Introducing the National Cervical Screening Program providing your




















- Slides: 47

Introducing the National Cervical Screening Program: providing your patients the right advice November 2017 Dr Hilary Bower Medical Coordinator Family Planning NSW

Aims of this session To understand the National Cervical Screening Program: implementation on December 1 st 2017 How to explain the National Cervical Screening Program to patients? How to access supporting resources?

Epidemiology of cervical cancer • Cervical cancer is the 15 th most commonly diagnosed cancer in Australian women, accounting for 1. 5% of cancers in Australian women • Since 1991, 50% decrease in mortality due to cervical cancer, largely due to our NCSP: a public health success story! • 80% of women with cervical cancer had not had a Pap smear in the previous 5 years Australian Institute of Health and Welfare. Cervical Screening in Australia 2013 -14. Canberra: AIHW 2016. Cancer Series no. 97 Cat. no. CAN 95. Australian Institute of Health and Welfare. Cancer in Australia 2017. Cancer series no. 101. Cat. no. CAN 100. Canberra: AIHW

Time for a change…. . Timely for Australia to take a lead with an evidence-based program based on: • new understanding of HPV & cancer • new testing technologies • a successful National HPV vaccination program

HPV: the ‘common cold’ of the genitals! • Up to 80% of people infected in their lifetime; usually resolves within 1 - 2 years • > 99% cervical cancer linked to oncogenic HPV subtypes • 14 oncogenic HPV types (16 & 18 more likely to persist detected in 70%– 80% cases cervical cancer) • Penetrative intercourse not strictly necessary; HPV can be transferred to the cervix from an infection at the introitus • Transmission via genital skin-to-skin contact, vaginal sex, oral sex & anal sex Ø Introduction of the Gardasil-9 in 2018!

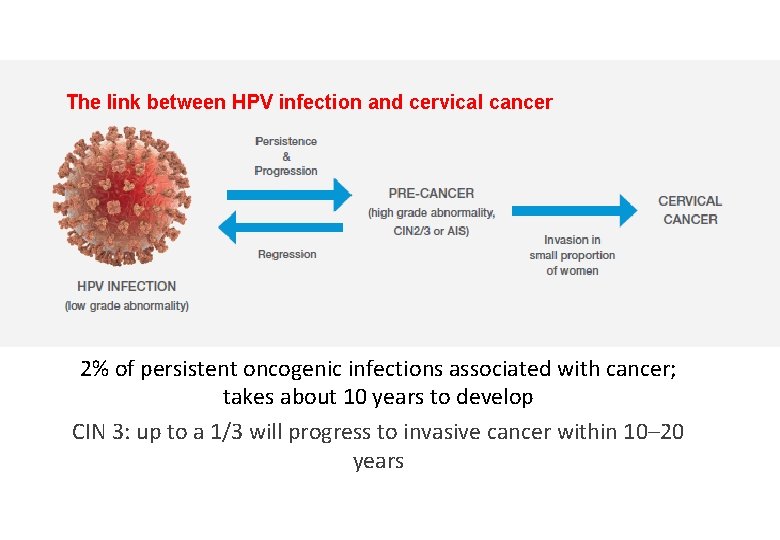
The link between HPV infection and cervical cancer 2% of persistent oncogenic infections associated with cancer; takes about 10 years to develop CIN 3: up to a 1/3 will progress to invasive cancer within 10– 20 years

National Cervical Screening Program from 1 st December 2017 ensuring all women (vaccinated and unvaccinated) have access to a program that is acceptable, effective and efficient and based on current evidence Aim for up to 36 % fewer cervical cancers www. msac. gov. au

Understanding the new National Cervical Screening Program • All women who have ever been sexually active will be invited for Cervical Screening Test at 25 years • Women will be managed using a risk-based approach that is dependent on the cervical screening test results • Cervical screening may cease between 70 and 74 if regular screening tests with –ve results and a –ve exit result • Routine screening carried out every 5 years for women with no symptoms or history suggestive of cervical cancer • Invitations and reminders will be sent by the National Register to screen-eligible women

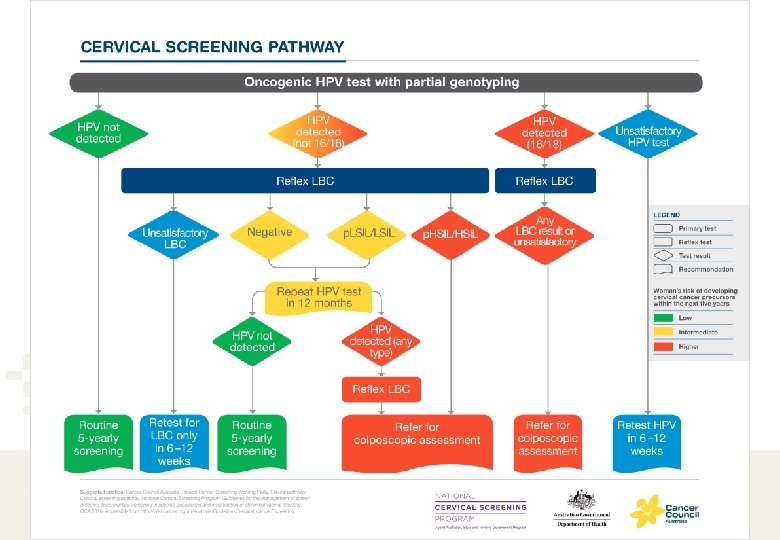
What is the Cervical Screening Test? The Cervical Screening Test has 2 components: 1. HPV DNA test with partial genotyping (to allow independent detection and reporting of HPV 16 and 18; other oncogenic HPV types are reported as a pooled result) 2. Reflex liquid based cytology (LBC) if the HPV test is +ve for any oncogenic HPV type (performed automatically on the sample); results of LBC used to inform colposcopy

Your essential guide…. “National Cervical Screening Program: Guidelines for the Management of Screen Detected Abnormalities, Screening in Specific Populations and Investigation of Abnormal Vaginal Bleeding. ” http: //wiki. cancer. org. au/australia/Guidelines: Cervical_cancer/Screening

Why is primary HPV testing replacing the Pap test? • A significant false-negative rate for Pap vs HPV tests (30% vs 23%) required more frequent screening to minimise failure to detect disease • Women who test HPV -ve are at very low risk of HSIL and cancer for at least 5 years • Compared with cytology, HPV testing provides 60– 70% greater protection against invasive cervical cancers; significantly reduced incidence of adenocarcinomas • Opportunity for self collection in under-screened populations Renshaw AA et al. Cancer Cytopathology 2001; 93: 106 -10 Dr Guglielmo Ronco et al. Lancet 2014; 383: 524 -32

Why use partial genotyping? • Partial genotyping allows for separate detection of HPV 16 and 18; other oncogenic HPV types will be reported as a pooled result • HPV 16 and 18 are associated with cervical abnormalities that are less likely to regress and more likely to progress to highgrade cervical abnormalities, compared with other oncogenic HPV genotypes • Improves risk stratification/assessment in the screening program Castle PE et al. Obstet Gynecol 2009; 113: 18 -25; Kjaer SK, et al. J Natl Cancer Inst 2010; 102: 1478 -88; Schiffman M, et al. Cancer Epidemiol Biomarkers Prev 2011; 20: 1398 -409

Explaining changes to the screening age: routine screening not recommended < 25 • Cervical cancer very rare in young women • Screening < 25 years has not reduced invasive cancer rates or deaths in this age group or in 25 -29 year olds • HPV vaccination already showing a reduction in screen-detected abnormalities in women <25 years of age • Cervical abnormalities common < 25 years and usually resolve; overdiagnosis and over-treatment not desirable • History of childhood sexual abuse or early sexual debut (< 14 years, prior to HPV vaccination): consider a HPV test between 20— 24 years • Symptomatic women of ANY age should be assessed with a co-test Peirson L et al Syst Rev 2013; 2: 35; Kyrgiou M, et al Lancet 2006; 367: 489 -98; Sasieni et al BMJ 2009; Schlect NF J Natl Cancer Inst 2003; 95: 1336 -43

A co-test is different to a Cervical Screening Test! A co-test is when a sample is tested for HPV DNA and cytology at the same time Not part of routine screening & must be specifically requested on the pathology request form: Ø at follow up of certain abnormalities eg glandular abnormalities after normal colposcopy Ø as part of Test of Cure (after treated CIN 2/3) Ø for some women post hysterectomy Ø for DES-exposed women Ø for women treated for adenocarcinoma in situ (AIS) Ø as part of Ix of abnormal vaginal bleeding or an abnormal looking cervix

What will change in clinical practice? • Discuss changes to the screening program for asymptomatic women • Transition clients to the new program • Collect the Cervical Screening Test using a liquid based medium (slides no longer used) • Order a CST on the pathology request form (or a co-test if indicated) Discuss results and risk assessment • Use the new management guidelines • Inform clients about the National Cancer Screening Register

The cervical screening pathway for asymptomatic women under National Cervical Screening Program (NCSP) from December 1 st 2017


What should you expect from a lab report? results are stratified by risk An overall cervical screening risk assessment: • Low risk – oncogenic HPV negative • Intermediate risk - e. g. HPV detected (not 16/18 and LBC negative or LSIL ) • Higher risk - e. g. HPV detected (16/18) A statement of test(s) performed and results: • HPV test result including LBC result (if performed) A recommendation for follow up: • taking account of result, screening history and clinical notes

Sara is +ve for HPV 16: what do you do? • Sara’s result is higher risk • referral to colposcopy regardless of LBC result • If LBC is unsatisfactory, repeat LBC at time of colposcopy • If LBC result predicts invasive disease referral within 2/52 • If LBC result PHSIL/HSIL referral within 8/52

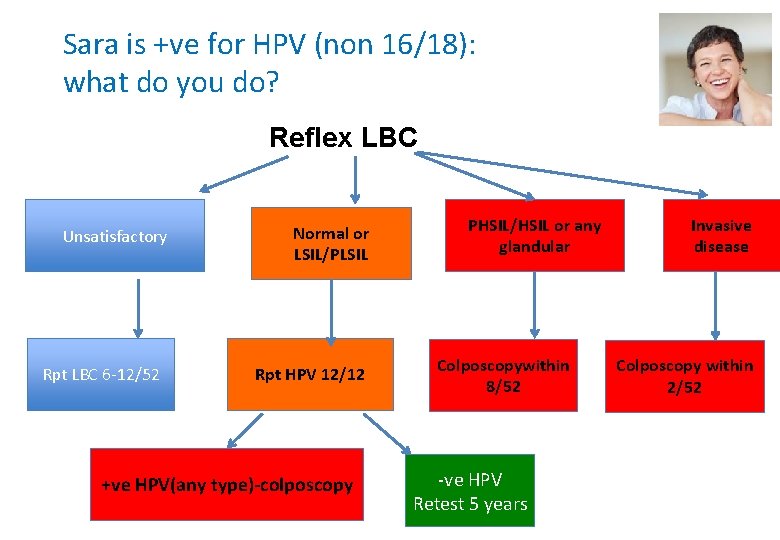
Sara is +ve for HPV (non 16/18): what do you do? Reflex LBC Unsatisfactory Rpt LBC 6 -12/52 Normal or LSIL/PLSIL Rpt HPV 12/12 +ve HPV(any type)-colposcopy PHSIL/HSIL or any glandular Colposcopywithin 8/52 -ve HPV Retest 5 years Invasive disease Colposcopy within 2/52

Sara has a glandular abnormality? All glandular abnormalities refer to an expert gynaecologist for colposcopy including “Atypical endocervical/glandular cells of undetermined significance” Follow up of completely excised AIS: • annual co testing indefinitely • any abnormal result refer for a colposcopy

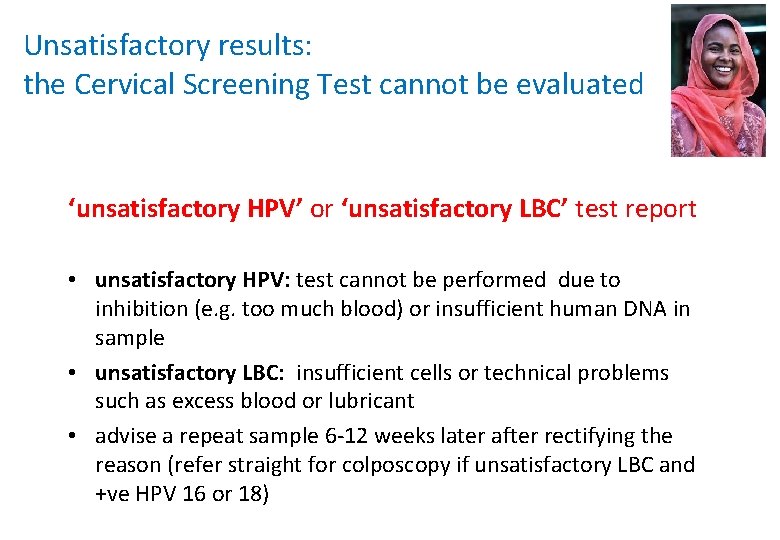
Unsatisfactory results: the Cervical Screening Test cannot be evaluated ‘unsatisfactory HPV’ or ‘unsatisfactory LBC’ test report • unsatisfactory HPV: test cannot be performed due to inhibition (e. g. too much blood) or insufficient human DNA in sample • unsatisfactory LBC: insufficient cells or technical problems such as excess blood or lubricant • advise a repeat sample 6 -12 weeks later after rectifying the reason (refer straight for colposcopy if unsatisfactory LBC and +ve HPV 16 or 18)

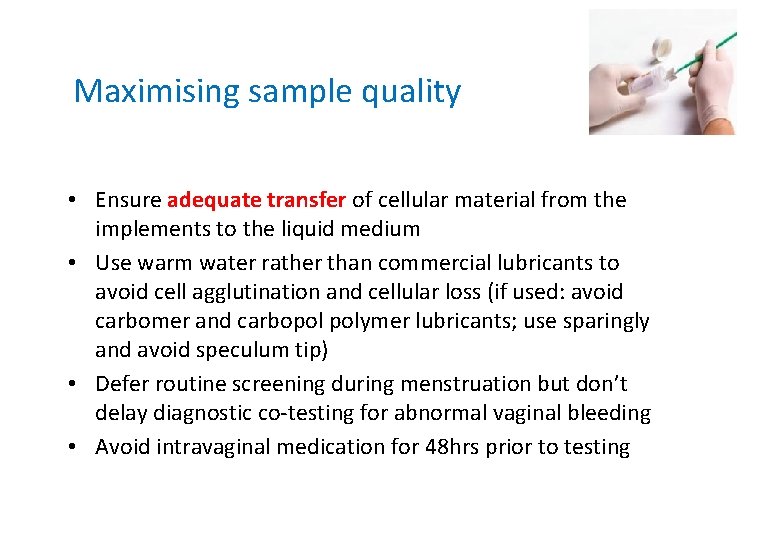
Maximising sample quality • Ensure adequate transfer of cellular material from the implements to the liquid medium • Use warm water rather than commercial lubricants to avoid cell agglutination and cellular loss (if used: avoid carbomer and carbopol polymer lubricants; use sparingly and avoid speculum tip) • Defer routine screening during menstruation but don’t delay diagnostic co-testing for abnormal vaginal bleeding • Avoid intravaginal medication for 48 hrs prior to testing

Self-collection of a vaginal HPV test http: //www. cancerscreening. gov. au/internet/screening/publishing. nsf/Content/re sources-menu

Self-collection of a vaginal HPV sample • Alternative for eligible under or never-screened women who have declined invitations to participate in conventional screening Eligibility: Ø 30 years + and never had cervical screening Ø 30 years + and overdue by 2 years or longer • Facilitated by a health professional within a healthcare clinic • Dry flocked swab self-inserted into vagina

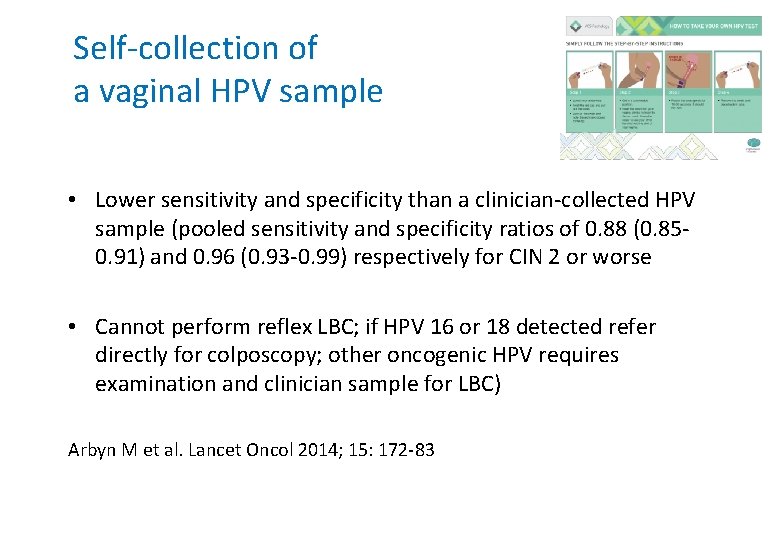
Self-collection of a vaginal HPV sample • Lower sensitivity and specificity than a clinician-collected HPV sample (pooled sensitivity and specificity ratios of 0. 88 (0. 850. 91) and 0. 96 (0. 93 -0. 99) respectively for CIN 2 or worse • Cannot perform reflex LBC; if HPV 16 or 18 detected refer directly for colposcopy; other oncogenic HPV requires examination and clinician sample for LBC) Arbyn M et al. Lancet Oncol 2014; 15: 172 -83

Transition into the National Cervical Screening Program • Women aged 25 to 74 will be invited for a Cervical Screening Test two years after their last Pap test (new migrants & refugees) • Women already screened under 25 will be a sent a letter advising rescreening at age 25; an invitation letter (and reminders) will be sent from the NCSR at age 24 and 9 m • Women overdue by 2 years of more and >30 years old will be invited to screen and self collection an option. • Women under surveillance for an abnormal Pap test: follow guidelines Provide cytology screening if due until Dec 1 st!

Screening for specific populations: Pregnancy & Postpartum Post-menopause Early sexual activity Immune-compromise (screen every 3 years) Post-hysterectomy DES-exposure in utero Transgender men with a cervix (consider a short course of vaginal oestrogen) • Women with abnormal vaginal bleeding or an abnormal appearing cervix • •

Screening for specific populations • Pregnancy – perform if due (use a broom-type sampler brush NOT a cytobrush or combi-brush) Ø Self-collection not recommended • Postpartum - screen > 6 weeks after delivery; if breastfeeding or no menses, consider prior short course vaginal oestrogen • Post-menopausal – no recommendation for routine vaginal oestrogen; consider if vaginal dryness/superficial dyspareunia or if reflex LBC unsatisfactory due to atrophy, insufficient cells or inflammation or prior to colposcopy

Screening in specific populations: immunocompromised Immune-deficiency - HIV +ve or solid organ transplant recipients screen 3 yearly if normal screening history Consider 3 yearly screening in other immune-deficient women: Øcongenital primary immune deficiency Ø immunosuppressant therapy Øbone marrow transplant recipients Øscreening young women(20 -24 years) if immune deficient for more than 5 years. +ve HPV (any type) referral for colposcopy (with experienced gynaecologist) regardless of LBC result. . ))

Screening in specific populations: Diethylstilboestrol (DES) exposure • Women exposed to DES in utero: should be offered an annual cotest (HPV + LBC) and expert colposcopy and examination of the cervix and vagina indefinitely. • Not for self collection • No evidence of increased risk for ‘DES granddaughters’- routine screening recommended ( 5 yearly HPV testing) 1 Pharmaceuticals WHO 2012

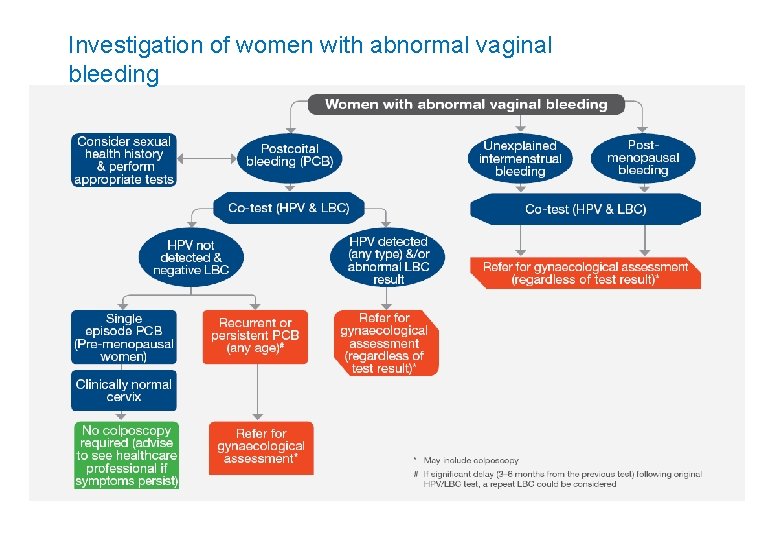
Investigation of women with abnormal vaginal bleeding

Abnormal vaginal bleeding (post-coital, intermenstrual, postmenopausal) • Malignancy uncommon but must be excluded; consider pregnancy, STIs, polyps, coagulopathies, ovulatory disorders, endometrial disorders, vaginal atrophy, hormonal contraception • Women of ANY age with signs or symptoms suggestive of cervical cancer should have a co-test • Co-test: a HPV DNA test AND liquid based cytology on the sample • Co-test has high negative predictive value for HSIL/CIN 3 • Do not delay co-test due to the presence of blood: co-testing improves reduced sensitivity of individual tests Management of co-test results……. .

Investigation of women with abnormal vaginal bleeding

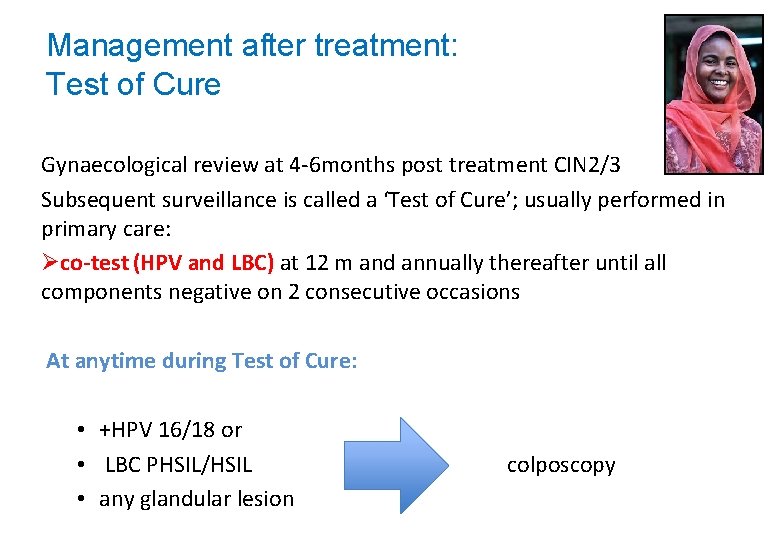
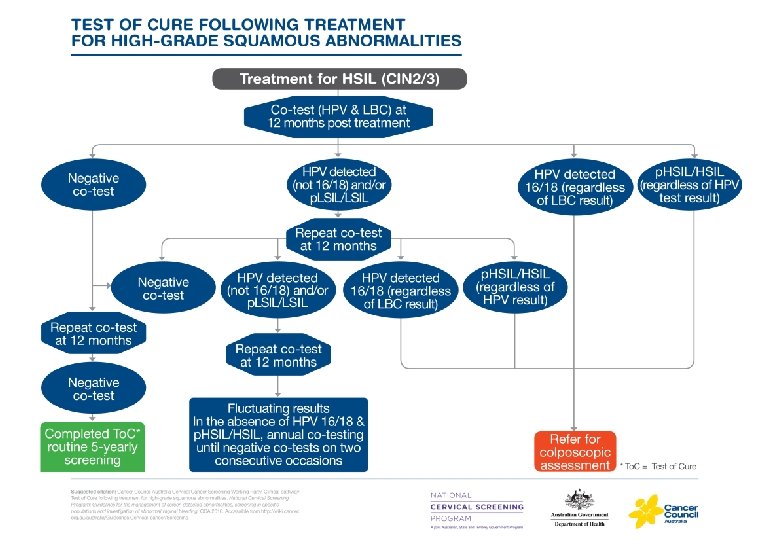
Management after treatment: Test of Cure Gynaecological review at 4 -6 months post treatment CIN 2/3 Subsequent surveillance is called a ‘Test of Cure’; usually performed in primary care: Øco-test (HPV and LBC) at 12 m and annually thereafter until all components negative on 2 consecutive occasions At anytime during Test of Cure: • +HPV 16/18 or • LBC PHSIL/HSIL colposcopy • any glandular lesion


Some groups are more likely to be under or never-screened • • • Aboriginal and Torres Strait Islander Culturally & Linguistically Diverse ( CALD) History of sexual trauma and/or domestic violence Living in a rural or remote areas, Identify as lesbian, bisexual, or same sex attracted Transgender men (with a cervix) Older women Women with disabilities (intellectual or physical) Women from lower socioeconomic status Women who have received the HPV vaccine

How to improve screening uptake? Ø Cultural awareness-including language barriers Ø Provide educational leaflets and posters Ø Stress importance of cervical screening as a preventative measure - prevents cancer Ø Asking if prefer to see a female colleague Ø Consider self-collection for eligible women

Understanding the National Cancer Screening Register Ø Single record for cervical & bowel cancer screening; will be linked to HPV vaccine registry Ø Records screening & colposcopy data; provides screening Hx to labs to inform recommendations Ø Health Care Provider portal Ø Consumer portal: limited information e. g. check due date for test or change address. Ø Sends letters of invitation and reminders; women can opt for reminders by mail, email or SMS; can nominate a personal representative Ø Women can ‘opt off’ by contacting the NCSR (initially by phone with or without support from clinician); previously via pathology request form and lab

The cervical screening consultation: what’s new and what’s the same • Discussion about screening; history taking; informed consent • Addressing screening barriers and opportunistically screening • Experience IS THE SAME FOR THE PATIENT with speculum examination • Sample of cells from squamo-columnar junction; no slides! • Opportunity for self-collection for eligible under-screened women • Completion of pathology request form for Cervical Screening Test (history, symptoms, examination) • Lab performs HPV test +/- reflex LBC • For specific indications order a co-test on the form

The cervical screening consultation: what’s new and what’s the same • Timely receipt of abnormal results; clear documentation of consultation and any follow-up • Discussion of results; risk assessment for cervical abnormality: Ø Low risk: invited to screen in 5 years Ø Intermediate risk: invited to screen in 12 m to check HPV clearance Ø Higher risk: referred for colposcopy • Using the new management guidelines • Informing women about the National Cancer Screening Register

Resources for women and health professionals http: //www. cancerscreening. gov. au/internet/screening/publishing. nsf/C ontent/resources-menu

Resources for health professionals Clinical guidelines wiki. cancer. org. au/australia/Guidelines: Cervical_cancer/Screening Clinical guidelines - Short Form Summary http: //wiki. cancer. org. au/australiawiki/images/2/2 e/National_Cervical_Screening_ Program_guidelines_short-form_PDF. pdf Clinical guidelines - Long Form Summary http: //wiki. cancer. org. au/australiawiki/images/a/ad/National_Cervical_Screening_ Program_guidelines_long-form_PDF. pdf

Resources Ø Background information on the Renewal www. msac. gov. au Ø NPS Medicine. Wise Learning modules on the changes to the National Cervical Screening Programhttps: //learn. nps. org. au/mod/page/view. php? id=7804 Ø National HPV Vaccination Program Register www. hpvregister. org. au Ø HPV vaccination www. cervicalcancervaccine. com. au

FPNSW resources • Talkline: phone 1300 658 886 • Reproductive and Sexual Health: an Australian Clinical Practice Handbook 3 rd edition • Fact Sheets: Cervical Screening and HPV vaccination https: //www. fpnsw. org. au/healthinformation/individuals/pap-test-and-cervical-screening • Everything you need to know about the changes to NSCP https: //www. fpnsw. org. au/changes

Key messages Ø Encourage eligible women (vaccinated and unvaccinated) to attend for cervical screening; opportunistically screen Ø New National Cervical Screening Program starts 1 st December; evidence-based tests and protocols Ø ‘More accurate and less harm’ Ø The Cervical Screening Test replaces the Pap test on 1 st December 2017 - no more slides! Ø Encourage primary prevention with HPV vaccination Ø Know where to go for information (including for specific populations)

Thank you and any questions?