HPV Vaccines Update on ACIP Recommendations National Immunization







- Slides: 42

HPV Vaccines Update on ACIP Recommendations National Immunization Conference April 20, 2010 Lauri E. Markowitz, MD Centers for Disease Control and Prevention

The findings and conclusions in this presentation are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention

Background Human Papillomavirus • >100 different types identified • ~40 types are sexually transmitted • “High-risk” types (16, 18, and others) • Cervical and other anogenital cancers; subset of oral cavity and oropharyngeal cancers • “Low-risk” types (6, 11, and others) • Genital warts and recurrent respiratory papillomatosis

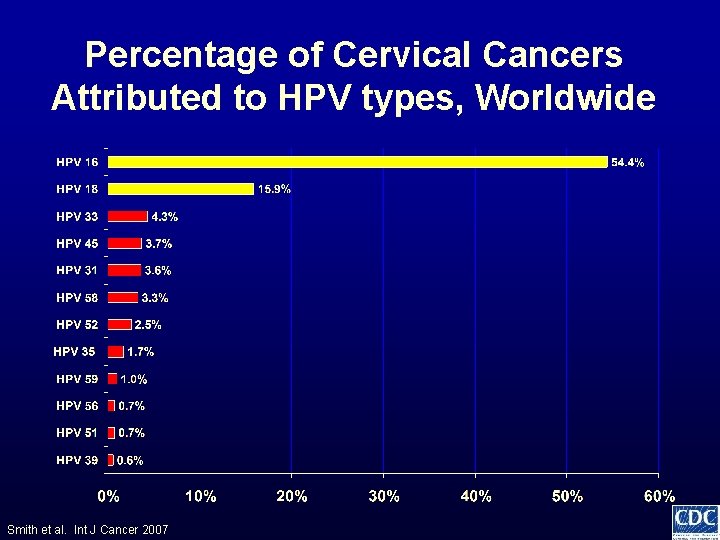
Percentage of Cervical Cancers Attributed to HPV types, Worldwide Smith et al. Int J Cancer 2007

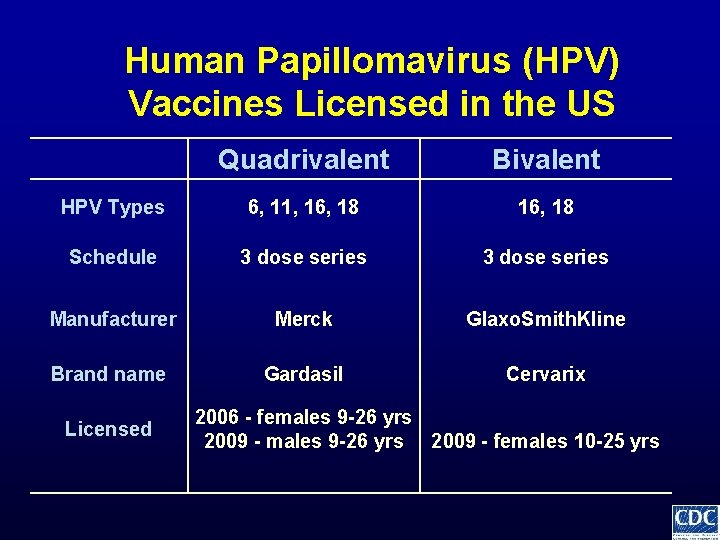
Human Papillomavirus (HPV) Vaccines Licensed in the US Quadrivalent Bivalent HPV Types 6, 11, 16, 18 Schedule 3 dose series Manufacturer Merck Glaxo. Smith. Kline Brand name Gardasil Cervarix Licensed 2006 - females 9 -26 yrs 2009 - females 10 -25 yrs

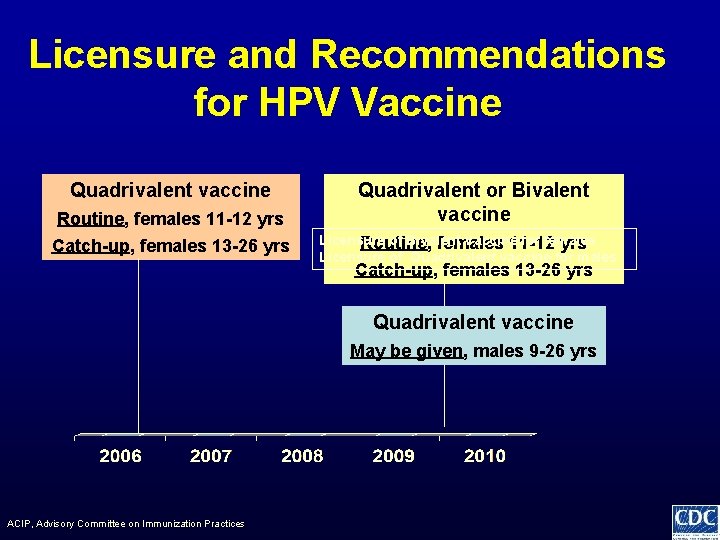
Licensure and Recommendations for HPV Vaccine Quadrivalent vaccine Routine, females 11 -12 yrs Catch-up, females 13 -26 yrs Quadrivalent or Bivalent vaccine Licensure of Bivalent Vaccine for females Routine, females 11 -12 yrs Licensure of Quadrivalent vaccine for males Catch-up, females 13 -26 yrs Quadrivalent vaccine May be given, males 9 -26 yrs ACIP, Advisory Committee on Immunization Practices

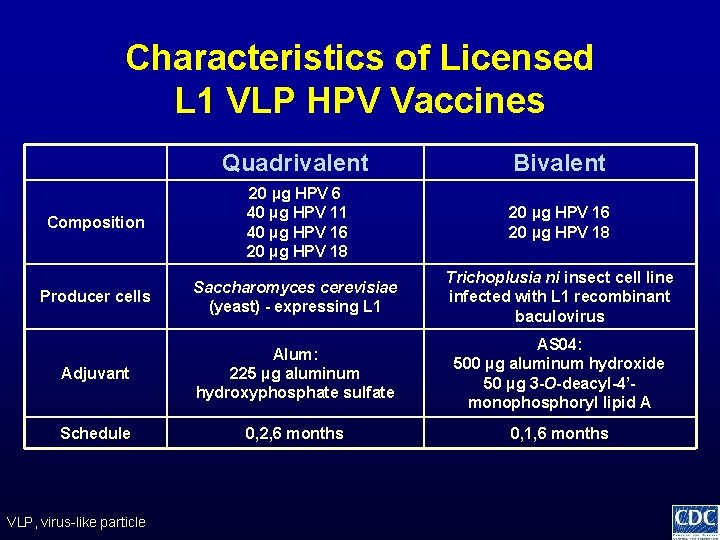
Characteristics of Licensed L 1 VLP HPV Vaccines Quadrivalent Bivalent Composition 20 µg HPV 6 40 µg HPV 11 40 µg HPV 16 20 µg HPV 18 20 µg HPV 16 20 µg HPV 18 Producer cells Saccharomyces cerevisiae (yeast) - expressing L 1 Trichoplusia ni insect cell line infected with L 1 recombinant baculovirus Adjuvant Alum: 225 µg aluminum hydroxyphosphate sulfate AS 04: 500 µg aluminum hydroxide 50 µg 3 -O-deacyl-4’monophosphoryl lipid A Schedule 0, 2, 6 months 0, 1, 6 months VLP, virus-like particle

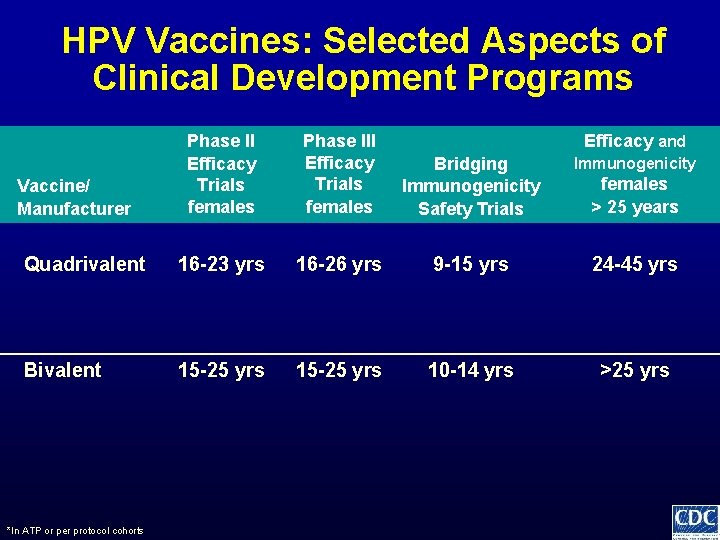
HPV Vaccines: Selected Aspects of Clinical Development Programs Phase II Efficacy Trials females Phase III Efficacy Trials females Bridging Immunogenicity Safety Trials Immunogenicity Quadrivalent 16 -23 yrs 16 -26 yrs 9 -15 yrs 24 -45 yrs Bivalent 15 -25 yrs 10 -14 yrs >25 yrs Vaccine/ Manufacturer *In ATP or per protocol cohorts Efficacy and females > 25 years

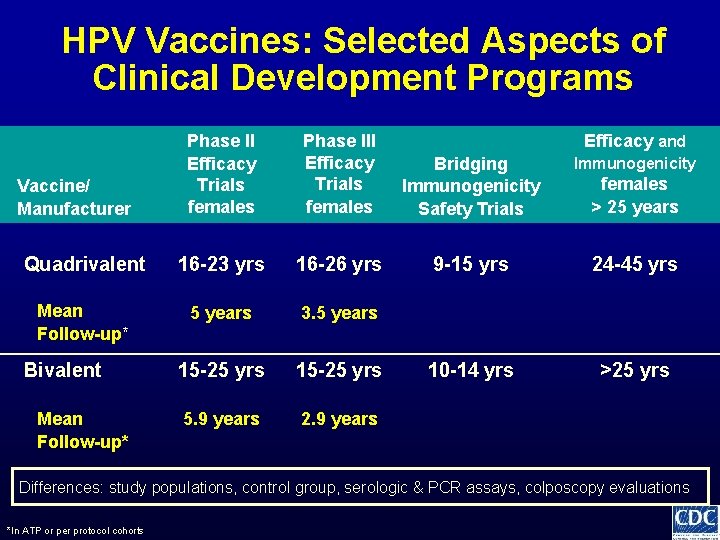
HPV Vaccines: Selected Aspects of Clinical Development Programs Phase II Efficacy Trials females Phase III Efficacy Trials females Bridging Immunogenicity Safety Trials Immunogenicity Quadrivalent 16 -23 yrs 16 -26 yrs 9 -15 yrs 24 -45 yrs Mean Follow-up* 5 years 3. 5 years 15 -25 yrs 10 -14 yrs >25 yrs 5. 9 years 2. 9 years Vaccine/ Manufacturer Bivalent Mean Follow-up* Efficacy and females > 25 years Differences: study populations, control group, serologic & PCR assays, colposcopy evaluations *In ATP or per protocol cohorts

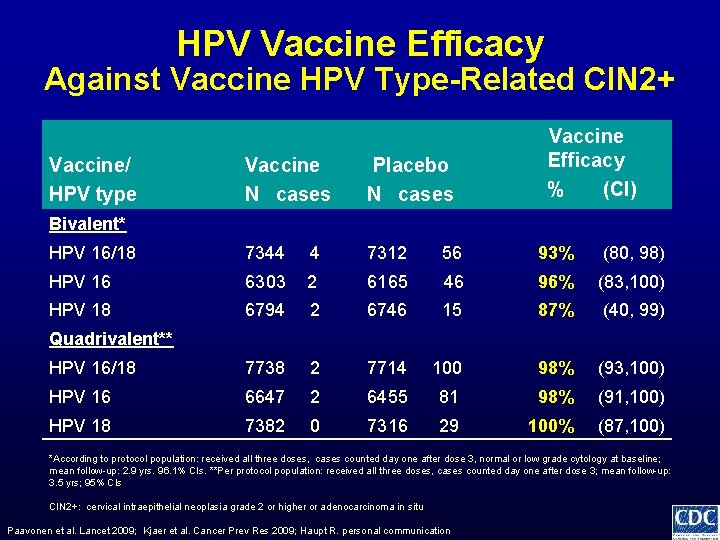
HPV Vaccine Efficacy Against Vaccine HPV Type-Related CIN 2+ Vaccine/ HPV type Vaccine Efficacy % (CI) Vaccine N cases Placebo N cases HPV 16/18 7344 4 7312 56 93% (80, 98) HPV 16 6303 2 6165 46 96% (83, 100) HPV 18 6794 2 6746 15 87% (40, 99) HPV 16/18 7738 2 7714 100 98% (93, 100) HPV 16 6647 2 6455 81 98% (91, 100) HPV 18 7382 0 7316 29 100% (87, 100) Bivalent* Quadrivalent** *According to protocol population: received all three doses, cases counted day one after dose 3, normal or low grade cytology at baseline; mean follow-up: 2. 9 yrs. 96. 1% CIs. **Per protocol population: received all three doses, cases counted day one after dose 3; mean follow-up: 3. 5 yrs; 95% CIs CIN 2+: cervical intraepithelial neoplasia grade 2 or higher or adenocarcinoma in situ Paavonen et al. Lancet 2009; Kjaer et al. Cancer Prev Res 2009; Haupt R. personal communication

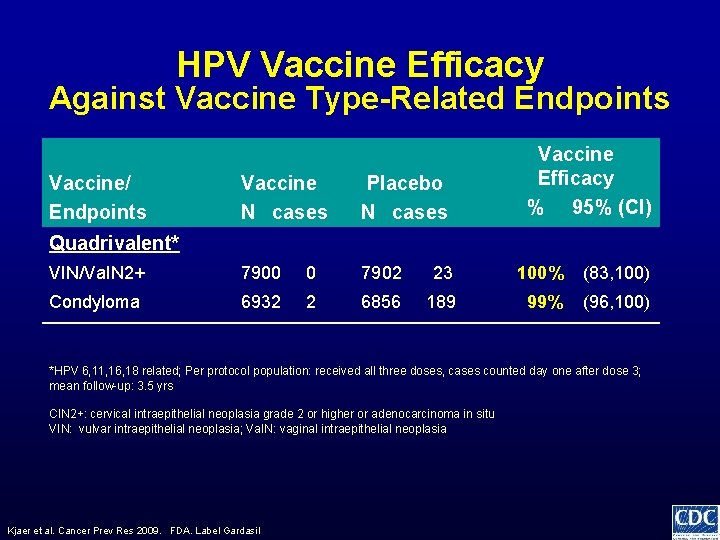
HPV Vaccine Efficacy Against Vaccine Type-Related Endpoints Vaccine/ Endpoints Vaccine Efficacy % 95% (CI) Vaccine N cases Placebo N cases VIN/Va. IN 2+ 7900 0 7902 23 100% (83, 100) Condyloma 6932 2 6856 189 99% (96, 100) Quadrivalent* *HPV 6, 11, 16, 18 related; Per protocol population: received all three doses, cases counted day one after dose 3; mean follow-up: 3. 5 yrs CIN 2+: cervical intraepithelial neoplasia grade 2 or higher or adenocarcinoma in situ VIN: vulvar intraepithelial neoplasia; Va. IN: vaginal intraepithelial neoplasia Kjaer et al. Cancer Prev Res 2009. FDA. Label Gardasil

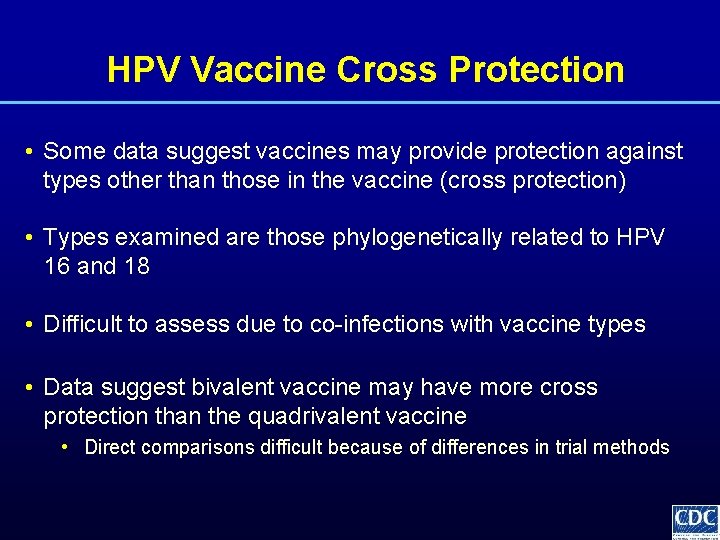
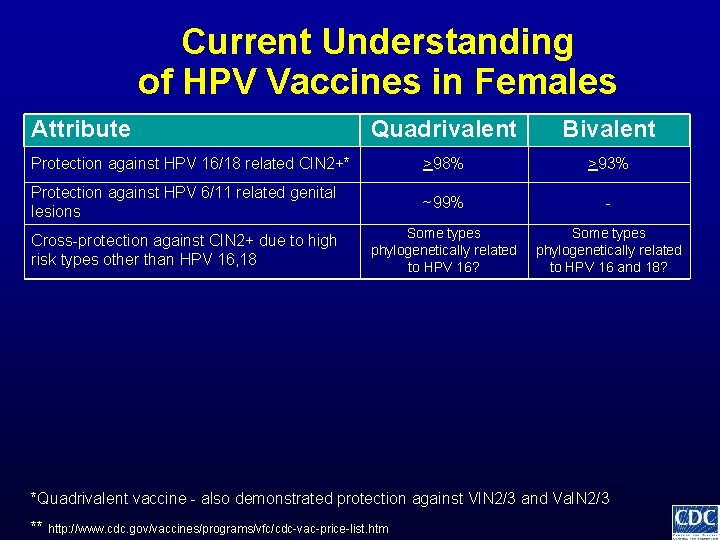
HPV Vaccine Cross Protection • Some data suggest vaccines may provide protection against types other than those in the vaccine (cross protection) • Types examined are those phylogenetically related to HPV 16 and 18 • Difficult to assess due to co-infections with vaccine types • Data suggest bivalent vaccine may have more cross protection than the quadrivalent vaccine • Direct comparisons difficult because of differences in trial methods

Bivalent HPV Vaccine Safety Assessment • Evaluations for • • • solicited symptoms unsolicited symptoms medically significant conditions new onset autoimmune disorders and chronic diseases serious adverse events pregnancy outcomes • Integrated safety analysis from 11 trials of bivalent HPV vaccine involving ~30, 000 females • Meta-analysis of autoimmune diseases from trials of AS 04 containing vaccines involving ~68, 000 subjects

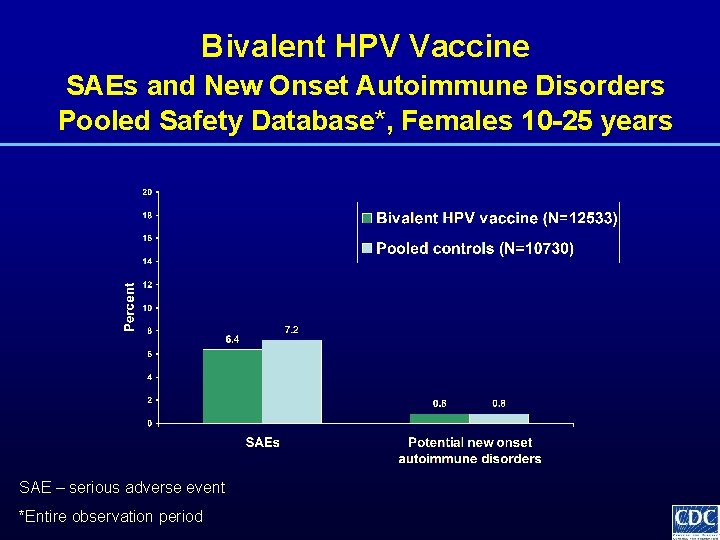
Bivalent HPV Vaccine SAEs and New Onset Autoimmune Disorders Pooled Safety Database*, Females 10 -25 years SAE – serious adverse event *Entire observation period

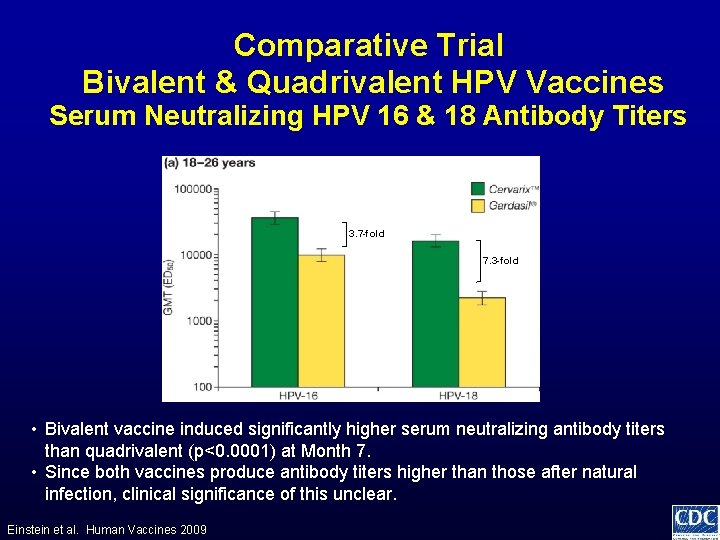
Comparative Trial Bivalent & Quadrivalent HPV Vaccines Serum Neutralizing HPV 16 & 18 Antibody Titers 3. 7 -fold 7. 3 -fold • Bivalent vaccine induced significantly higher serum neutralizing antibody titers than quadrivalent (p<0. 0001) at Month 7. • Since both vaccines produce antibody titers higher than those after natural infection, clinical significance of this unclear. Einstein et al. Human Vaccines 2009

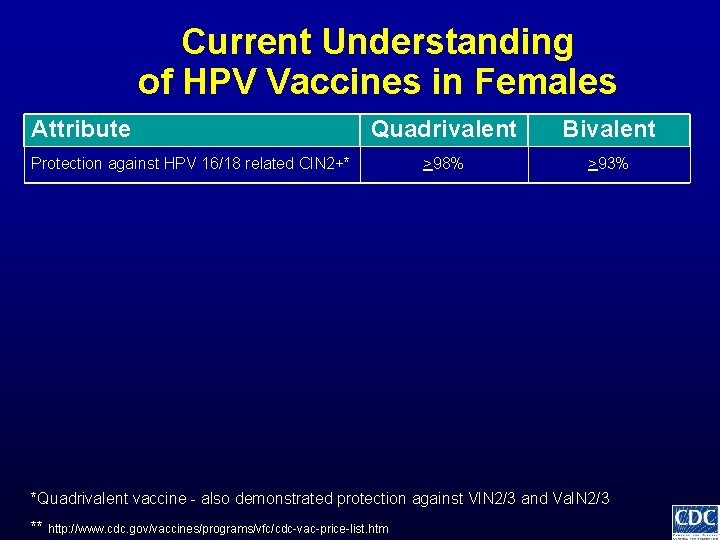
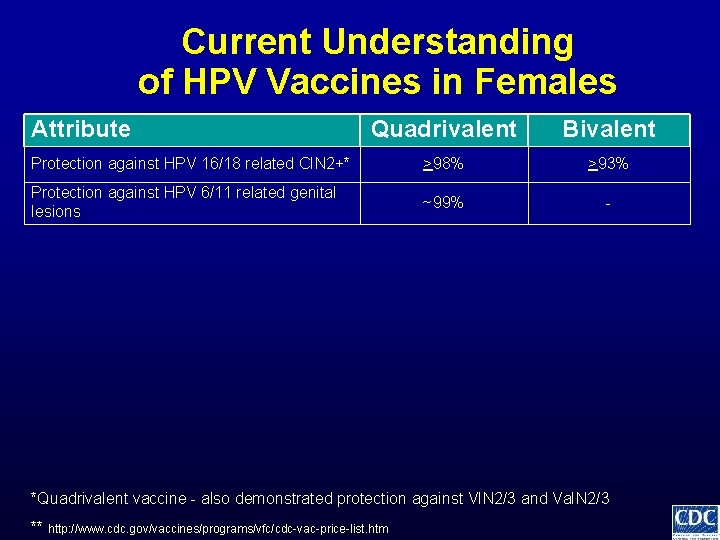
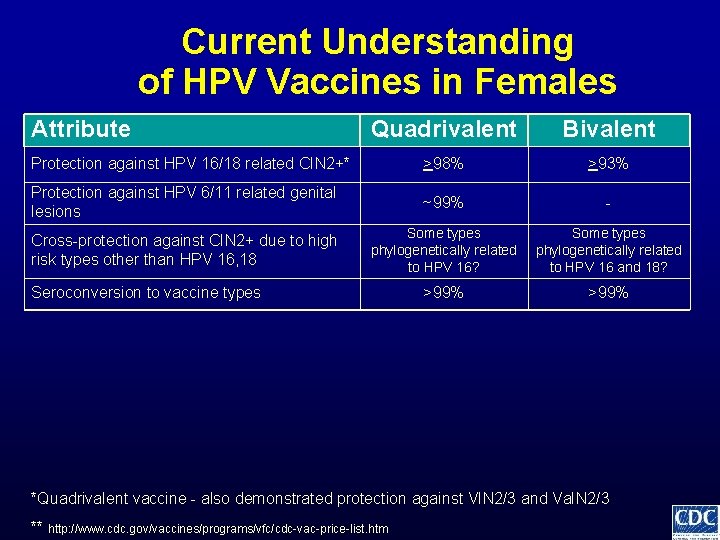
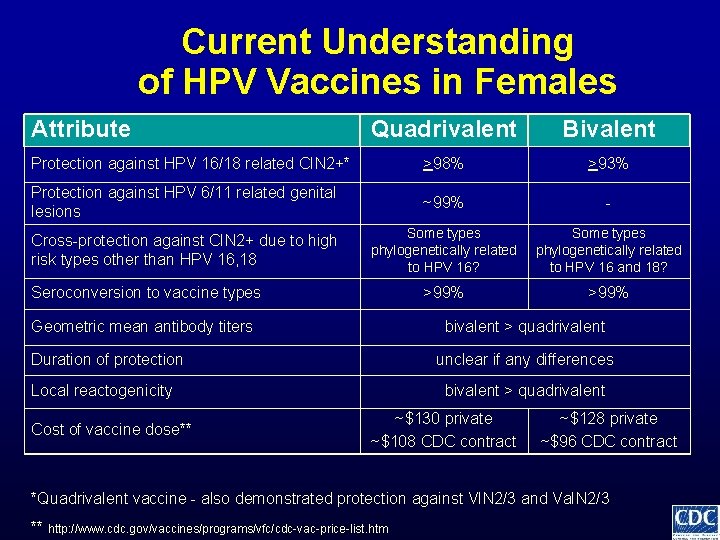
Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent >98% >93% Protection against HPV 16/18 related CIN 2+* *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

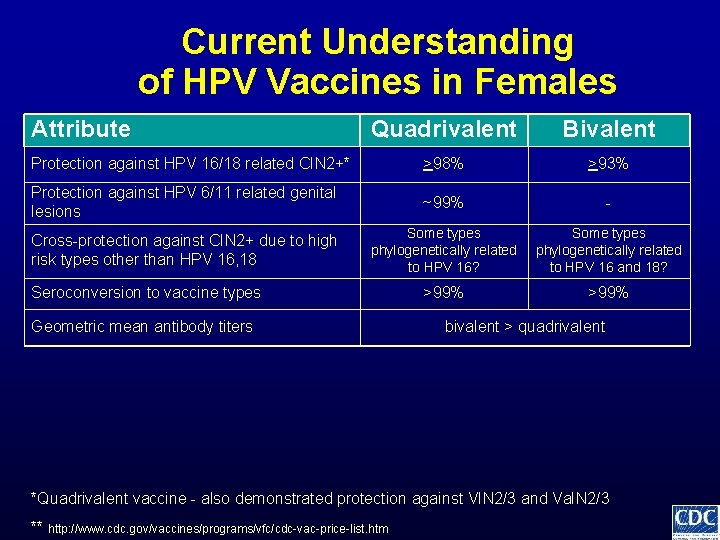
Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

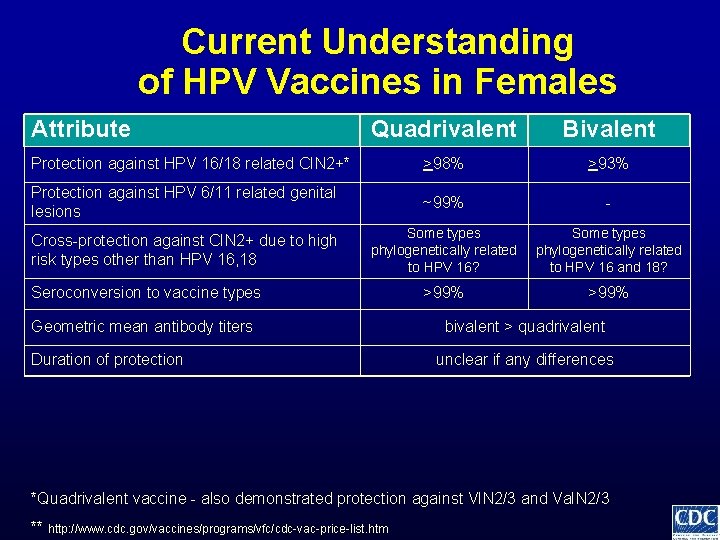
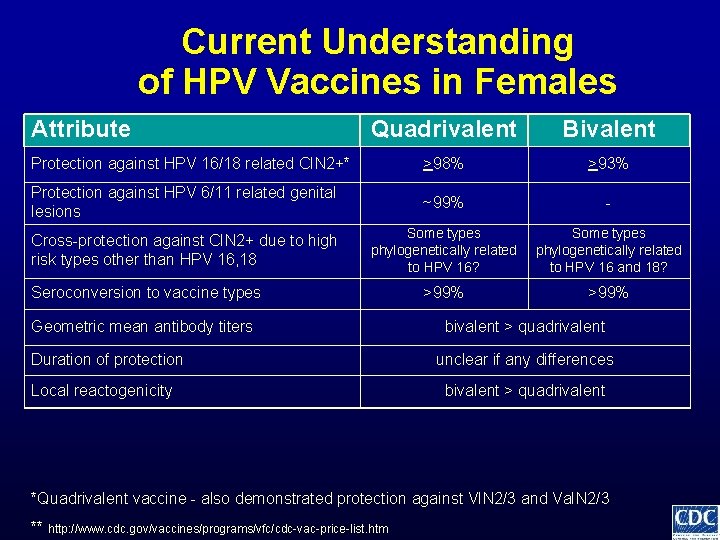
Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - Cross-protection against CIN 2+ due to high risk types other than HPV 16, 18 Some types phylogenetically related to HPV 16? Some types phylogenetically related to HPV 16 and 18? *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - Cross-protection against CIN 2+ due to high risk types other than HPV 16, 18 Some types phylogenetically related to HPV 16? Some types phylogenetically related to HPV 16 and 18? >99% Seroconversion to vaccine types *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - Cross-protection against CIN 2+ due to high risk types other than HPV 16, 18 Some types phylogenetically related to HPV 16? Some types phylogenetically related to HPV 16 and 18? >99% Seroconversion to vaccine types Geometric mean antibody titers bivalent > quadrivalent *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - Cross-protection against CIN 2+ due to high risk types other than HPV 16, 18 Some types phylogenetically related to HPV 16? Some types phylogenetically related to HPV 16 and 18? >99% Seroconversion to vaccine types Geometric mean antibody titers Duration of protection bivalent > quadrivalent unclear if any differences *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - Cross-protection against CIN 2+ due to high risk types other than HPV 16, 18 Some types phylogenetically related to HPV 16? Some types phylogenetically related to HPV 16 and 18? >99% Seroconversion to vaccine types Geometric mean antibody titers Duration of protection Local reactogenicity bivalent > quadrivalent unclear if any differences bivalent > quadrivalent *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

Current Understanding of HPV Vaccines in Females Attribute Quadrivalent Bivalent Protection against HPV 16/18 related CIN 2+* >98% >93% Protection against HPV 6/11 related genital lesions ~99% - Cross-protection against CIN 2+ due to high risk types other than HPV 16, 18 Some types phylogenetically related to HPV 16? Some types phylogenetically related to HPV 16 and 18? >99% Seroconversion to vaccine types Geometric mean antibody titers bivalent > quadrivalent Duration of protection unclear if any differences Local reactogenicity Cost of vaccine dose** bivalent > quadrivalent ~$130 private ~$108 CDC contract ~$128 private ~$96 CDC contract *Quadrivalent vaccine - also demonstrated protection against VIN 2/3 and Va. IN 2/3 ** http: //www. cdc. gov/vaccines/programs/vfc/cdc-vac-price-list. htm

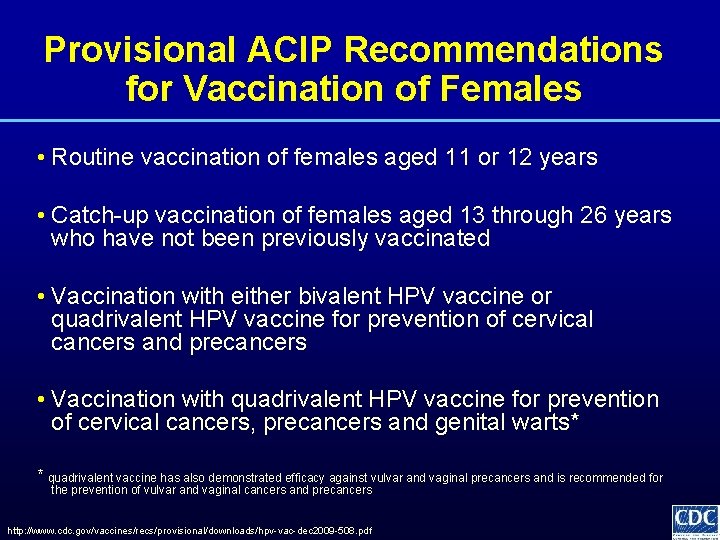
Provisional ACIP Recommendations for Vaccination of Females • Routine vaccination of females aged 11 or 12 years • Catch-up vaccination of females aged 13 through 26 years who have not been previously vaccinated • Vaccination with either bivalent HPV vaccine or quadrivalent HPV vaccine for prevention of cervical cancers and precancers • Vaccination with quadrivalent HPV vaccine for prevention of cervical cancers, precancers and genital warts* * quadrivalent vaccine has also demonstrated efficacy against vulvar and vaginal precancers and is recommended for the prevention of vulvar and vaginal cancers and precancers http: //www. cdc. gov/vaccines/recs/provisional/downloads/hpv-vac-dec 2009 -508. pdf

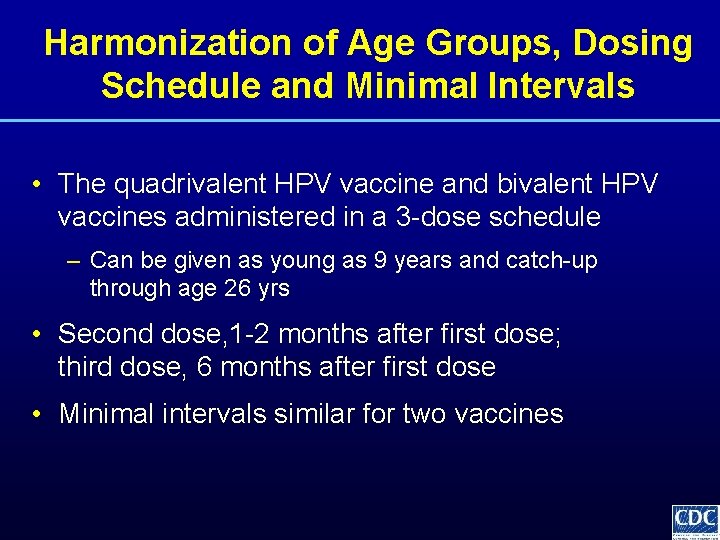
Harmonization of Age Groups, Dosing Schedule and Minimal Intervals • The quadrivalent HPV vaccine and bivalent HPV vaccines administered in a 3 -dose schedule – Can be given as young as 9 years and catch-up through age 26 yrs • Second dose, 1 -2 months after first dose; third dose, 6 months after first dose • Minimal intervals similar for two vaccines

Interchangeability of HPV Vaccines ACIP recommends that the HPV vaccine product be used for the entire vaccination series If vaccination provider does not know or have available the HPV vaccine product previously administered, either HPV vaccine product can be used to complete the series to provide protection against HPV 16 & 18

Precautions and Contraindications • Not recommended for use in pregnant women; pregnancy testing is not needed before vaccination – Any exposure to vaccine during pregnancy should be reported to the appropriate vaccine pregnancy registry: • 1 -800 -986 -8999 (quadrivalent HPV vaccine) • 1 -888 -452 -9622 (bivalent HPV vaccine) • Contraindicated for persons with immediate hypersensitivity to any vaccine component – Quadrivalent HPV vaccine - yeast – Bivalent HPV vaccine in prefilled syringes - latex

Quadrivalent HPV Vaccine for Males

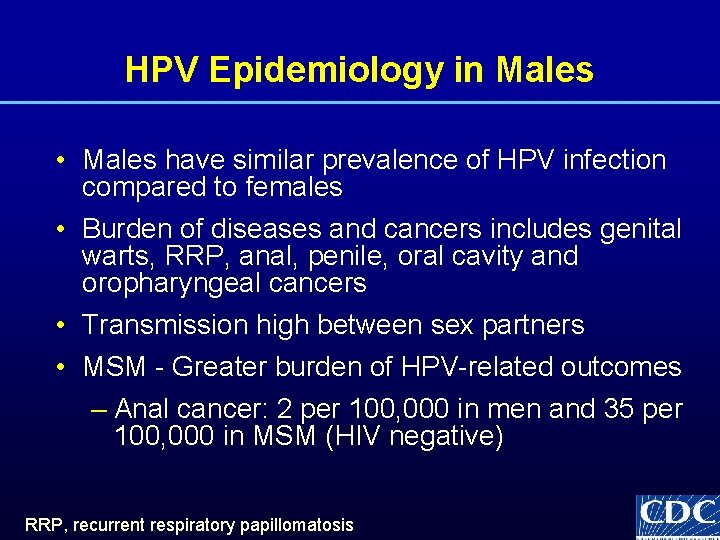
HPV Epidemiology in Males • Males have similar prevalence of HPV infection compared to females • Burden of diseases and cancers includes genital warts, RRP, anal, penile, oral cavity and oropharyngeal cancers • Transmission high between sex partners • MSM - Greater burden of HPV-related outcomes – Anal cancer: 2 per 100, 000 in men and 35 per 100, 000 in MSM (HIV negative) RRP, recurrent respiratory papillomatosis

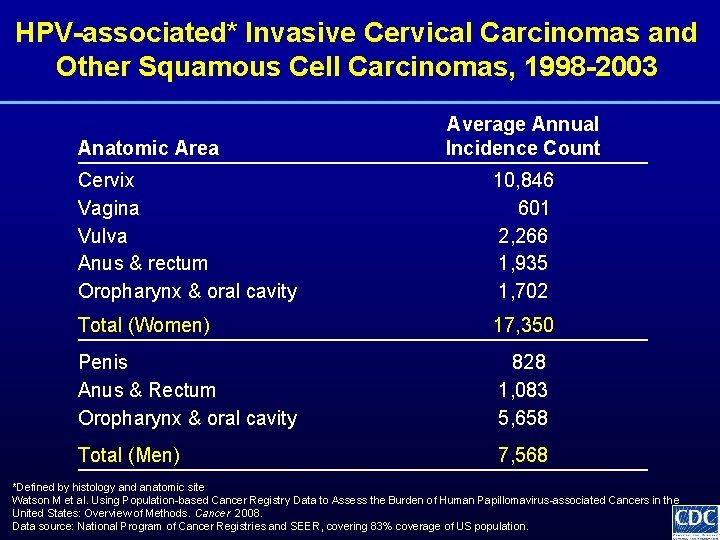
HPV-associated* Invasive Cervical Carcinomas and Other Squamous Cell Carcinomas, 1998 -2003 Anatomic Area Average Annual Incidence Count Cervix Vagina Vulva Anus & rectum Oropharynx & oral cavity 10, 846 601 2, 266 1, 935 1, 702 Total (Women) 17, 350 Penis Anus & Rectum Oropharynx & oral cavity 828 1, 083 5, 658 Total (Men) 7, 568 *Defined by histology and anatomic site Watson M et al. Using Population-based Cancer Registry Data to Assess the Burden of Human Papillomavirus-associated Cancers in the United States: Overview of Methods. Cancer 2008. Data source: National Program of Cancer Registries and SEER, covering 83% coverage of US population.

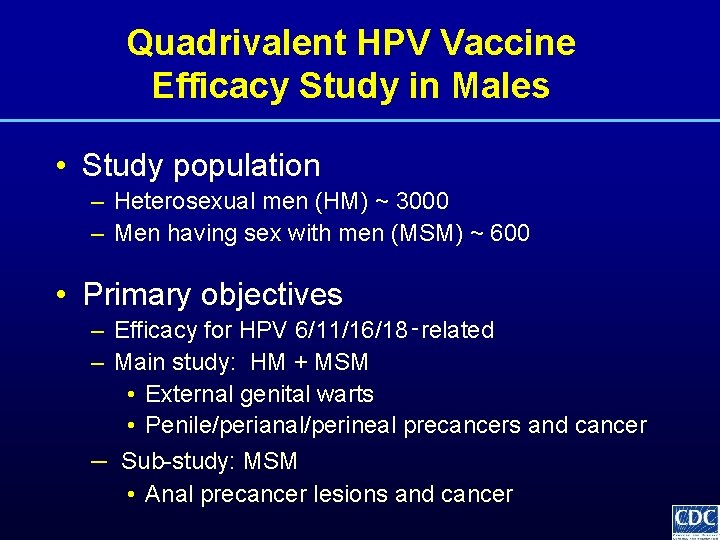
Quadrivalent HPV Vaccine Efficacy Study in Males • Study population – Heterosexual men (HM) ~ 3000 – Men having sex with men (MSM) ~ 600 • Primary objectives – Efficacy for HPV 6/11/16/18‑related – Main study: HM + MSM • External genital warts • Penile/perianal/perineal precancers and cancer – Sub-study: MSM • Anal precancer lesions and cancer

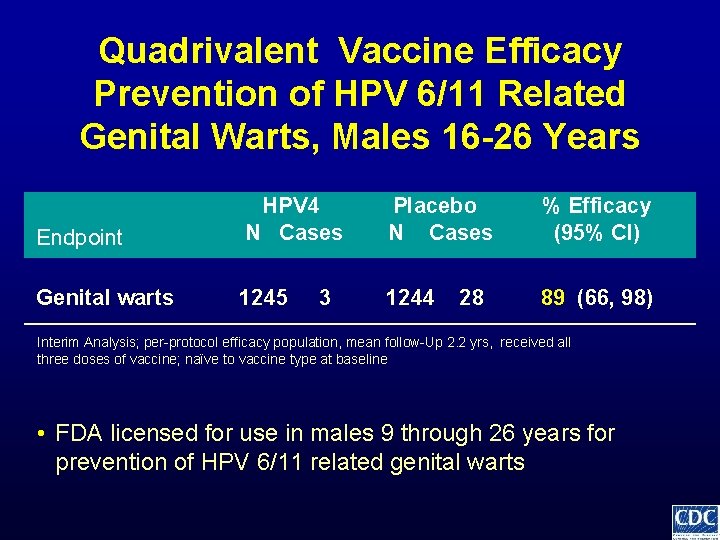
Quadrivalent Vaccine Efficacy Prevention of HPV 6/11 Related Genital Warts, Males 16 -26 Years Endpoint HPV 4 N Cases Placebo N Cases % Efficacy (95% CI) Genital warts 1245 1244 89 (66, 98) 3 28 Interim Analysis; per-protocol efficacy population, mean follow-Up 2. 2 yrs, received all three doses of vaccine; naïve to vaccine type at baseline • FDA licensed for use in males 9 through 26 years for prevention of HPV 6/11 related genital warts

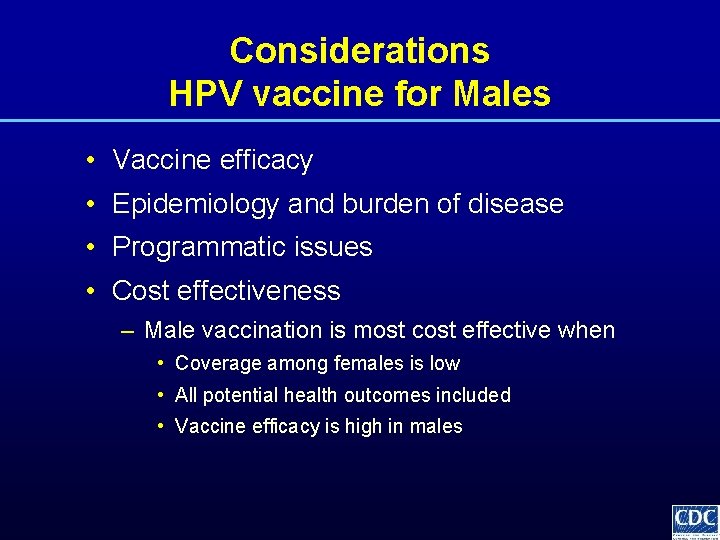
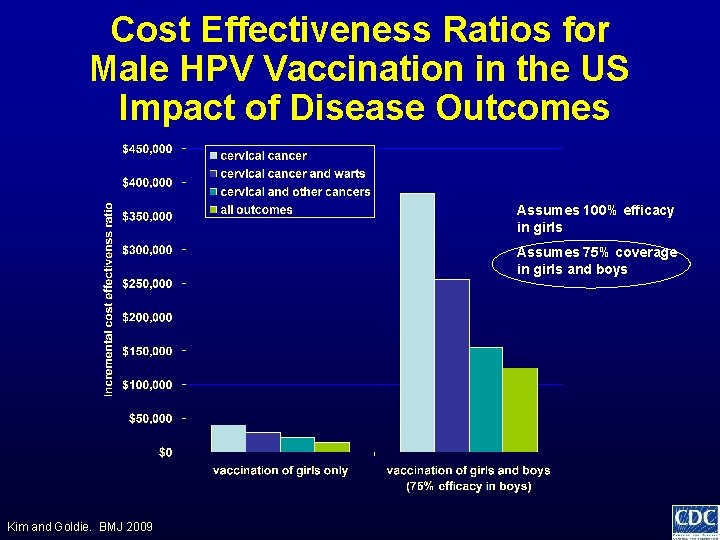
Considerations HPV vaccine for Males • Vaccine efficacy • Epidemiology and burden of disease • Programmatic issues • Cost effectiveness – Male vaccination is most cost effective when • Coverage among females is low • All potential health outcomes included • Vaccine efficacy is high in males

Cost Effectiveness Ratios for Male HPV Vaccination in the US Impact of Disease Outcomes Assumes 100% efficacy in girls Assumes 75% coverage in girls and boys Kim and Goldie. BMJ 2009

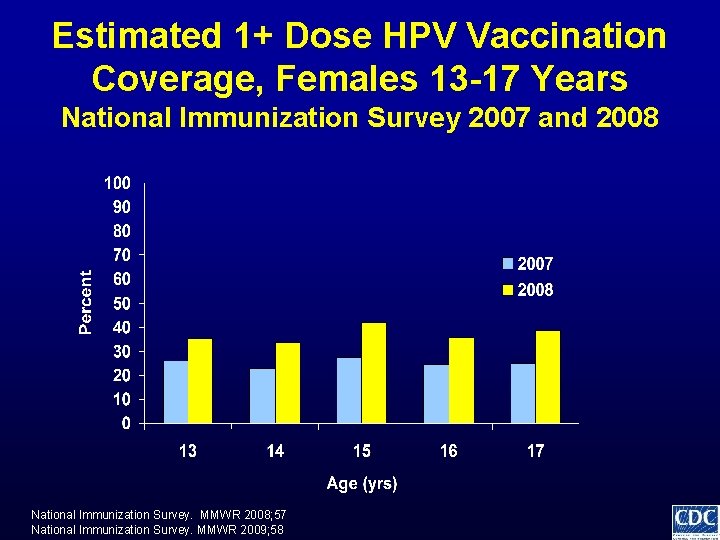
Estimated 1+ Dose HPV Vaccination Coverage, Females 13 -17 Years National Immunization Survey 2007 and 2008 National Immunization Survey. MMWR 2008; 57 National Immunization Survey. MMWR 2009; 58

HPV Vaccine Use in Males: Discussions at October 2009 ACIP • Programmatic challenges at state and local level • Most of the burden of disease/cancers in females • Priority should be given to vaccinating females to reduce the overall burden of disease/cancers • Outstanding information for future consideration: – Efficacy for prevention of AIN – Cost effectiveness in MSM – Vaccine price

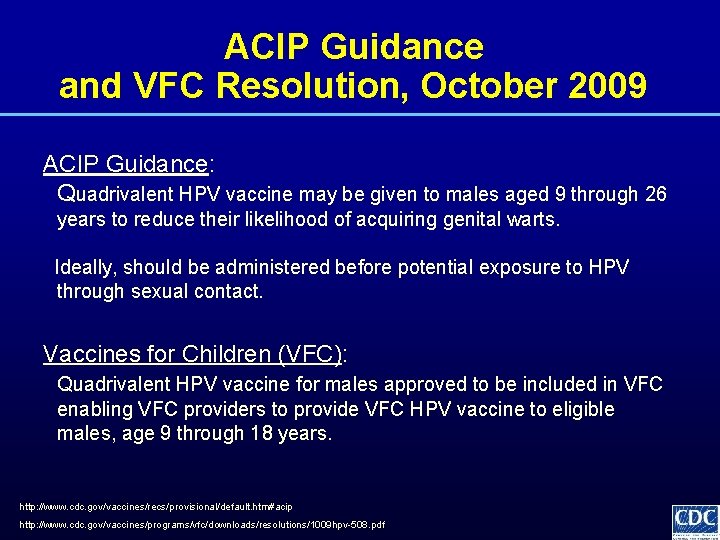
ACIP Guidance and VFC Resolution, October 2009 ACIP Guidance: Quadrivalent HPV vaccine may be given to males aged 9 through 26 years to reduce their likelihood of acquiring genital warts. Ideally, should be administered before potential exposure to HPV through sexual contact. Vaccines for Children (VFC): Quadrivalent HPV vaccine for males approved to be included in VFC enabling VFC providers to provide VFC HPV vaccine to eligible males, age 9 through 18 years. http: //www. cdc. gov/vaccines/recs/provisional/default. htm#acip http: //www. cdc. gov/vaccines/programs/vfc/downloads/resolutions/1009 hpv-508. pdf

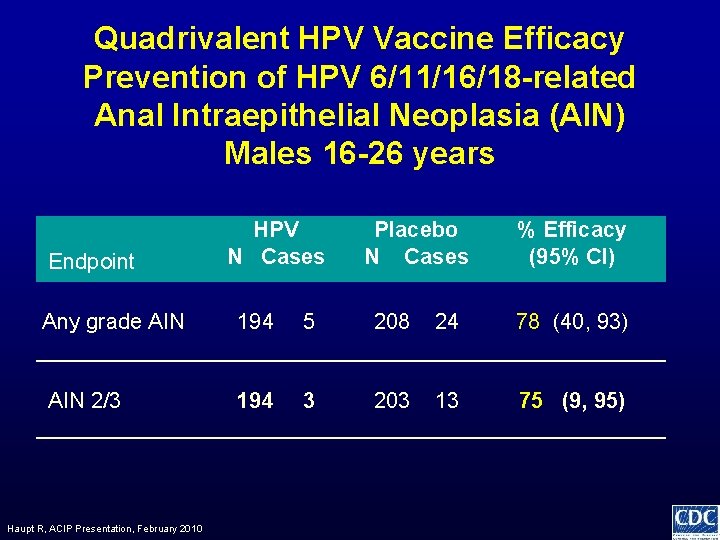
Quadrivalent HPV Vaccine Efficacy Prevention of HPV 6/11/16/18 -related Anal Intraepithelial Neoplasia (AIN) Males 16 -26 years Endpoint HPV N Cases Placebo N Cases % Efficacy (95% CI) Any grade AIN 194 5 208 24 78 (40, 93) AIN 2/3 194 3 203 13 75 (9, 95) Haupt R, ACIP Presentation, February 2010

HPV Vaccine for Males • Further consideration of – Vaccine efficacy data – Cost effectiveness analyses with different efficacy and coverage assumptions – Programmatic issues, equity issues – Epidemiology and cost effectiveness of HPV vaccine in MSM – Feasibility of reaching MSM when they would most benefit from vaccination

Quadrivalent HPV vaccine for Women Older than 26 Years? • Trials conducted in women age 24 -45 years • Relatively small impact of vaccination of adult women –Incidence of new infections decreases –% of women with previous infection increases • Models show potential for high cost per QALY

Summary – ACIP recommendations first made in 2006 and updated in October 2009 • Two vaccines available for females 9 through 26 years – Either vaccine recommended at 11 or 12 years – Catch up through age 26 years • Quadrivalent HPV vaccine may be given to males ages 9 through 26 years – Further data/policy issues in next year • Quadrivalent HPV vaccine in males • HPV vaccine for women >age 26 years

Thank you