Transfusion in children Packed cell platelet FFP cryoprecipitate

































- Slides: 52


Transfusion in children Packed cell, platelet, FFP, cryoprecipitate, WBC

Packed red cell Transfusion

Packed red cells • Average hematocrit of a unit is 65 -75% ( concentrated) • Estimated unit size : 250 -350 cc • Stored at 2 -6 C • Mixed with preservation ( shelf-life of 35 days) • Infusion should take maximum 4 hours

General guidelines • Oncology: Hb, 8 gr/dl increased O 2 requirement patient symptomatic • Bone marrow failure: Hb<7 gr/dl increased O 2 requirement • Hemoglobinopathies: clinical situation(thalassemia major or intermediate, sickle cell

Contraindication: • Anemia that can be corrected by nontransfusion therapy( iron, recombinants erythropoietin) • Hypovolemia

Recommendation • CMV seronegative, irradiated, leukopoor preparation should be used per clinical guidelines

Platelet transfusion

Descriptions: • 1 random donor unit is concentrate of platelet separated from a unite of whole blood (5. 5× 1010 platelet in 40 -70 cc plasma) • A single donor unit is a unit collected by apheresis that contain 4 -8 time the number of platelet in 1 random donor unit (3 × 1011 platelet in 100 -500 cc plasma)

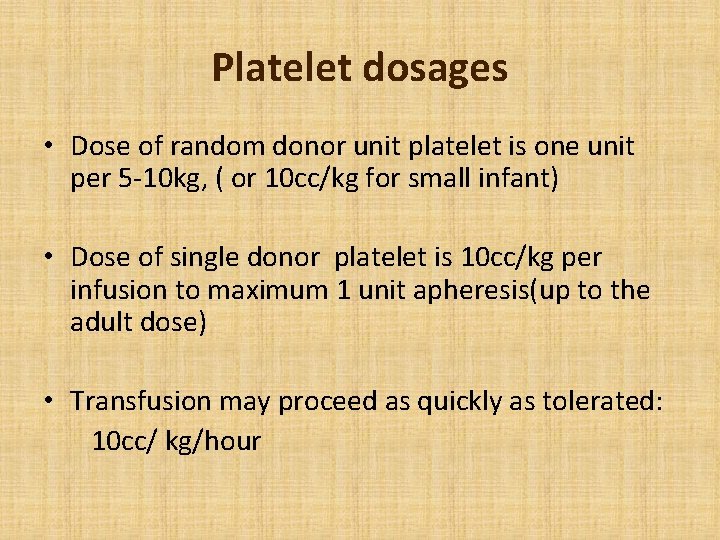
Platelet dosages • Dose of random donor unit platelet is one unit per 5 -10 kg, ( or 10 cc/kg for small infant) • Dose of single donor platelet is 10 cc/kg per infusion to maximum 1 unit apheresis(up to the adult dose) • Transfusion may proceed as quickly as tolerated: 10 cc/ kg/hour

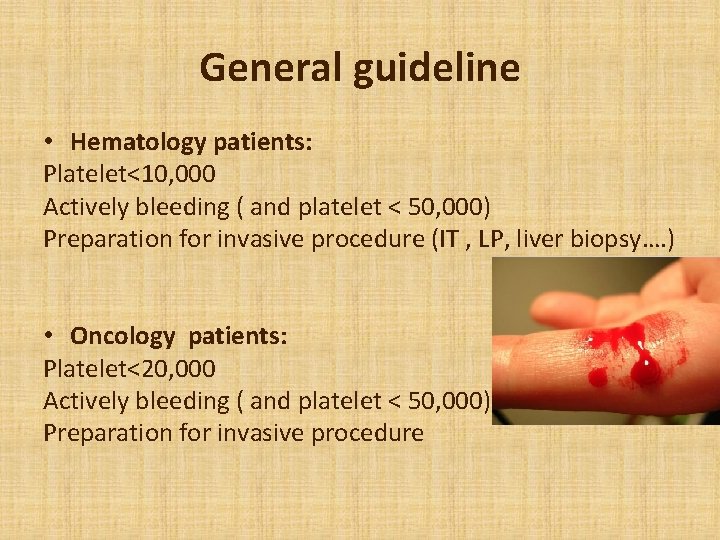
General guideline • Hematology patients: Platelet<10, 000 Actively bleeding ( and platelet < 50, 000) Preparation for invasive procedure (IT , LP, liver biopsy…. ) • Oncology patients: Platelet<20, 000 Actively bleeding ( and platelet < 50, 000) Preparation for invasive procedure

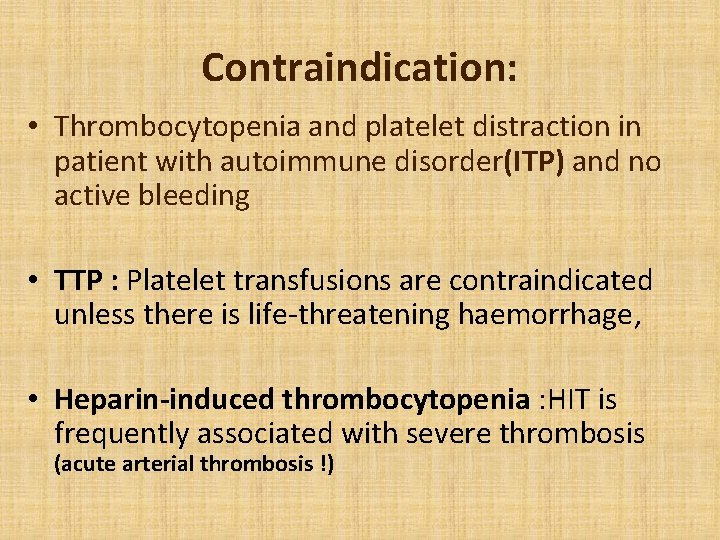
Contraindication: • Thrombocytopenia and platelet distraction in patient with autoimmune disorder(ITP) and no active bleeding • TTP : Platelet transfusions are contraindicated unless there is life-threatening haemorrhage, • Heparin-induced thrombocytopenia : HIT is frequently associated with severe thrombosis (acute arterial thrombosis !)

Recommendation: • Infuse relatively quickly(10 ccc/kg/hour) to reduce clumping and adherence in bag and tubing • If concern over platelet response, obtain posttransfusion platelet count at 15 minute to 1 hour

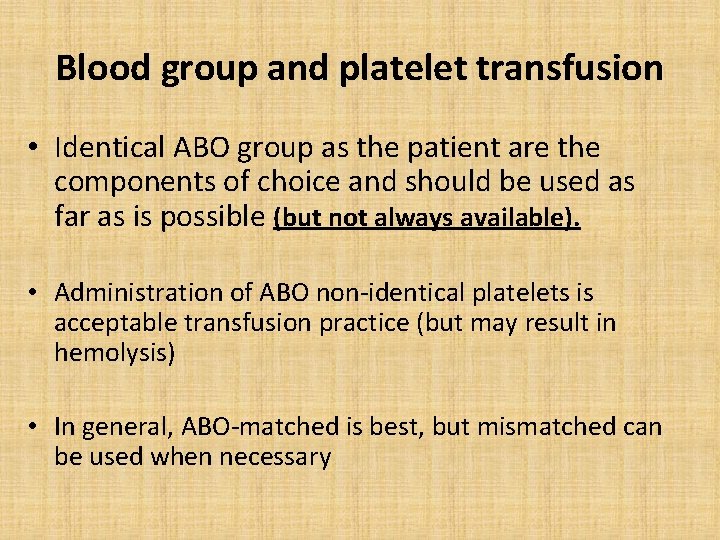
Blood group and platelet transfusion • Identical ABO group as the patient are the components of choice and should be used as far as is possible (but not always available). • Administration of ABO non-identical platelets is acceptable transfusion practice (but may result in hemolysis) • In general, ABO-matched is best, but mismatched can be used when necessary

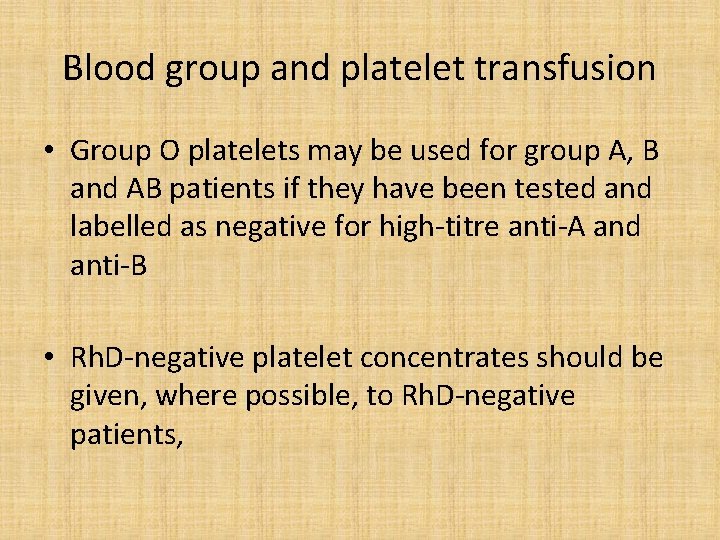
Blood group and platelet transfusion • Group O platelets may be used for group A, B and AB patients if they have been tested and labelled as negative for high-titre anti-A and anti-B • Rh. D-negative platelet concentrates should be given, where possible, to Rh. D-negative patients,

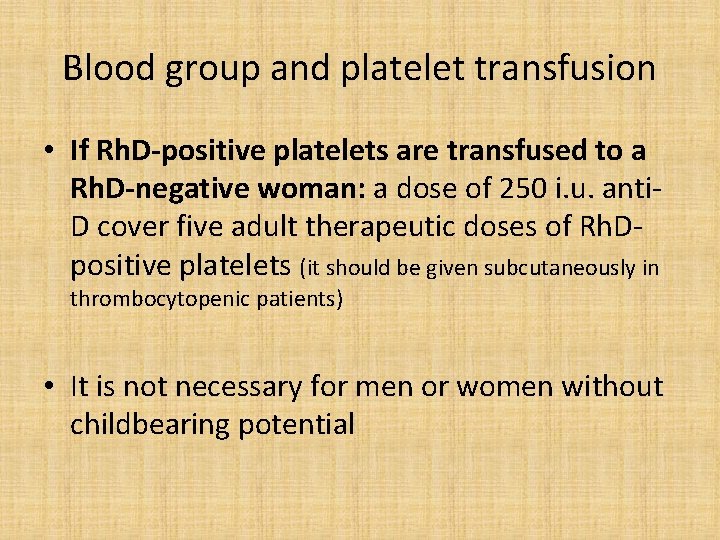
Blood group and platelet transfusion • If Rh. D-positive platelets are transfused to a Rh. D-negative woman: a dose of 250 i. u. anti. D cover five adult therapeutic doses of Rh. Dpositive platelets (it should be given subcutaneously in thrombocytopenic patients) • It is not necessary for men or women without childbearing potential

Fresh Frozen Plasma Transfusion

Fresh Frozen Plasma(FFP) • Contain coagulation factors in physiologically amounts( each ml contain I IU of each coagulation factor • Contain anticoagulation's such as protein C and S • Contain albumin, immunoglobulins, and complement proteins

Indications for FFP • Patients who require replacement of multiple plasma coagulation factors( e. g. liver disease, DIC. . ) • Massive transfusion ( have clinically significant coagulation deficiencies) • Patient taking warfarin who are bleeding or need to undergo an invasive procedure before Vit. K could reverse the warfarin effect • Transfusion or plasma exchange in patient with TTP • Management of patients with selected coagulation factor deficiencies , for which no specific coagulation concentrates are available

Contraindications FFP • Don’t use when coagulopathy can be corrected with specific therapy( Vit. K, cryoprecipitate, coagulation factor concentrate) • Don’t use when blood volume can be safely and adequately replaced with other volume expander • Don’t use as a source of albumin

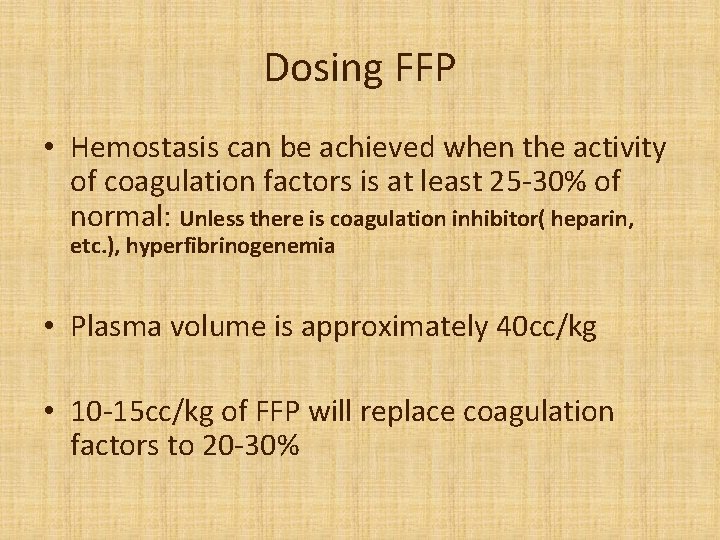
Dosing FFP • Hemostasis can be achieved when the activity of coagulation factors is at least 25 -30% of normal: Unless there is coagulation inhibitor( heparin, etc. ), hyperfibrinogenemia • Plasma volume is approximately 40 cc/kg • 10 -15 cc/kg of FFP will replace coagulation factors to 20 -30%

FFP storage • Frozen at -18 C for 1 year or at -65 for 7 years • Once thawed should be infused within 4 hours

Cryoprecipitate

Cryoprecipitate • Cold soluble remnant of FFE • Concentrated preparation that contain : Factor 8 (80 -100 IU/bag), Fibrinogen(200 mg/bag), Factor 13, von Willwberand factor

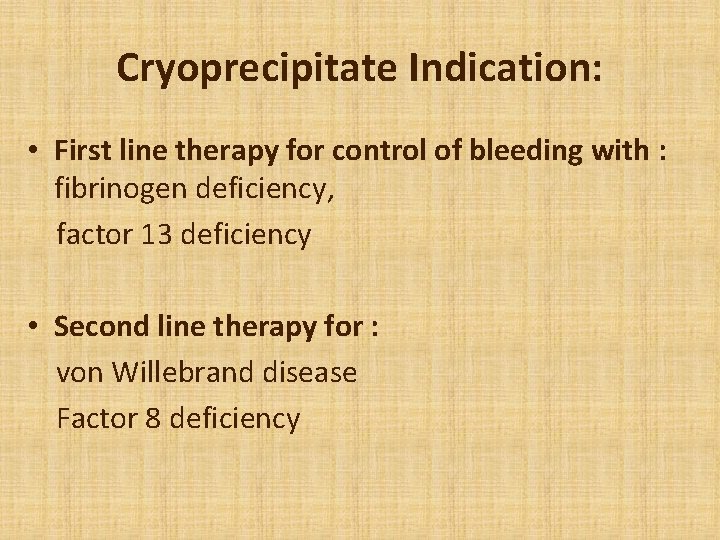
Cryoprecipitate Indication: • First line therapy for control of bleeding with : fibrinogen deficiency, factor 13 deficiency • Second line therapy for : von Willebrand disease Factor 8 deficiency

Cryoprecipitate Contraindications: • Don’t use unless result of laboratory studies indicate a specific hemostasis defect for which this product indicated • Don’t use if virus inactivated factor 8 concentrates or recombinants factor preparation are available for patient with v. W. disease or hemophilia A • Don’t use for DIC( dose not contain all necessary factors(factor 5)

Cryoprecipitate dose • Hyperfibrinogenemia: 0. 2 bag /kg( increase fibrinogen approximately 50 -100 mg/dl) • Factor 13 deficiency: 1 bag/10 kg once • Bleeding in v. WD : 1 bag/10 kg every 6 -12 hours

WBC transfusion

WBCs • Administered as soon after collection as possible • If stored, maintain at room temperature(20 -24 C) without agitation, for no more than 24 hours • Donor preparation with G-CSF increased harvest yield

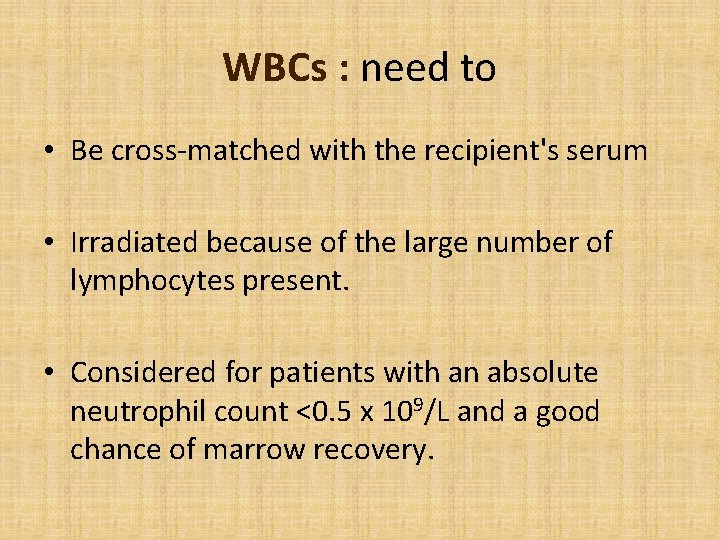
WBCs : need to • Be cross-matched with the recipient's serum • Irradiated because of the large number of lymphocytes present. • Considered for patients with an absolute neutrophil count <0. 5 x 109/L and a good chance of marrow recovery.

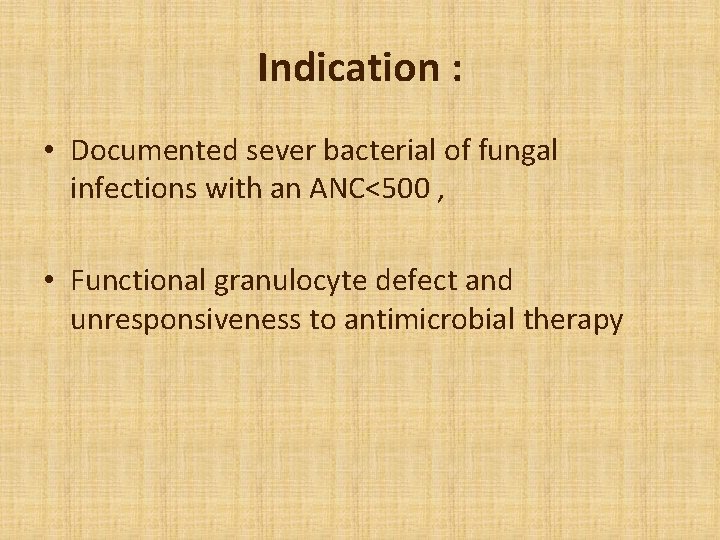
Indication : • Documented sever bacterial of fungal infections with an ANC<500 , • Functional granulocyte defect and unresponsiveness to antimicrobial therapy

Contraindication: • Irreversible BM failure • Prophylaxis's in non infected patients

Pediatric dosage • 1 -2× 109 cell/kg • Once initiated, WBC therapy should continue on daily basis until infection is cured, patient defervesce, or ANC is>500

Processing Leukodepletion, Gamma irradiation, washing, CMV negative

Processing: Leukodepletion

Processing: Leukodepletion • Leukodepletion is a technical term for the removal of leucocytes (white blood cells) from blood components using special filters. • Leukodepletion of blood components removes ≥ 99% of contaminating leucocytes • Prestorage or bedside filter?

Processing: Leukodepletion • Reduced risk of platelet refractoriness(HLA alloimmunization) • Reduced risk of febrile non-haemolytic transfusion reactions • Reduced risk of CMV transmission • Possible reduced risk of transfusion-associated GVHD (reduce risk but not prevent)

Processing: Gamma irradiation

Processing: Gamma irradiation • Gamma irradiation of blood product to stop donor lymphocyte proliferation • Prevent transfusion induced GVHD (100% fatal)

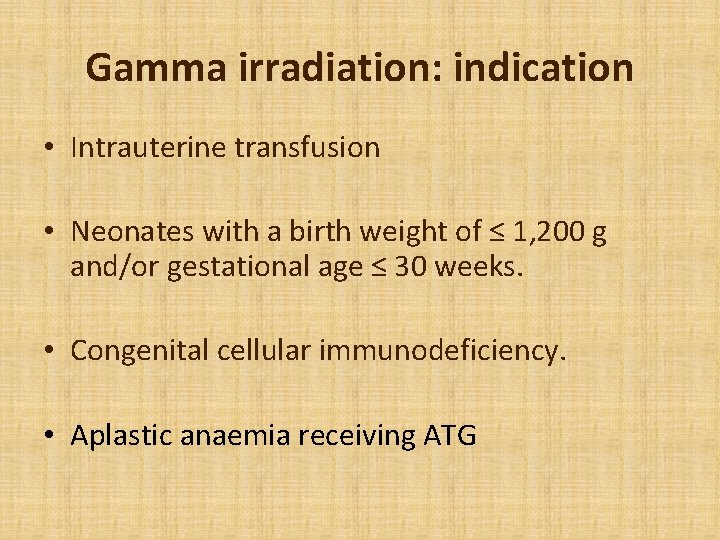
Gamma irradiation: indication • Intrauterine transfusion • Neonates with a birth weight of ≤ 1, 200 g and/or gestational age ≤ 30 weeks. • Congenital cellular immunodeficiency. • Aplastic anaemia receiving ATG

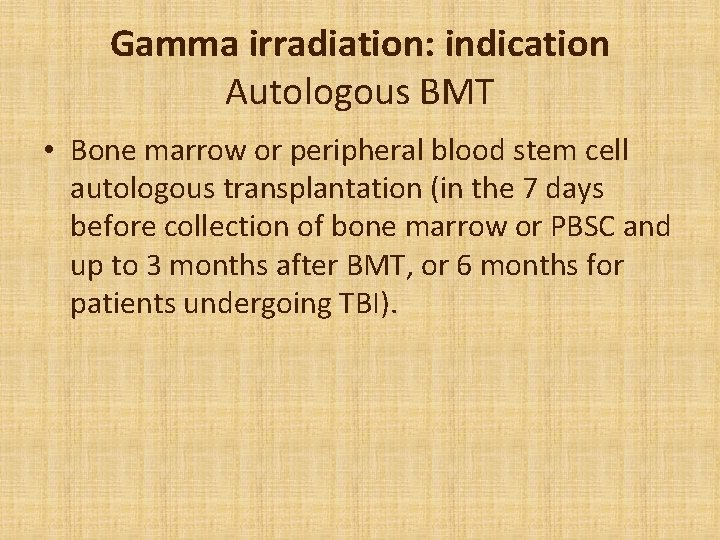
Gamma irradiation: indication Autologous BMT • Bone marrow or peripheral blood stem cell autologous transplantation (in the 7 days before collection of bone marrow or PBSC and up to 3 months after BMT, or 6 months for patients undergoing TBI).

Gamma irradiation: indication Allogeneic BMT • All recipients of allogeneic haemopoietic stem cell transplantation (SCT) must receive irradiated blood components from the time of initiation of conditioning chemoradiotherapy • Irradiated components should be continued while the patient continues to receive GVHD prophylaxis (until the end of GVHD prophylaxis) • Allogeneic blood transfused to bone marrow and peripheral blood stem cell donors 7 days prior to or during the harvest should also be irradiated.

Gamma irradiation: indication chemotherapy • Hodgkin’s lymphoma • chemotherapy (should be decided on an individual basis) • It is not necessary to irradiate red cells or platelets for adults or children with acute leukaemia, except for HLA-selected platelets or donations from first- or second-degree relatives

Gamma irradiation: indication other indications • All transfusions from first- or second-degree relatives should be irradiated, even if the patient is immunocompetent • All HLA-selected platelets should be irradiated, even if the patient is immunocompetent. • All granulocytes should be irradiated before issue and transfused with minimum delay

Gamma irradiation: not necessary When none of the above conditions are present, it is not necessary to irradiate blood components transfused to: • HIV infection, • Aplastic anaemia • Solid organ transplantation, • Chemotherapy for NHL, acute leukaemia and solid tumours • It is not necessary to irradiate red cells for routine 'top-up' transfusions of premature or term infants unless eithere has been a previous IUT, or the donation has come from a first- or second-degree relative

Processing: washing

Processing: washing • Help to remove extra K from red cell • Remove Ig. A form plasma • Extra plasma containing antigens and cytokine • Should be used within 24 hr.

Processing: washing Ø Indication : • Patients with Ig. A deficiency • Prevention of allergic reactions • Post-transfusion febrile reactions, present even when leukodepleted RBCs are used

Processing: CMV negative components

CMV negative components: Are recommended for the following recipients to reduce the risk of CMV transmission : Ø CMV negative recipients of allogeneic stem cell and bone marrow transplants CMV negative pregnant women Ø Intrauterine transfusions Ø Infants weighing less than 1200 g at birth

CMV negative components : May be recommended for CMV negative individuals: Ø HIV infection Ø Conditions likely to require an BMT or Solid organ transplant recipients Ø Severe neutropenia

 Whats a platelet transfusion
Whats a platelet transfusion Cryoprecipetate
Cryoprecipetate Platelet transfusion indication
Platelet transfusion indication Cryoprecipitate dose
Cryoprecipitate dose Cryoprecipitate components
Cryoprecipitate components Platelets transfusion indication
Platelets transfusion indication Blood transfusion in children
Blood transfusion in children Transfusion reaction
Transfusion reaction Dr alicia kalamas
Dr alicia kalamas Fresh frozen plasma contents
Fresh frozen plasma contents Trapped plasma
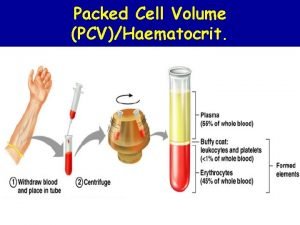
Trapped plasma Packed cell volume
Packed cell volume Packed cell volume
Packed cell volume An enzyme- packed wrecking balls of the cell.
An enzyme- packed wrecking balls of the cell. Mch blood test high
Mch blood test high Packed cell volume
Packed cell volume Cryopercipitate
Cryopercipitate Laura cooling
Laura cooling Hemarthrosis
Hemarthrosis Cryoprecipitate dose
Cryoprecipitate dose Rbc shape
Rbc shape Platelet
Platelet Platelet satellitosis
Platelet satellitosis Rees and ecker method procedure illustration
Rees and ecker method procedure illustration Cv range
Cv range Platelet aggregation test
Platelet aggregation test Ecchumosis
Ecchumosis Platelet ribbon
Platelet ribbon Romiplostim
Romiplostim Lines of zahn is seen in coralline thrombus
Lines of zahn is seen in coralline thrombus Sol gel zone
Sol gel zone Ristocetin test
Ristocetin test Process of platelet plug formation
Process of platelet plug formation Cbc platelet count low
Cbc platelet count low Cbc platelet count low
Cbc platelet count low Promyelocytic
Promyelocytic Platelet
Platelet Platelet satellitosis
Platelet satellitosis Platelet aggregation test
Platelet aggregation test Types of forces diagram
Types of forces diagram Steep sided volcano made of loosely packed tephra
Steep sided volcano made of loosely packed tephra What is close packed direction
What is close packed direction Arthrokinematic motion
Arthrokinematic motion Cbe150
Cbe150 Steep-sided volcano made of loosely packed tephra
Steep-sided volcano made of loosely packed tephra Closely packed elongated cells with corners thickened
Closely packed elongated cells with corners thickened Who packed your parachute poem
Who packed your parachute poem Density of air
Density of air Closed packed position of hip
Closed packed position of hip Prefix doubling
Prefix doubling Attribute packed c
Attribute packed c Packing efficiency for body centered cubic
Packing efficiency for body centered cubic Close packed direction
Close packed direction