How do you know how many atoms and

























- Slides: 62


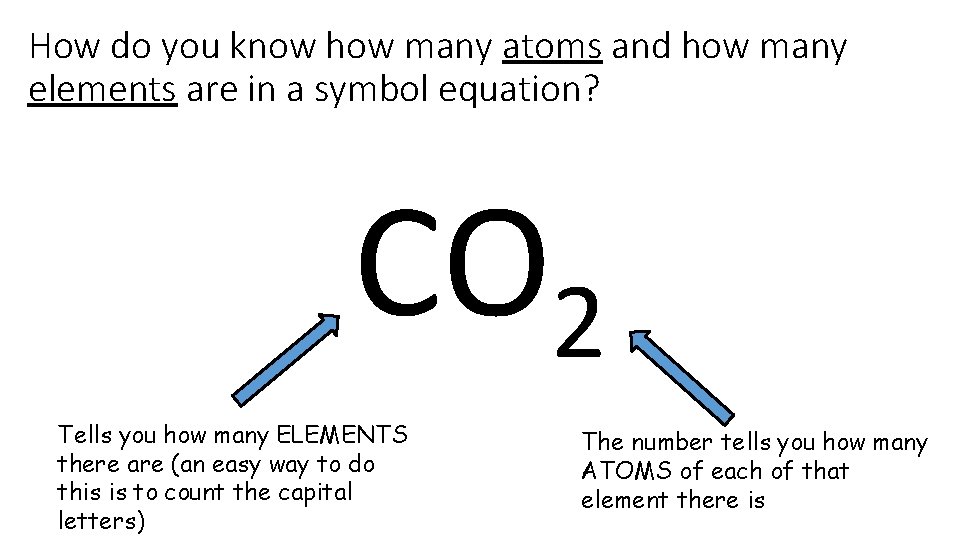
How do you know how many atoms and how many elements are in a symbol equation? CO 2 Tells you how many ELEMENTS there are (an easy way to do this is to count the capital letters) The number tells you how many ATOMS of each of that element there is

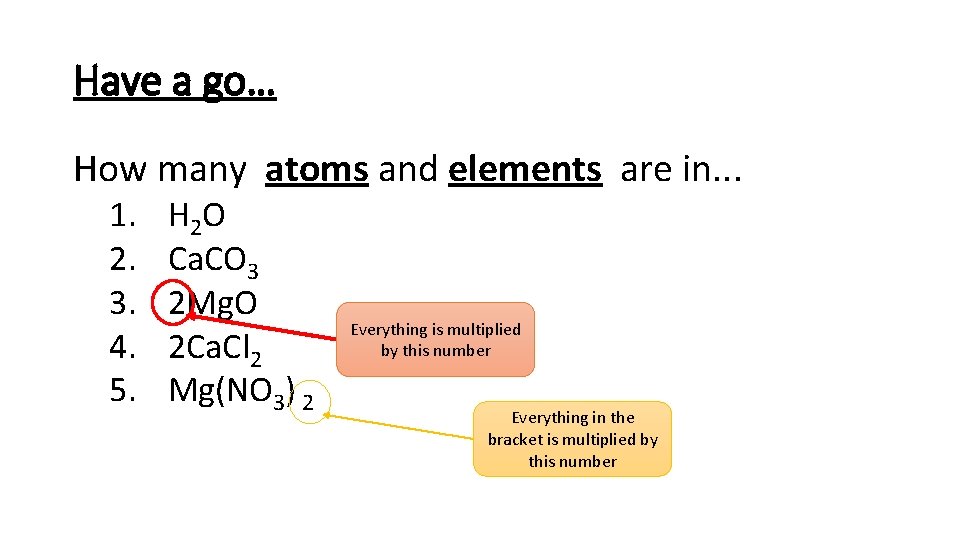
Have a go… How many atoms and elements are in. . . 1. 2. 3. 4. 5. H 2 O Ca. CO 3 2 Mg. O 2 Ca. Cl 2 Mg(NO 3) 2 Everything is multiplied by this number Everything in the bracket is multiplied by this number


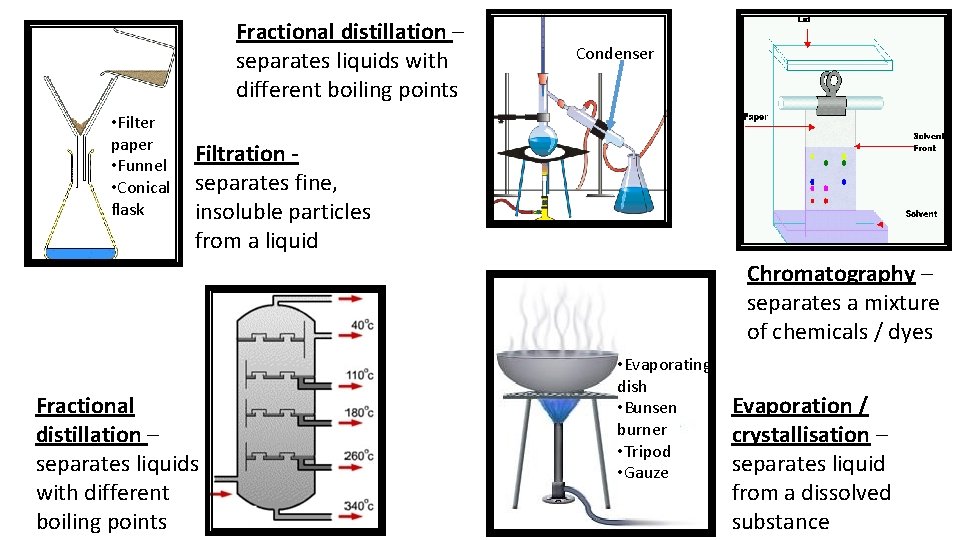
Fractional distillation – separates liquids with different boiling points • Filter paper • Funnel • Conical flask Condenser Filtration separates fine, insoluble particles from a liquid Chromatography – separates a mixture of chemicals / dyes Fractional distillation – separates liquids with different boiling points • Evaporating dish • Bunsen burner • Tripod • Gauze Evaporation / crystallisation – separates liquid from a dissolved substance



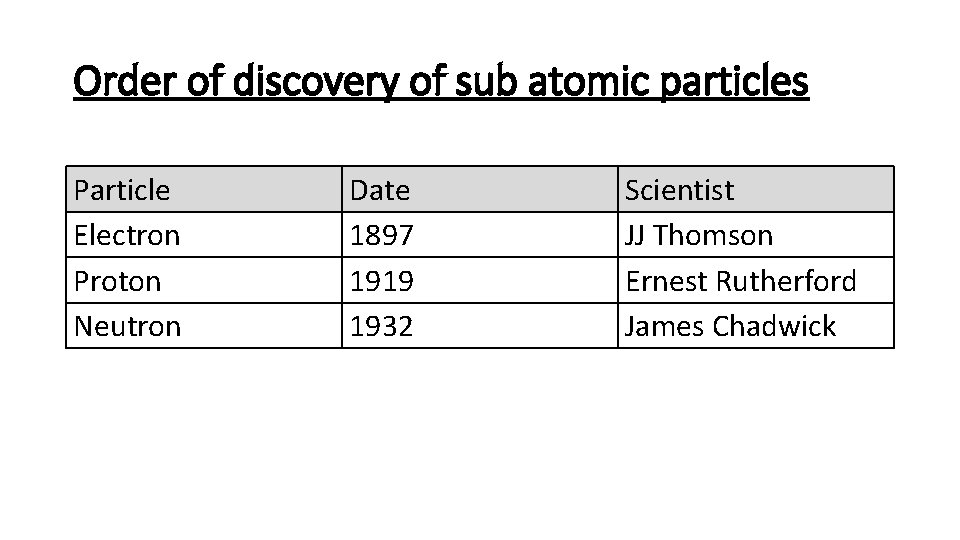
Order of discovery of sub atomic particles Particle Electron Proton Neutron Date 1897 1919 1932 Scientist JJ Thomson Ernest Rutherford James Chadwick

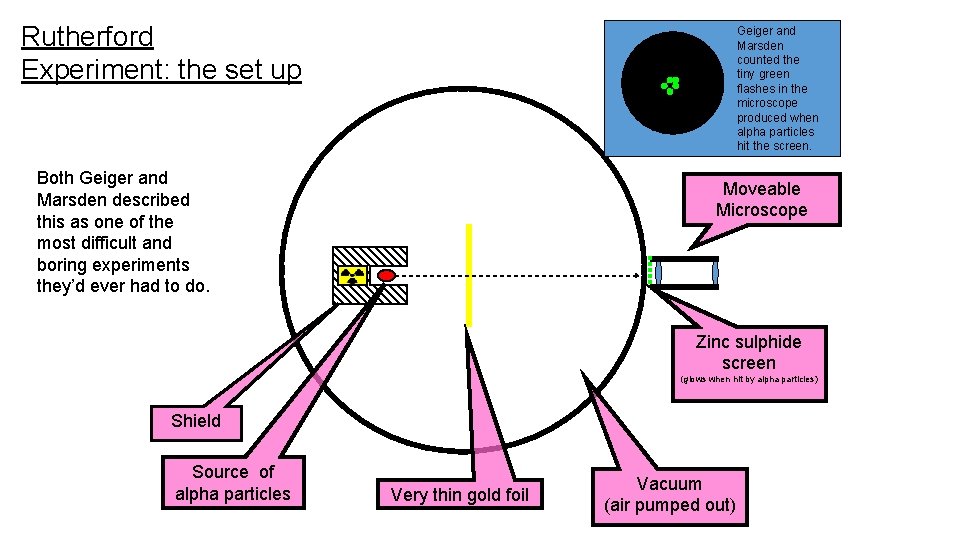
Rutherford Experiment: the set up Geiger and Marsden counted the tiny green flashes in the microscope produced when alpha particles hit the screen. Both Geiger and Marsden described this as one of the most difficult and boring experiments they’d ever had to do. Moveable Microscope Zinc sulphide screen (glows when hit by alpha particles) Shield Source of alpha particles Very thin gold foil Vacuum (air pumped out)

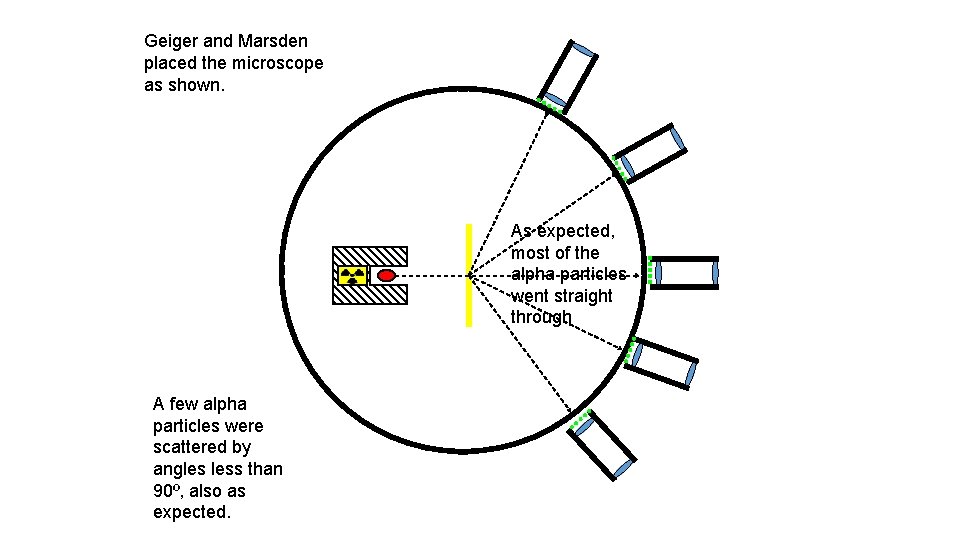
Geiger and Marsden placed the microscope as shown. As expected, most of the alpha particles went straight through A few alpha particles were scattered by angles less than 90º, also as expected.

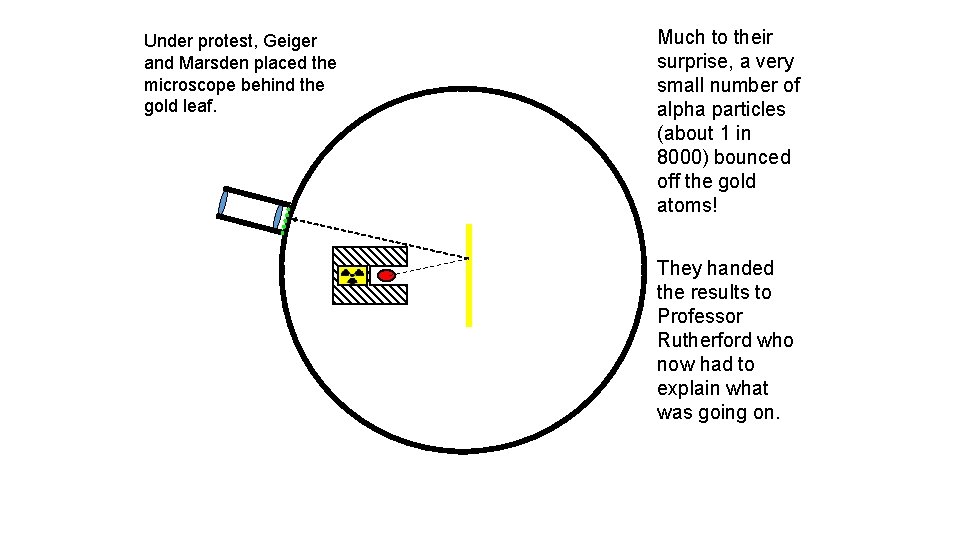
Under protest, Geiger and Marsden placed the microscope behind the gold leaf. Much to their surprise, a very small number of alpha particles (about 1 in 8000) bounced off the gold atoms! They handed the results to Professor Rutherford who now had to explain what was going on.

Observations Rutherford’s experiment finished with 3 observations. You need to explain what each observation tells us about the atom as we know it today • Most of the fast, highly charged alpha particles went whizzing straight through un-deflected. • Some of the alpha particles were deflected through a small angles • A very small number of alpha particles were deflected backwards!

Conclusions Most of the fast, highly charged alpha particles went whizzing straight through undeflected. SUGGESTS THAT MOST OF THE ATOM IS EMPTY SPACE!!

Conclusions Some of the alpha particles were deflected through a small angles! SUGGESTS THAT THERE IS A CONCENTRATED POSITIVE MASS SOMEWHERE IN THE ATOM.

Conclusions A very small number of alpha particles were deflected backwards! SUGGESTS THAT THE CONCENTRATED MASS IS MINISCULE COMPARED TO THE SIZE OF THE REST OF THE ATOM, BUT CONTAINS MOST OF THE MASS

What did Bohr do? (1913) 1. Realised that electrons should be attracted in to the nucleus 2. Used mathematical models to show that electrons occupy fixed energy levels (or shells) around the nucleus


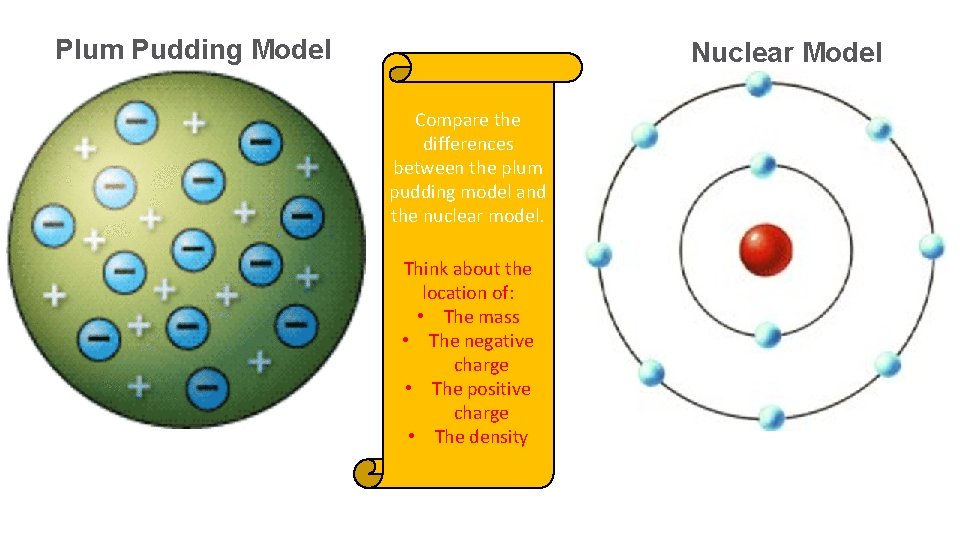
Plum Pudding Model Nuclear Model Compare the differences between the plum pudding model and the nuclear model. Think about the location of: • The mass • The negative charge • The positive charge • The density



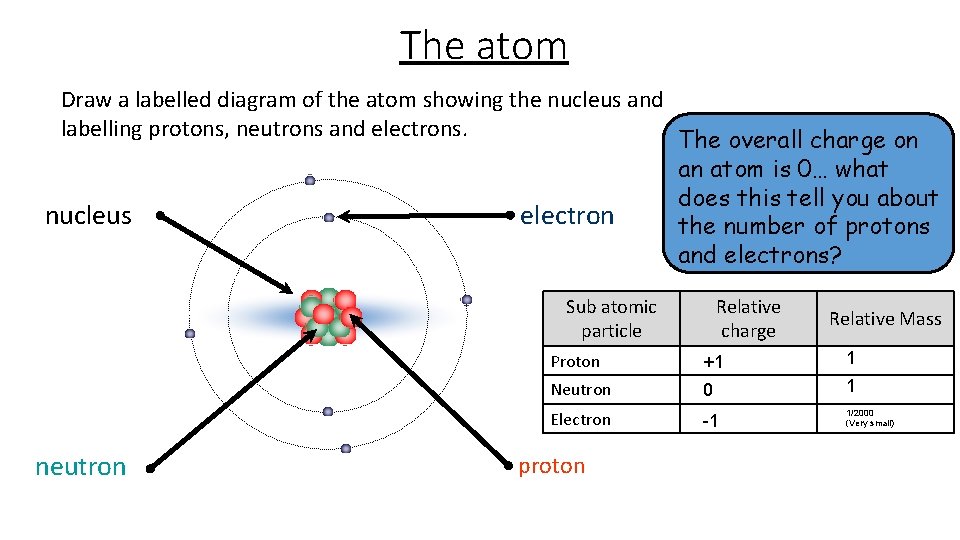
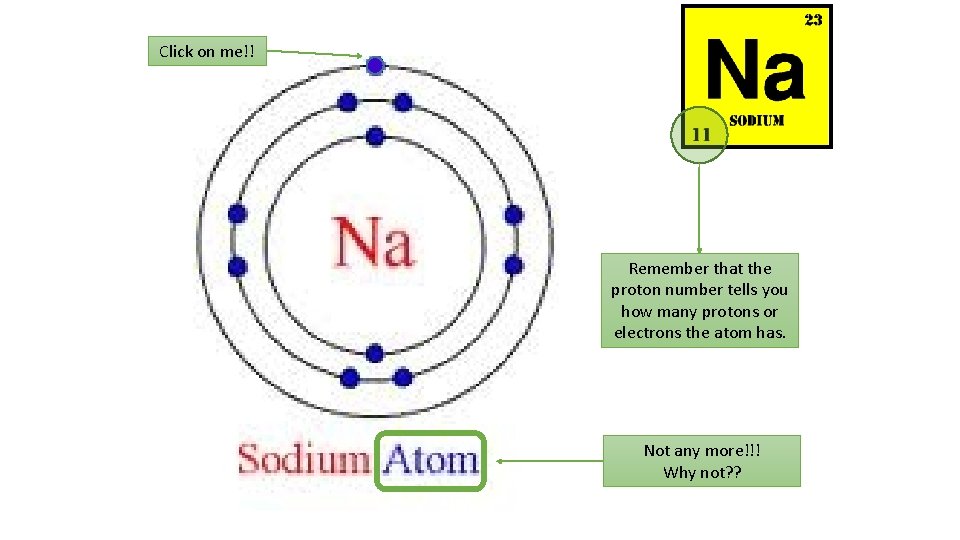
The atom Draw a labelled diagram of the atom showing the nucleus and labelling protons, neutrons and electrons. The overall charge on an atom is 0… what does this tell you about nucleus electron the number of protons and electrons? Sub atomic particle neutron Relative charge Relative Mass Proton +1 1 Neutron 0 1 Electron -1 1/2000 (Very small) proton


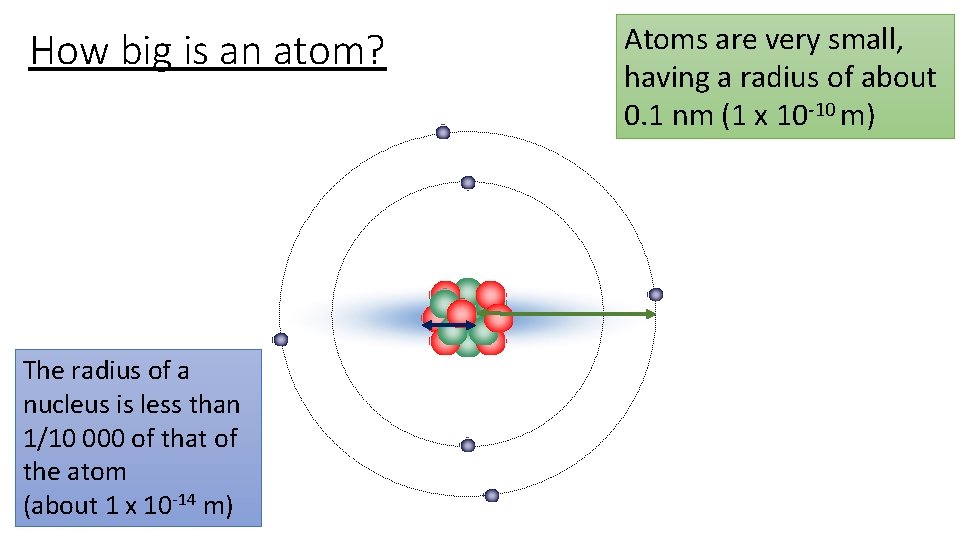
How big is an atom? The radius of a nucleus is less than 1/10 000 of that of the atom (about 1 x 10 -14 m) Atoms are very small, having a radius of about 0. 1 nm (1 x 10 -10 m)

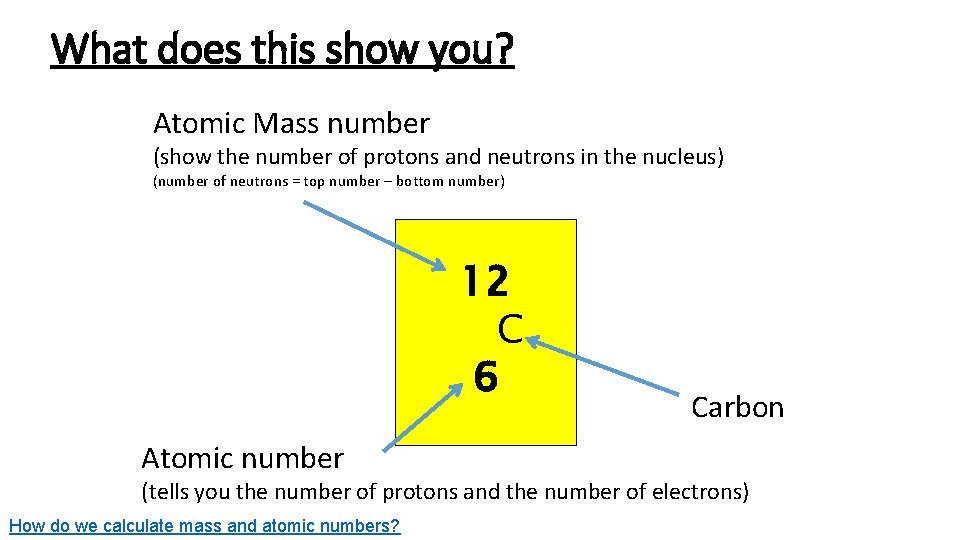
What does this show you? Atomic Mass number (show the number of protons and neutrons in the nucleus) (number of neutrons = top number – bottom number) 12 C 6 Atomic number Carbon (tells you the number of protons and the number of electrons) How do we calculate mass and atomic numbers?

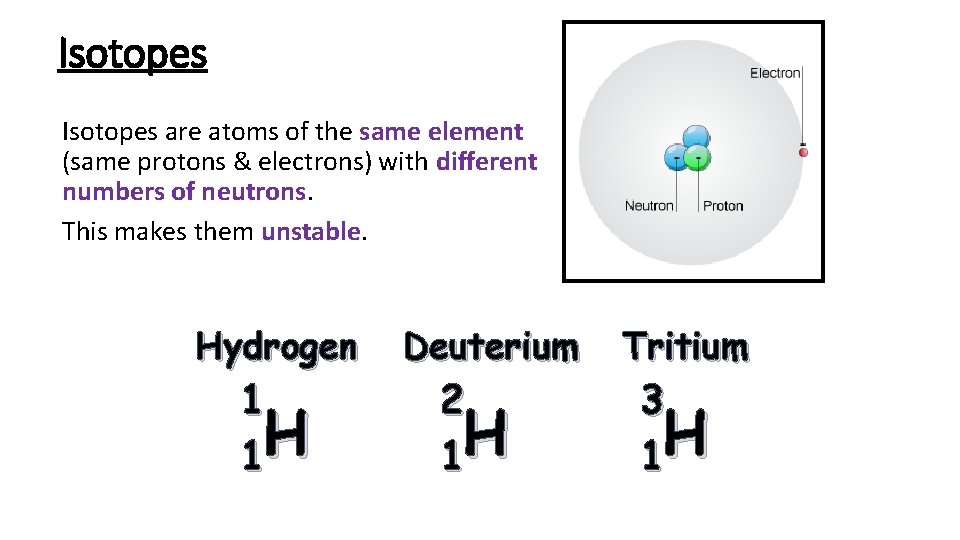
Isotopes are atoms of the same element (same protons & electrons) with different numbers of neutrons. This makes them unstable. Hydrogen 1 H 1 Deuterium 2 H 1 Tritium 3 H 1


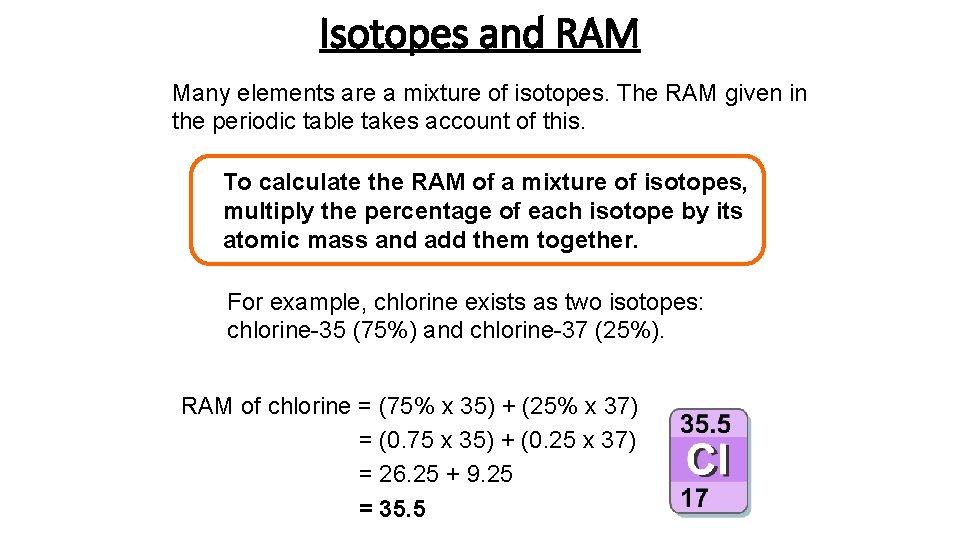
Isotopes and RAM Many elements are a mixture of isotopes. The RAM given in the periodic table takes account of this. To calculate the RAM of a mixture of isotopes, multiply the percentage of each isotope by its atomic mass and add them together. For example, chlorine exists as two isotopes: chlorine-35 (75%) and chlorine-37 (25%). RAM of chlorine = (75% x 35) + (25% x 37) = (0. 75 x 35) + (0. 25 x 37) = 26. 25 + 9. 25 = 35. 5


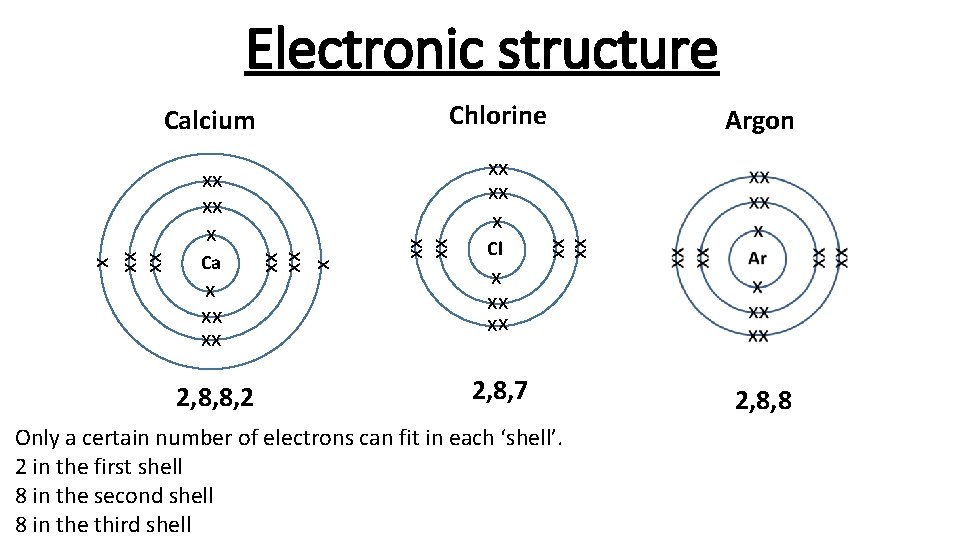
Electronic structure Calcium x xx xx 2, 8, 8, 2 Cl xx xx xx xx Argon xx xx x Ca Chlorine x xx xx 2, 8, 7 Only a certain number of electrons can fit in each ‘shell’. 2 in the first shell 8 in the second shell 8 in the third shell 2, 8, 8


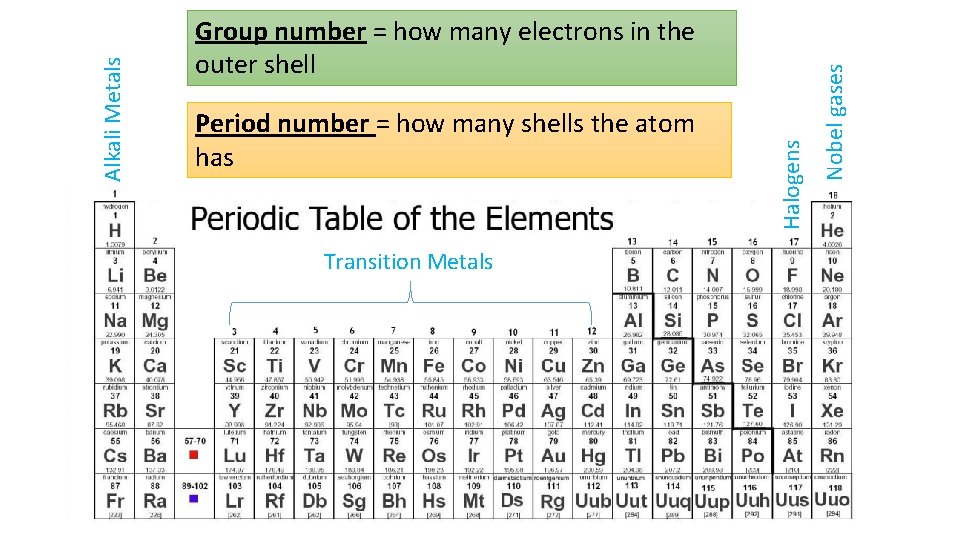
Transition Metals Nobel gases Period number = how many shells the atom has Halogens Alkali Metals Group number = how many electrons in the outer shell


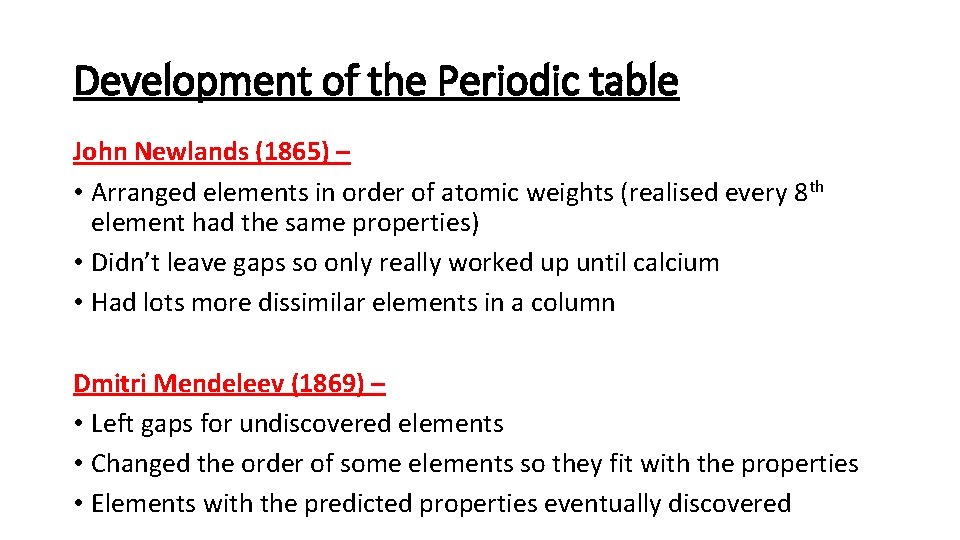
Development of the Periodic table John Newlands (1865) – • Arranged elements in order of atomic weights (realised every 8 th element had the same properties) • Didn’t leave gaps so only really worked up until calcium • Had lots more dissimilar elements in a column Dmitri Mendeleev (1869) – • Left gaps for undiscovered elements • Changed the order of some elements so they fit with the properties • Elements with the predicted properties eventually discovered


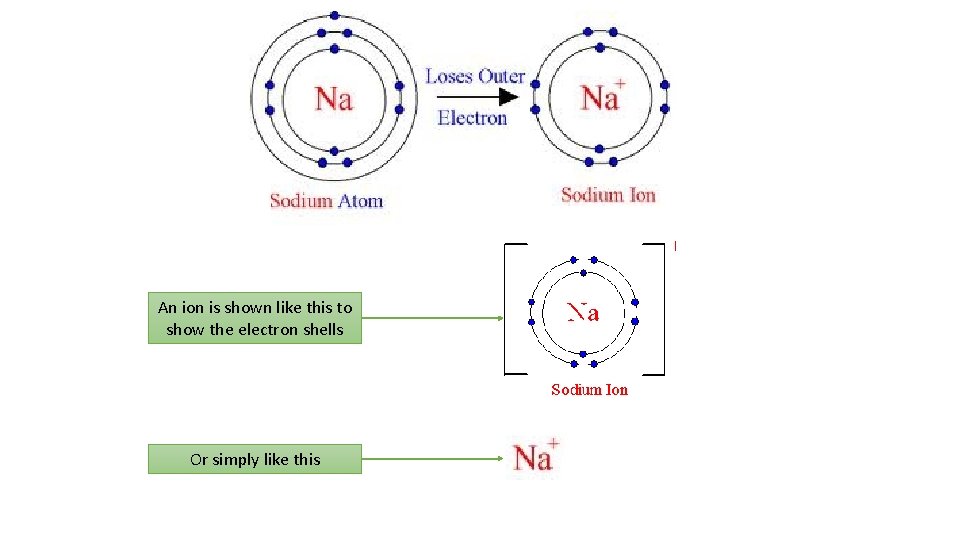
What is an ION? Video • An ION is a ‘charged’ particle. • Atoms 'like' to have a full shell of electrons. They are more stable if they have a full electron shell. Atoms will lose or gain electrons in order to gain a full shell. • It has therefore either LOST or GAINED electrons.

Click on me!! Remember that the proton number tells you how many protons or electrons the atom has. Not any more!!! Why not? ?

An ion is shown like this to show the electron shells Or simply like this

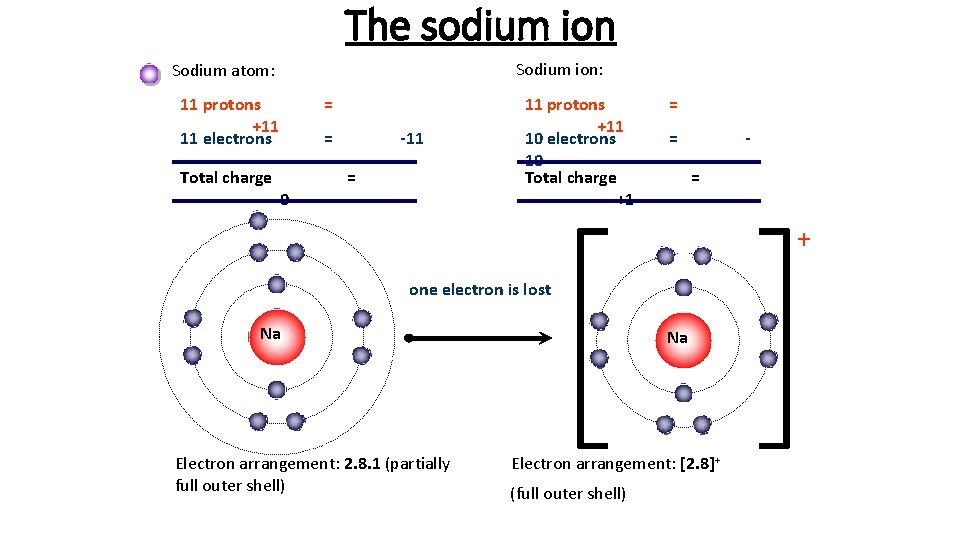
The sodium ion Sodium ion: Sodium atom: 11 protons +11 11 electrons Total charge = = 0 -11 = 11 protons +11 10 electrons 10 Total charge +1 = = = + one electron is lost Na Electron arrangement: 2. 8. 1 (partially full outer shell) Na Electron arrangement: [2. 8]+ (full outer shell)

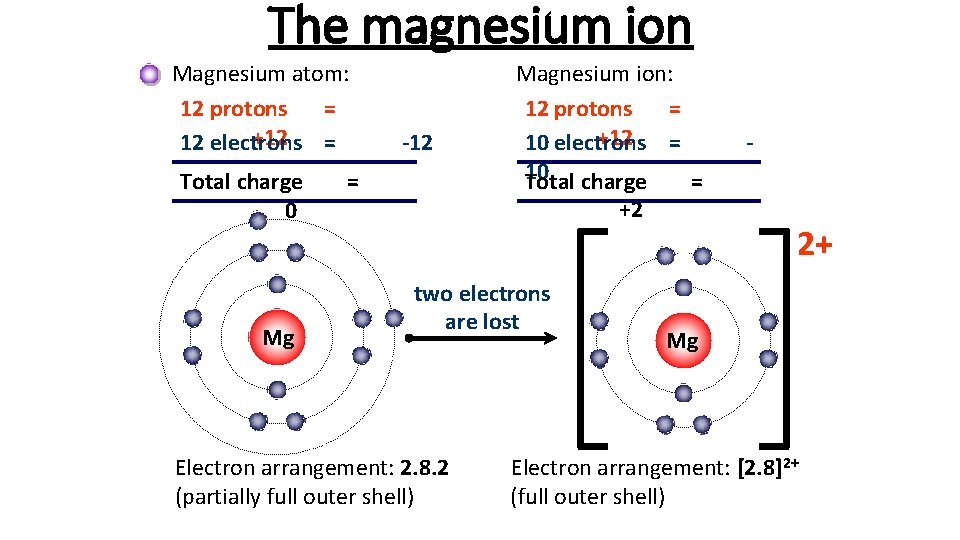
The magnesium ion Magnesium atom: 12 protons = +12 = 12 electrons Total charge 0 Mg -12 = Magnesium ion: 12 protons = +12 = 10 electrons 10 charge Total = +2 two electrons are lost Electron arrangement: 2. 8. 2 (partially full outer shell) - 2+ Mg Electron arrangement: [2. 8]2+ (full outer shell)

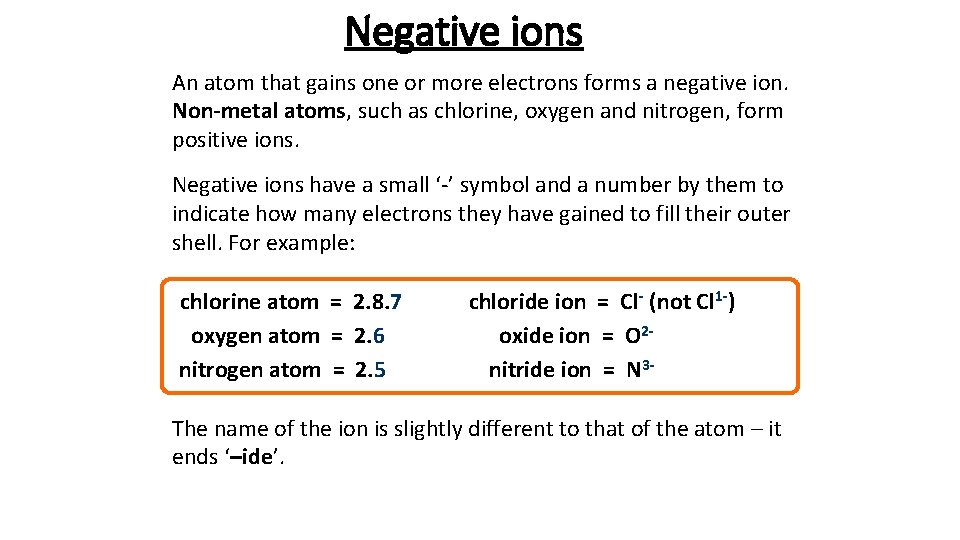
Negative ions An atom that gains one or more electrons forms a negative ion. Non-metal atoms, such as chlorine, oxygen and nitrogen, form positive ions. Negative ions have a small ‘-’ symbol and a number by them to indicate how many electrons they have gained to fill their outer shell. For example: chlorine atom = 2. 8. 7 oxygen atom = 2. 6 nitrogen atom = 2. 5 chloride ion = Cl- (not Cl 1 -) oxide ion = O 2 nitride ion = N 3 - The name of the ion is slightly different to that of the atom – it ends ‘–ide’.

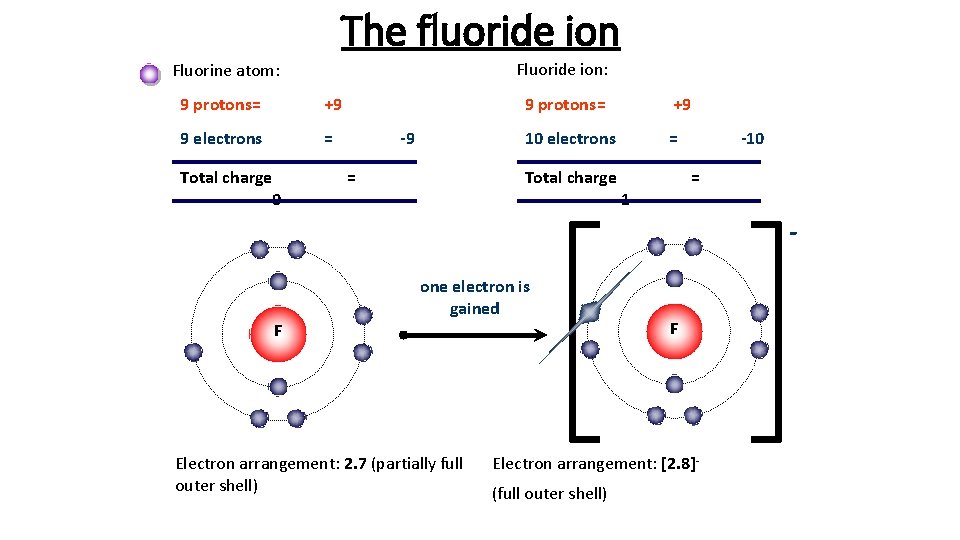
The fluoride ion Fluoride ion: Fluorine atom: 9 protons= +9 9 electrons = Total charge 0 -9 9 protons= +9 10 electrons = Total charge -1 = -10 = one electron is gained F Electron arrangement: 2. 7 (partially full outer shell) F Electron arrangement: [2. 8](full outer shell)

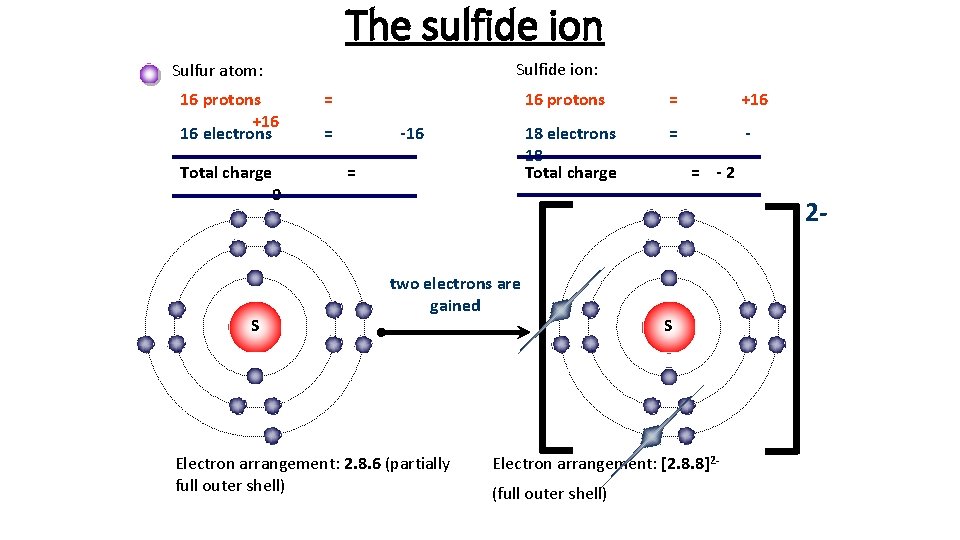
The sulfide ion Sulfide ion: Sulfur atom: 16 protons +16 16 electrons Total charge 0 S = = -16 = 16 protons = +16 18 electrons 18 Total charge = = -2 2 two electrons are gained Electron arrangement: 2. 8. 6 (partially full outer shell) S Electron arrangement: [2. 8. 8]2(full outer shell)

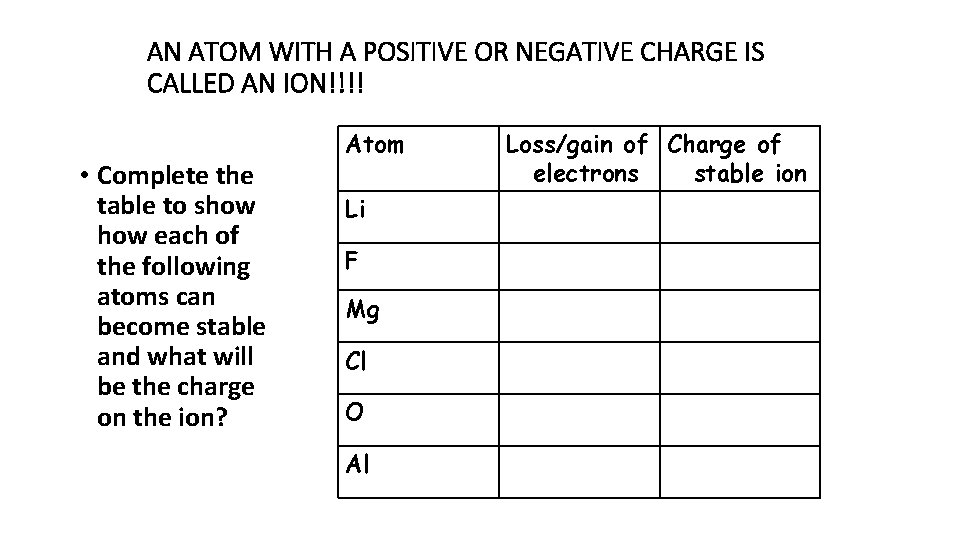
AN ATOM WITH A POSITIVE OR NEGATIVE CHARGE IS CALLED AN ION!!!! • Complete the table to show each of the following atoms can become stable and what will be the charge on the ion? Atom Li F Mg Cl O Al Loss/gain of Charge of electrons stable ion


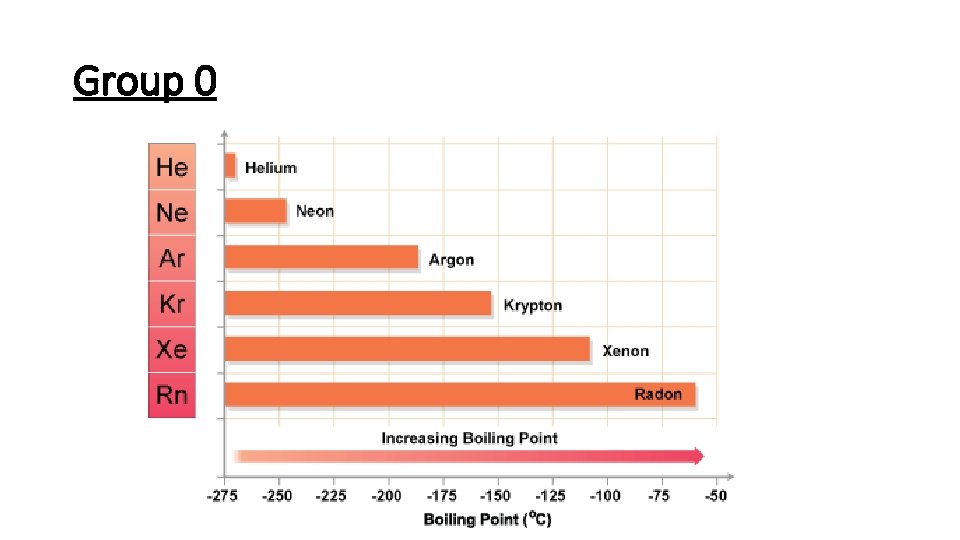
Group 0


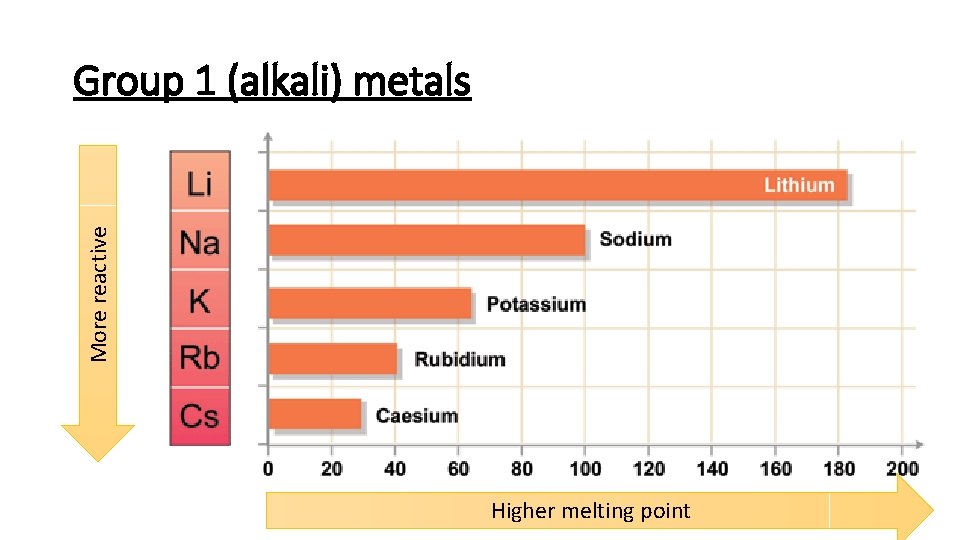
More reactive Group 1 (alkali) metals Higher melting point

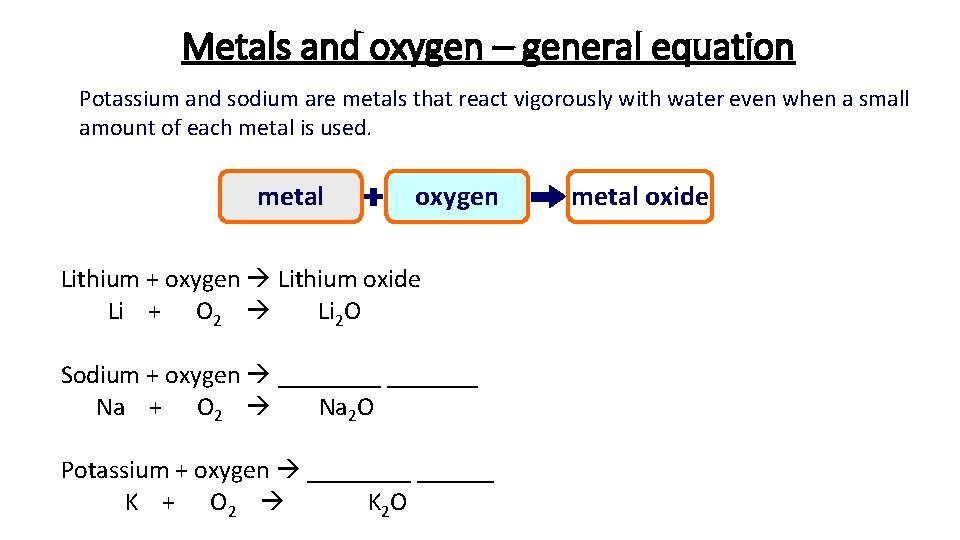
Metals and oxygen – general equation Potassium and sodium are metals that react vigorously with water even when a small amount of each metal is used. metal oxygen Lithium + oxygen Lithium oxide Li + O 2 Li 2 O Sodium + oxygen _______ Na + O 2 Na 2 O Potassium + oxygen ______ K + O 2 K 2 O metal oxide

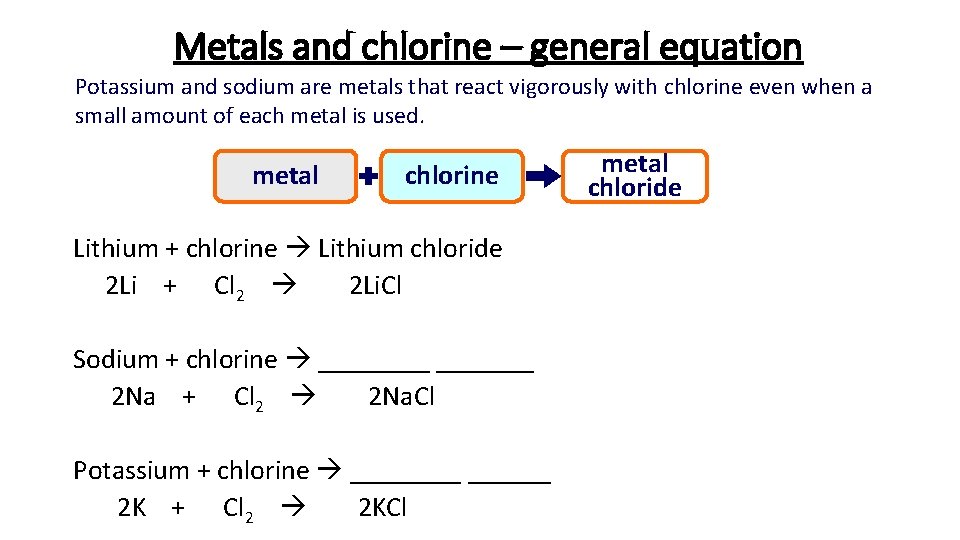
Metals and chlorine – general equation Potassium and sodium are metals that react vigorously with chlorine even when a small amount of each metal is used. metal chlorine Lithium + chlorine Lithium chloride 2 Li + Cl 2 2 Li. Cl Sodium + chlorine _______ 2 Na + Cl 2 2 Na. Cl Potassium + chlorine ______ 2 K + Cl 2 2 KCl metal chloride

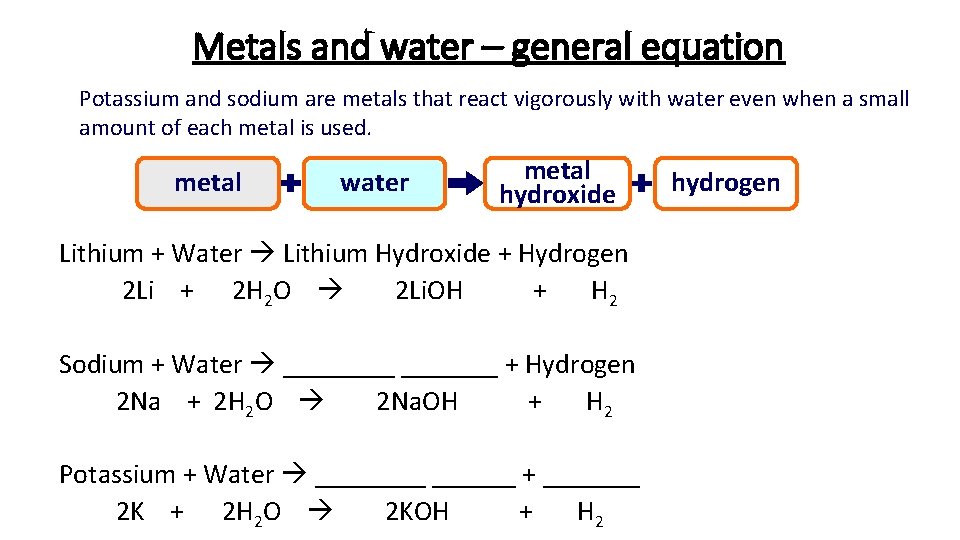
Metals and water – general equation Potassium and sodium are metals that react vigorously with water even when a small amount of each metal is used. metal water metal hydroxide Lithium + Water Lithium Hydroxide + Hydrogen 2 Li + 2 H 2 O 2 Li. OH + H 2 Sodium + Water _______ + Hydrogen 2 Na + 2 H 2 O 2 Na. OH + H 2 Potassium + Water ______ + _______ 2 K + 2 H 2 O 2 KOH + H 2 hydrogen



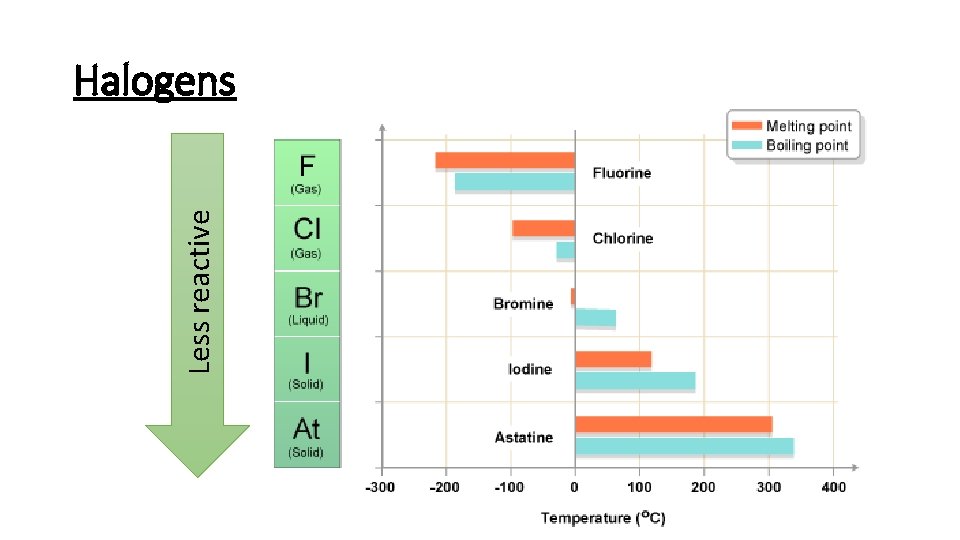
Less reactive Halogens

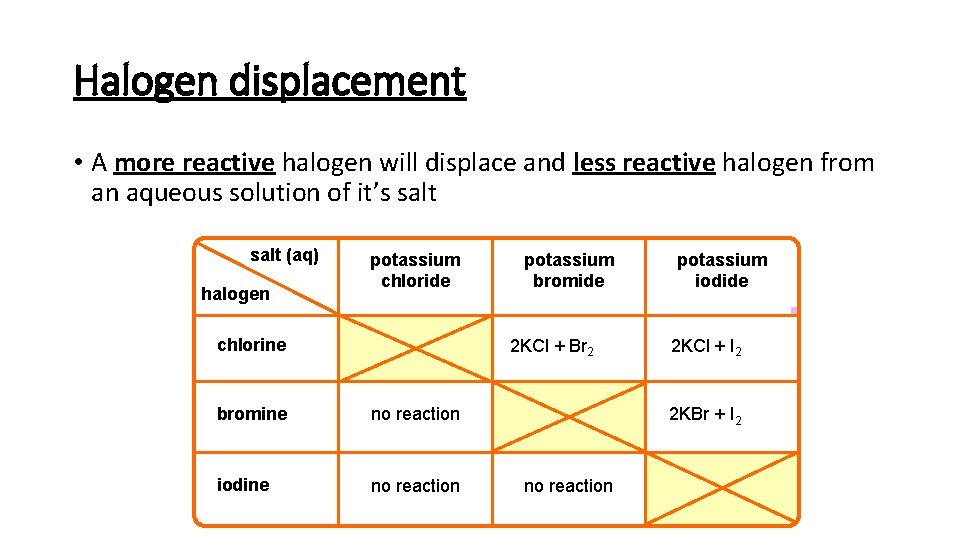
Halogen displacement • A more reactive halogen will displace and less reactive halogen from an aqueous solution of it’s salt (aq) halogen potassium chloride chlorine potassium bromide 2 KCl + Br 2 bromine no reaction iodine no reaction potassium iodide 2 KCl + I 2 2 KBr + I 2 no reaction

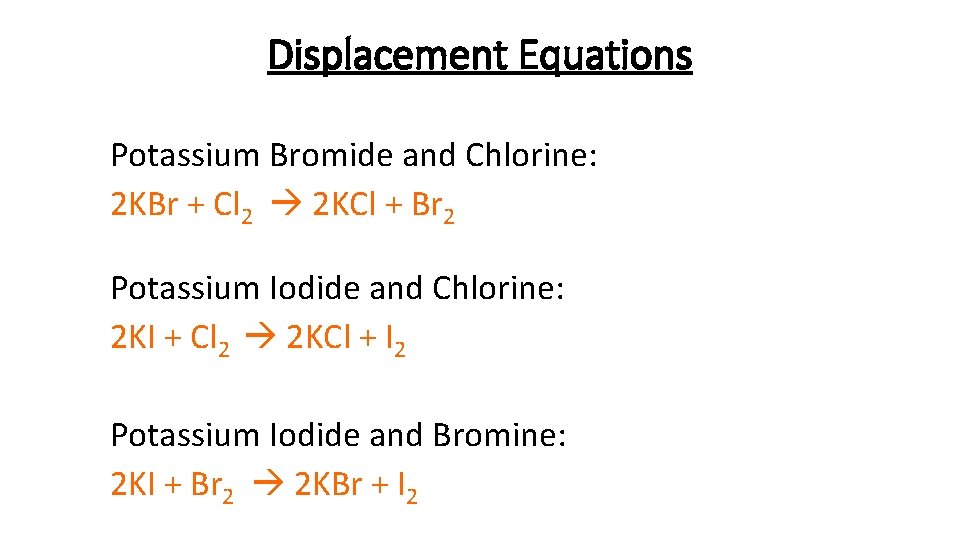
Displacement Equations Potassium Bromide and Chlorine: 2 KBr + Cl 2 2 KCl + Br 2 Potassium Iodide and Chlorine: 2 KI + Cl 2 2 KCl + I 2 Potassium Iodide and Bromine: 2 KI + Br 2 2 KBr + I 2

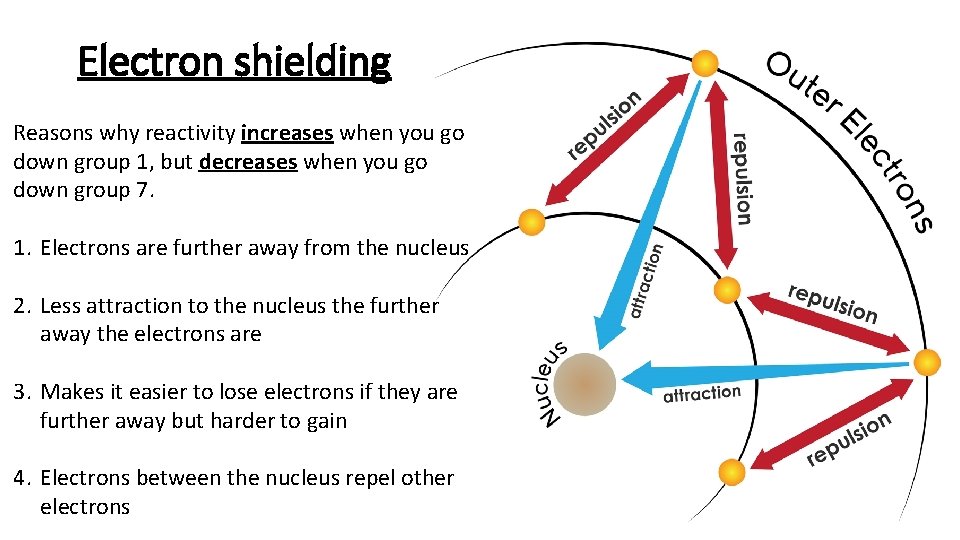
Electron shielding Reasons why reactivity increases when you go down group 1, but decreases when you go down group 7. 1. Electrons are further away from the nucleus 2. Less attraction to the nucleus the further away the electrons are 3. Makes it easier to lose electrons if they are further away but harder to gain 4. Electrons between the nucleus repel other electrons



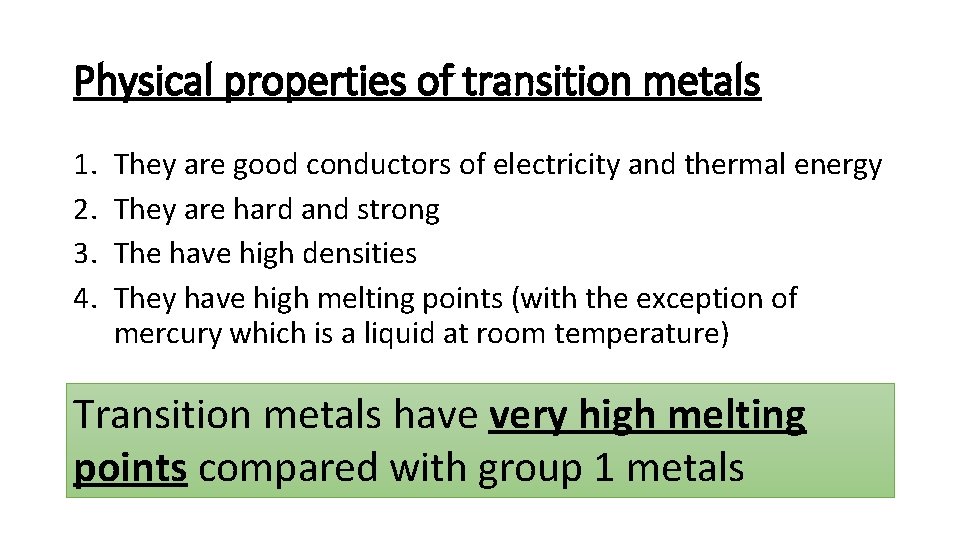
Physical properties of transition metals 1. 2. 3. 4. They are good conductors of electricity and thermal energy They are hard and strong The have high densities They have high melting points (with the exception of mercury which is a liquid at room temperature) Transition metals have very high melting points compared with group 1 metals

Chemical properties of transition metals • Don’t react as strongly with oxygen, chlorine or water as the alkali metals do. • Most transition metals react very slowly with oxygen and water (e. g iron rusts very slowly over time) Transition metals are much less reactive compared with group 1 metals


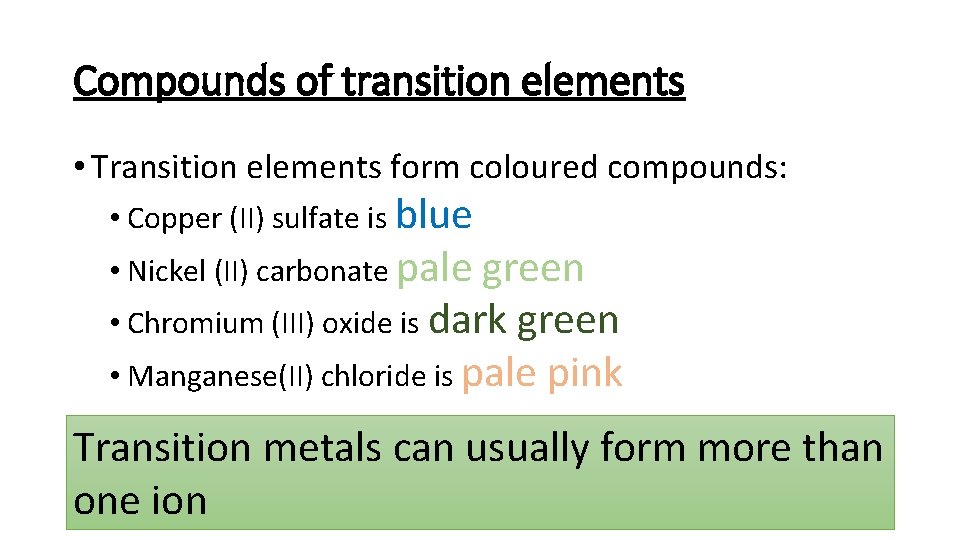
Compounds of transition elements • Transition elements form coloured compounds: • Copper (II) sulfate is blue • Nickel (II) carbonate pale green • Chromium (III) oxide is dark green • Manganese(II) chloride is pale pink Transition metals can usually form more than one ion