Human platelet 150 x 109 400 x 109











- Slides: 33


Human platelet • 150 x 109 ~ 400 x 109 / liter • Life span; 10 days • Blood volume of 5 liters • One-third of platelet pooled in the spleen • Produce approximately 1 x 1011, each day • Maintain a count under steady-state conditions • A level of production increase more than 10 -fold under condition of increased demand

Thrombocytopenia • Increase the risk of bleeding • Limit the dose of chemotherapeutic agents • Delay the time of treatment Platelet transfusion

Platelet transfusion • Immune or cytokine mediated febrile reaction • Bacteremia • Graft-versus-host disease • Acute pulmonary injury • Inadequate platelet response due to HLA alloimmunization • Platelet transfusions are expensive

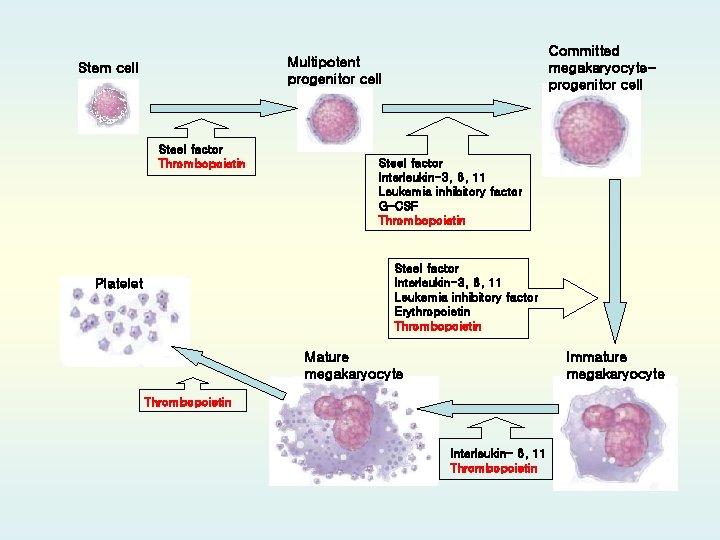
Committed megakaryocyteprogenitor cell Multipotent progenitor cell Stem cell Steel factor Thrombopoietin Steel factor Interleukin-3, 6, 11 Leukemia inhibitory factor G-CSF Thrombopoietin Steel factor Interleukin-3, 6, 11 Leukemia inhibitory factor Erythropoietin Thrombopoietin Platelet Mature megakaryocyte Immature megakaryocyte Thrombopoietin Interleukin- 6, 11 Thrombopoietin

Thrombopoietin (TPO, THPO) The primary regulator of platelet production

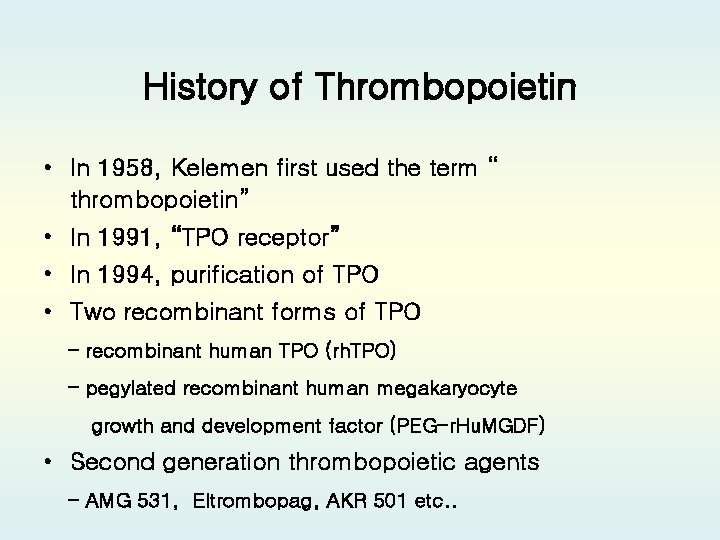
History of Thrombopoietin • In 1958, Kelemen first used the term “ thrombopoietin” • In 1991, “TPO receptor” • In 1994, purification of TPO • Two recombinant forms of TPO - recombinant human TPO (rh. TPO) - pegylated recombinant human megakaryocyte growth and development factor (PEG-r. Hu. MGDF) • Second generation thrombopoietic agents - AMG 531, Eltrombopag, AKR 501 etc. .

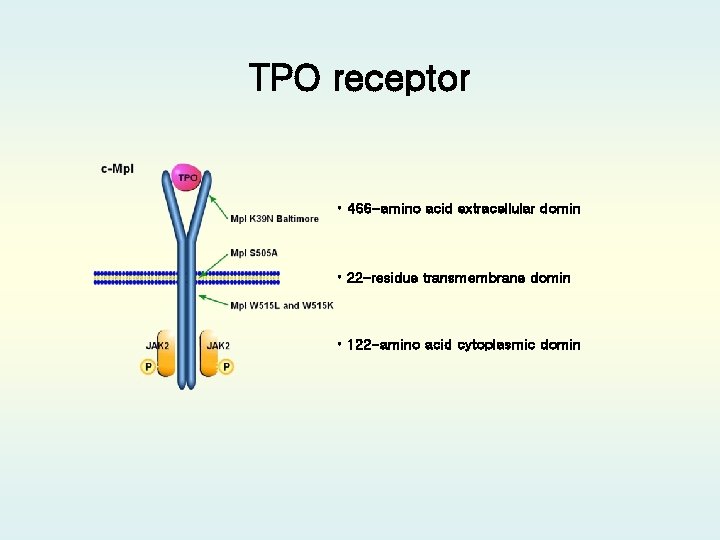
TPO receptor • 466 -amino acid extracellular domin • 22 -residue transmembrane domin • 122 -amino acid cytoplasmic domin

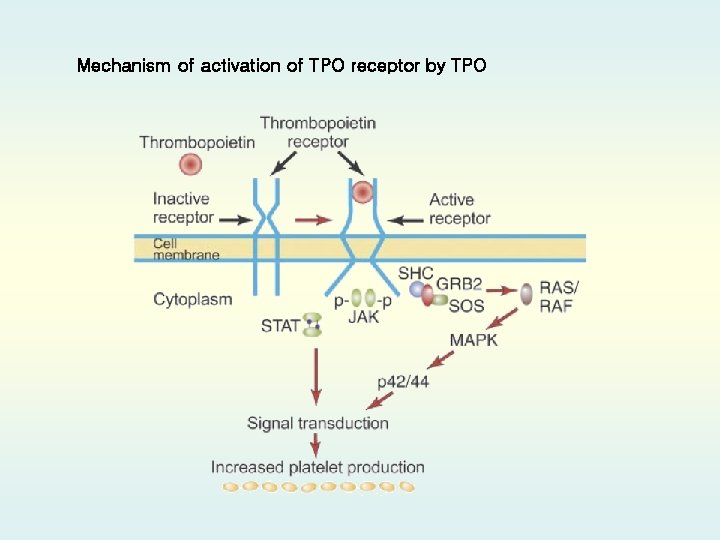
Mechanism of activation of TPO receptor by TPO

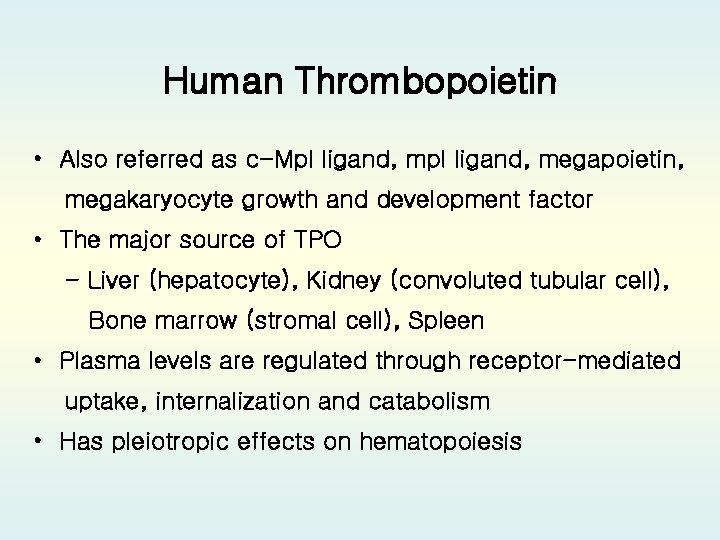
Human Thrombopoietin • Also referred as c-Mpl ligand, megapoietin, megakaryocyte growth and development factor • The major source of TPO - Liver (hepatocyte), Kidney (convoluted tubular cell), Bone marrow (stromal cell), Spleen • Plasma levels are regulated through receptor-mediated uptake, internalization and catabolism • Has pleiotropic effects on hematopoiesis

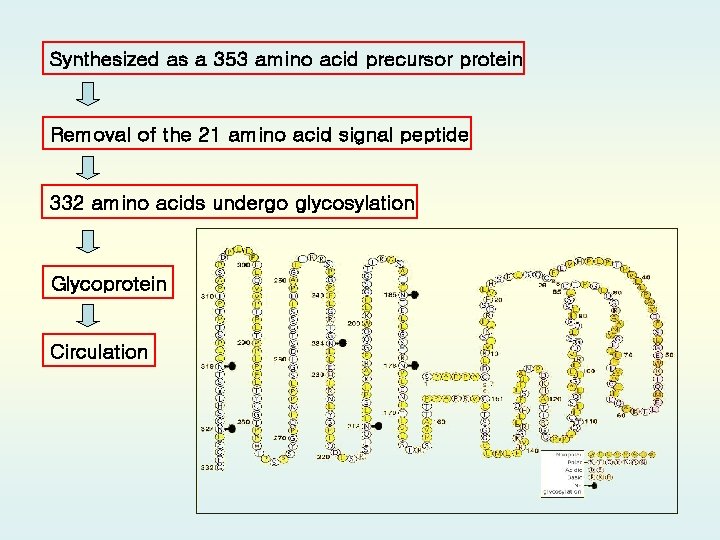
Synthesized as a 353 amino acid precursor protein Removal of the 21 amino acid signal peptide 332 amino acids undergo glycosylation Glycoprotein Circulation

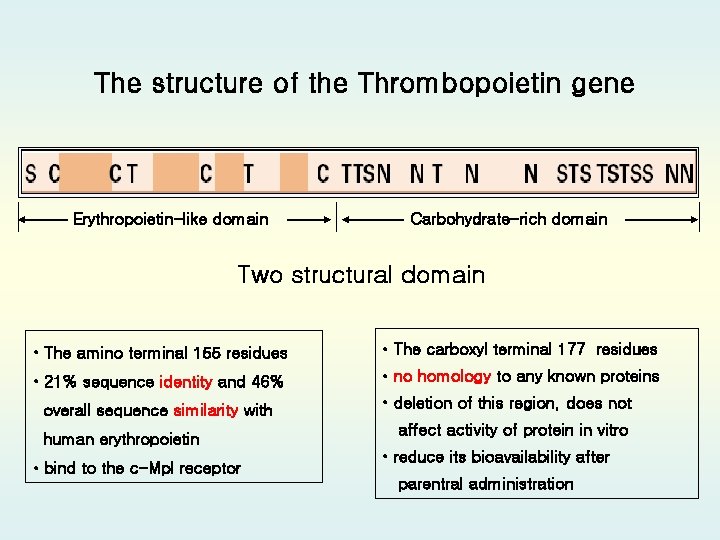
The structure of the Thrombopoietin gene Erythropoietin-like domain Carbohydrate-rich domain Two structural domain • The amino terminal 155 residues • The carboxyl terminal 177 residues • 21% sequence identity and 46% • no homology to any known proteins overall sequence similarity with human erythropoietin • bind to the c-Mpl receptor • deletion of this region, does not affect activity of protein in vitro • reduce its bioavailability after parentral administration

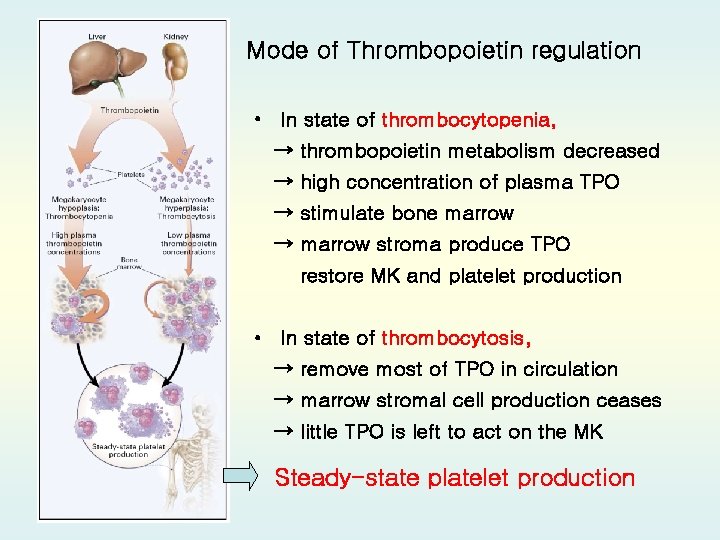
Mode of Thrombopoietin regulation • In state of thrombocytopenia, → thrombopoietin metabolism decreased → high concentration of plasma TPO → stimulate bone marrow → marrow stroma produce TPO restore MK and platelet production • In state of thrombocytosis, → remove most of TPO in circulation → marrow stromal cell production ceases → little TPO is left to act on the MK Steady-state platelet production

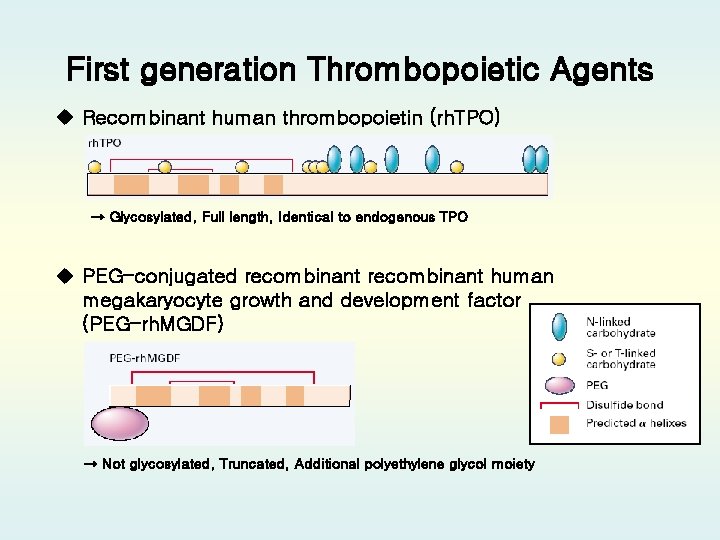
First generation Thrombopoietic Agents u Recombinant human thrombopoietin (rh. TPO) → Glycosylated, Full length, Identical to endogenous TPO u PEG-conjugated recombinant human megakaryocyte growth and development factor (PEG-rh. MGDF) → Not glycosylated, Truncated, Additional polyethylene glycol moiety

Clinical trials of first generation Thrombopoietic Agent

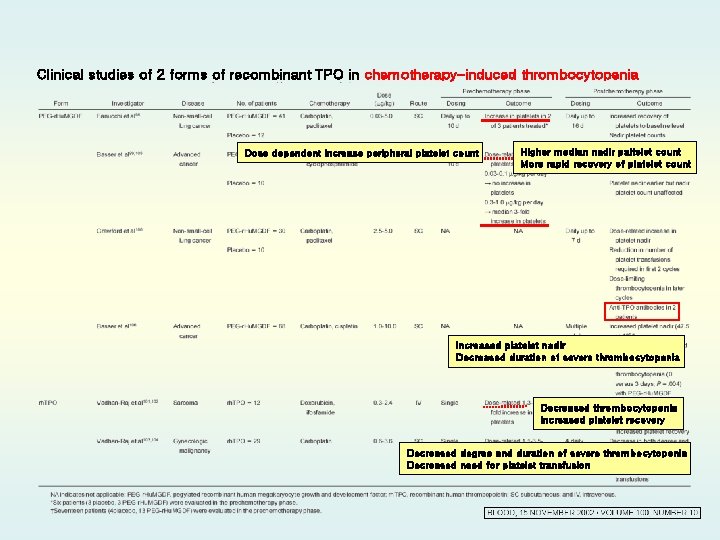
Clinical studies of 2 forms of recombinant TPO in chemotherapy-induced thrombocytopenia Dose dependent increase peripheral platelet count Higher median nadir paltelet count More rapid recovery of platelet count Increased platelet nadir Decreased duration of severe thrombocytopenia Decreased thrombocytopenia Increased platelet recovery Decreased degree and duration of severe thrombocytopenia Decreased need for platelet transfusion

Clinical studies of 2 forms of recombinant TPO as adjunct to induction/consoidation chemotherapy in patients with newly diadnosed acute myelogenous leukemia No difference in platelet recovery time No difference in platelet transfusion need

Clinical studies of 2 forms of recombinant TPO in patients receiving myeloablative chemotherapy followed HSCT Dose related increased in median platelet count No difference in platelet recovery time No difference platelet transfusion requirement No difference in platelet recovery time No difference in thrombocytopenia No difference platelet transfusion requirement No effect in platelet recovery time No effect in platelet transfusion requirement Reduced time to platelet recovery and platelet transfusion requirement with G-CSF Dose related increased in median platelet count No difference in platelet recovery time No difference platelet transfusion requirement

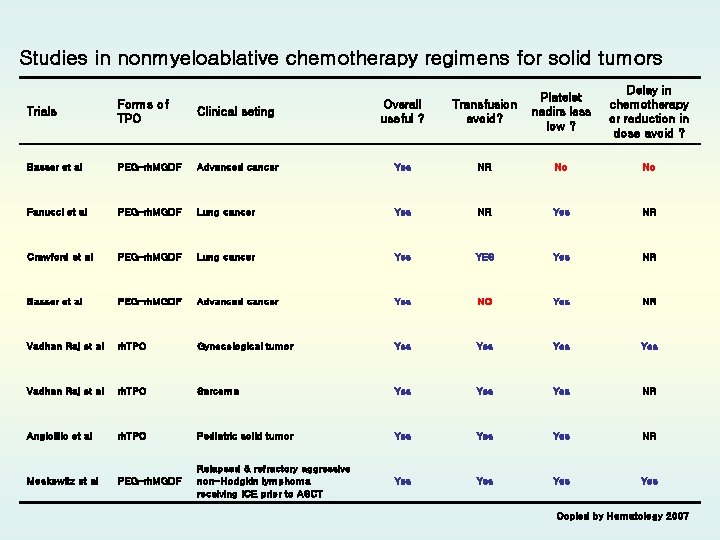
Studies in nonmyeloablative chemotherapy regimens for solid tumors Delay in chemotherapy or reduction in dose avoid ? Trials Forms of TPO Clinical seting Overall useful ? Transfusion avoid? Platelet nadirs less low ? Basser et al PEG-rh. MGDF Advanced cancer Yes NR No No Fanucci et al PEG-rh. MGDF Lung cancer Yes NR Crawford et al PEG-rh. MGDF Lung cancer Yes YES Yes NR Basser et al PEG-rh. MGDF Advanced cancer Yes NO Yes NR Vadhan Raj et al rh. TPO Gynecological tumor Yes Yes Vadhan Raj et al rh. TPO Sarcoma Yes Yes NR Angiolillo et al rh. TPO Pediatric solid tumor Yes Yes NR Moskowitz et al PEG-rh. MGDF Relapsed & refractory aggressive non-Hodgkin lymphoma receiving ICE prior to ASCT Yes Yes Copied by Hematology 2007

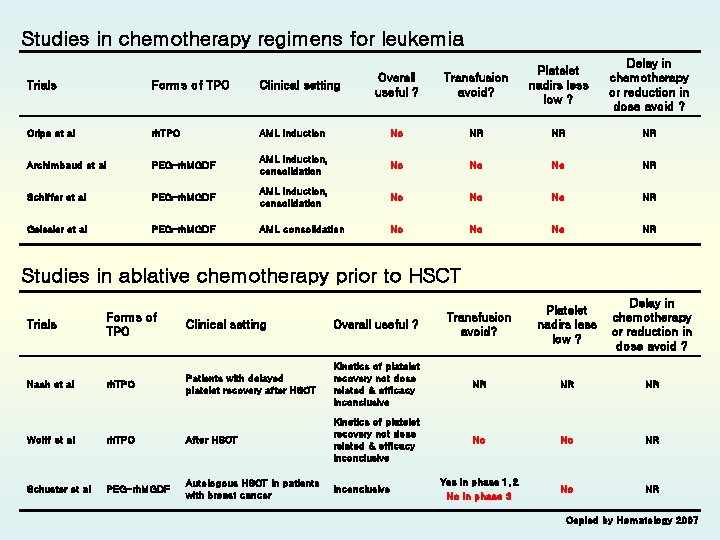
Studies in chemotherapy regimens for leukemia Delay in chemotherapy or reduction in dose avoid ? Overall useful ? Transfusion avoid? Platelet nadirs less low ? AML induction No NR NR NR PEG-rh. MGDF AML induction, consolidation No No No NR Schiffer et al PEG-rh. MGDF AML induction, consolidation No No No NR Geissler et al PEG-rh. MGDF AML consolidation No No No NR Trials Forms of TPO Clinical setting Cripe et al rh. TPO Archimbaud et al Studies in ablative chemotherapy prior to HSCT Trials Nash et al Delay in chemotherapy or reduction in dose avoid ? Forms of TPO Clinical setting Overall useful ? Transfusion avoid? Platelet nadirs less low ? rh. TPO Patients with delayed platelet recovery after HSCT Kinetics of platelet recovery not dose related & efficacy inconclusive NR NR NR No No NR Yes in phase 1, 2 No in phase 3 No NR Wolff et al rh. TPO After HSCT Kinetics of platelet recovery not dose related & efficacy inconclusive Schuster et al PEG-rh. MGDF Autologous HSCT in patients with breast cancer Inconclusive Copied by Hematology 2007

Potential problems of first generation thrombopoietic agent (PEG-rh. MGDF) u Development of neutralizing antibodies → Neutralized both the recombinant and endogenous TPO → Severe thrombocytopenia u discontinuation of the clinical use of first generation growth factor Search a nonimmunogenic thrombopoietins Develop second generation thrombopoietic agents

Second generation Thrombopoietins • TPO Peptide Mimetics bind to c-Mpl receptor no identical or similar peptide sequence of native TPO Fc carrier domain + peptide receptor binding domain AMG-531, Romiplostim • TPO Nonpeptide Mimetics binding to the transmembrane region small chemical structure, orally administered bind only to TPO receptors in human and chimpanzees eltrombopag, AKR-501, LGD 4665 • TPO Antibody Mimetics TPO minibody, MA 01 G 4344

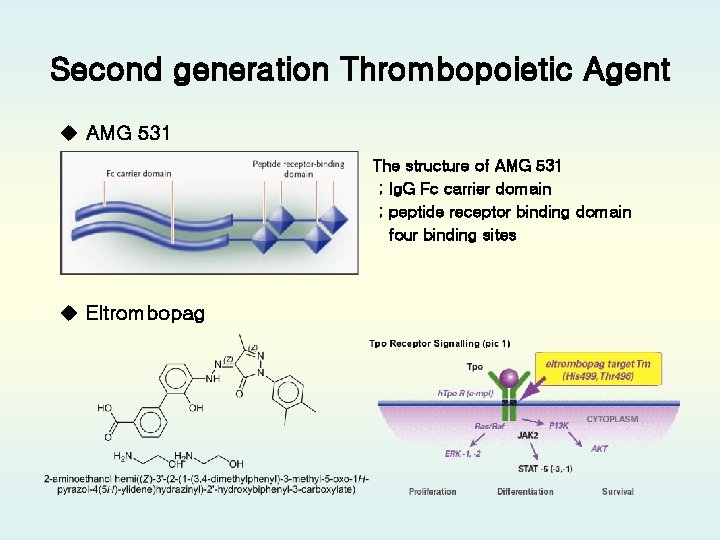
Second generation Thrombopoietic Agent u AMG 531 The structure of AMG 531 ; Ig. G Fc carrier domain ; peptide receptor binding domain four binding sites u Eltrombopag

Clinical trials of Second generation Thrombopoietc Agent





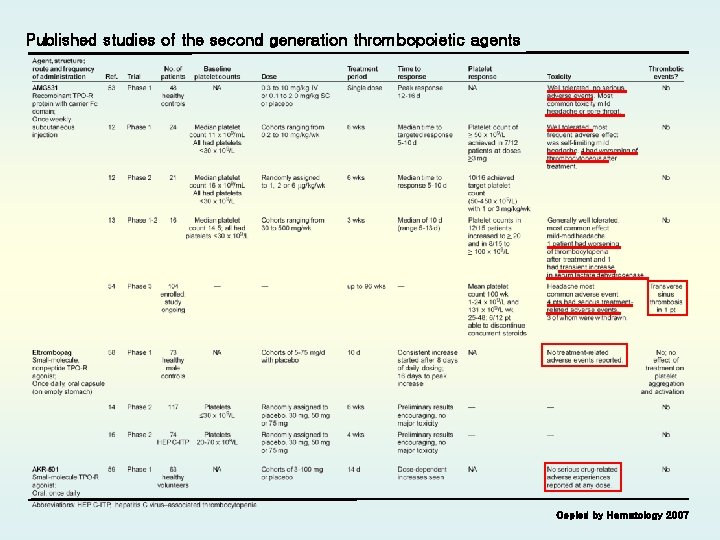
Published studies of the second generation thrombopoietic agents Copied by Hematology 2007

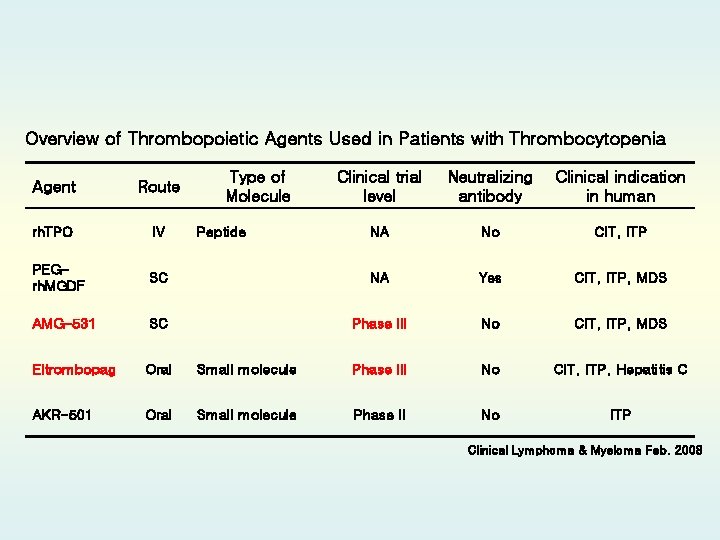
Overview of Thrombopoietic Agents Used in Patients with Thrombocytopenia Clinical trial level Neutralizing antibody Clinical indication in human NA No CIT, ITP SC NA Yes CIT, ITP, MDS AMG-531 SC Phase III No CIT, ITP, MDS Eltrombopag Oral Small molecule Phase III No CIT, ITP, Hepatitis C AKR-501 Oral Small molecule Phase II No ITP Agent Route rh. TPO IV PEGrh. MGDF Type of Molecule Peptide Clinical Lymphoma & Myeloma Feb. 2008

• Thrombocytopenia is a common medical problems in the management of patient with cancer and other conditions that affect hematopoietic cell. • Although first generation thrombopoietic agents were successful in increasing platelet count and reducing platelet transfusion requirements. • Because of unexpected side effect “neutralizing antiboy”, clinical trials of first generation thrombopoietic agents were discontinued.

• To overcome the problem of antigenecity, several second generation thrombopoietic agents have been developed and many clinical trials were tried and on going. • Many successful results are reported. • I hope that we will have a effective, safe, easily and widely used thrombopoietic agnet in recent future.

Thank you very much for your attention