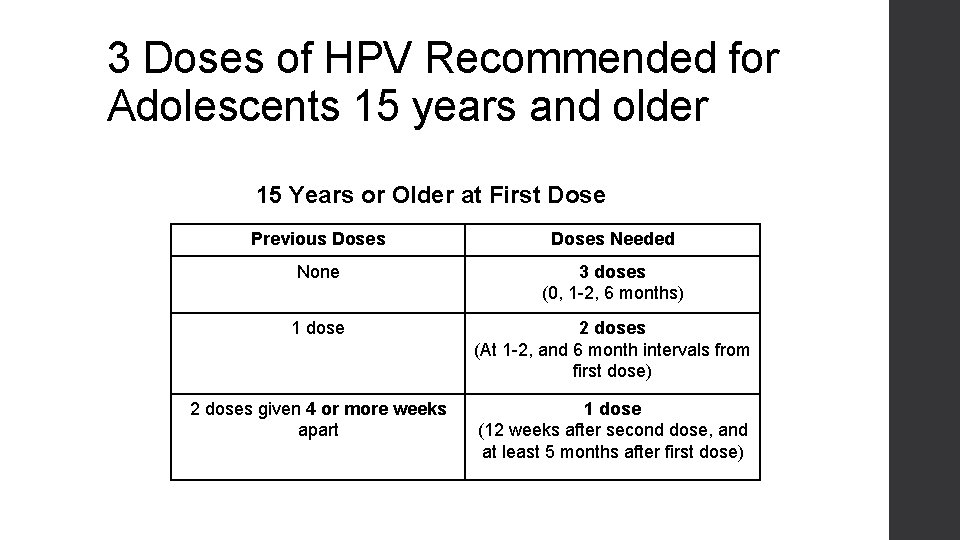
3 Doses of HPV Recommended for Adolescents 15 years and older 15 Years or Older at First Dose Previous Doses Needed None 3 doses (0, 1 -2, 6 months) 1 dose 2 doses (At 1 -2, and 6 month intervals from first dose) 2 doses given 4 or more weeks apart 1 dose (12 weeks after second dose, and at least 5 months after first dose)

23

24
![HPV Coverage in Minnesota 90 80 VALUE 70 60 VALUE 50 40 30 20 HPV Coverage in Minnesota 90 80 [VALUE]% 70 60 [VALUE]% 50 40 30 20](https://slidetodoc.com/presentation_image/d6e1b82e5714f50d6a156a4586c25ae3/image-25.jpg)
HPV Coverage in Minnesota 90 80 [VALUE]% 70 60 [VALUE]% 50 40 30 20 10 0 Tdap MCV 4 13 -17 year olds, MIIC data April 2017 HPV

References: • Baxter, R. , Bartlett, J. , Fireman, B. , Lewis, E. , & Klein, N. P. (2017). Effectiveness of Vaccination During Pregnancy to Prevent Infant Pertussis. Pediatrics, e 20164091. • Bright, H. , Malloy, R. (2017). Update on Status of Investigation of Reduced LAIV Effectiveness. February 2017. https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2017 -02/influenza-04 bright-mallory. pdf • Flannery, B. (2017). Interim estimates of 2016– 17 seasonal influenza vaccine effectiveness—United States, February 2017. MMWR. Morbidity and Mortality Weekly Report, 66. • Grohskopf, L. A. (2016). Prevention and control of seasonal influenza with vaccines. MMWR. Recommendations and Reports, 65. • Liang, J. (2016). Guidance on the use of Tdap during pregnancy. October 2016. https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2016 -10/pertussis-02 -liang. pdf • Robinson, C. Kim, D. (2017). Current Issues in Immunization Netconference. March 2017. https: //www 2. cdc. gov/vaccines/ed/pickup/ciinc/2017/CIINC_3_29_2017. pdf • Schillie, S. (2016). Revised ACIP Hepatitis B (Hep. B) Vaccine Recommendations. October 2016. https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2016 -10/hepatitis-02 -schillie-october 2016. pdf