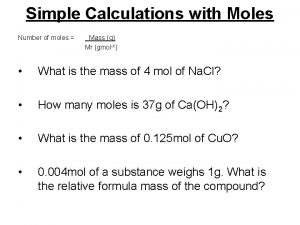
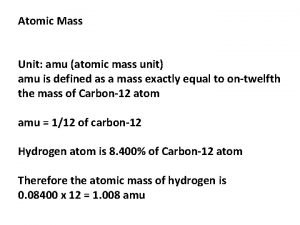Moles Quiz I say atom you say amu




















- Slides: 26

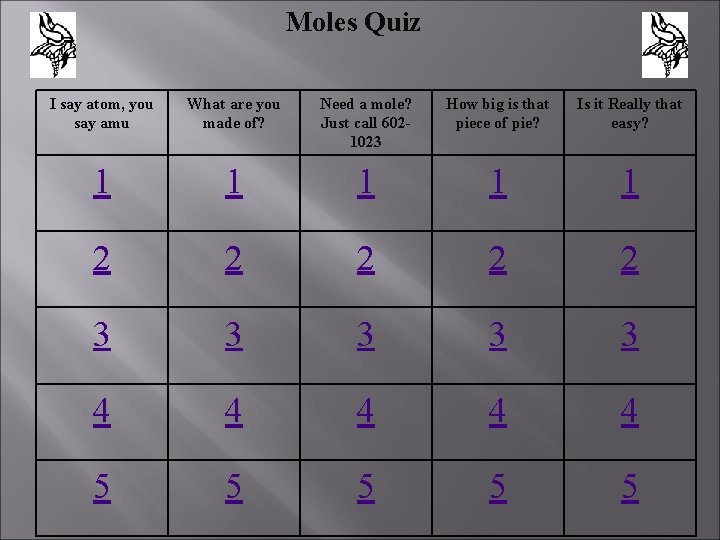
Moles Quiz I say atom, you say amu What are you made of? Need a mole? Just call 6021023 How big is that piece of pie? Is it Really that easy? 1 1 1 2 2 2 3 3 3 4 4 4 5 5 5

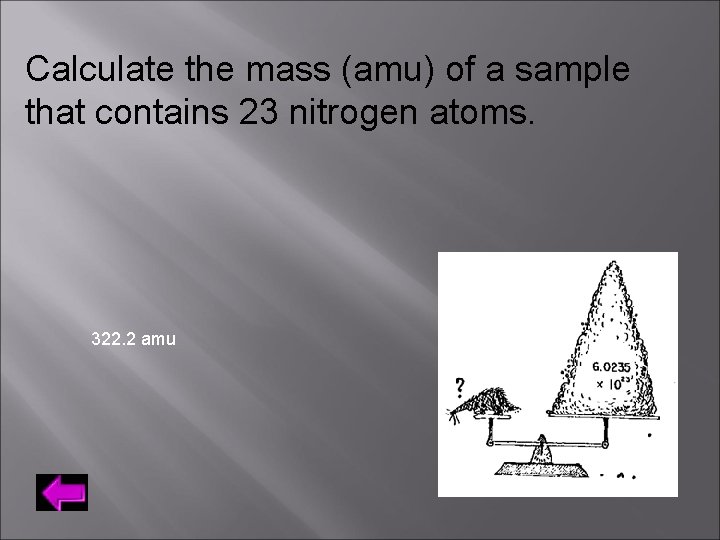
Calculate the mass (amu) of a sample that contains 23 nitrogen atoms. 322. 2 amu

Calculate the number of oxygen atoms in a sample that has a mass of 288 amu. 18. 0 atoms

Calculate the mass (in amu) of a sample of Bromine that has the same number of atoms as a 343 amu sample of Calcium. 684 amu

Calculate the number of atoms in a 5. 321 amu sample of molybdenum. 5. 546 x 10 -2 atoms

How many grams is 1 amu? 1. 66 x 10 -24 g

What is the percent composition of the elements found in carbon tetrafluoride? 13. 65% C and 86. 35%F

What is the percent composition of the elements in Cu. Cl 2? 47. 27%Cu and 52. 73% Cl

What is the mass percent of chlorine in sodium chloride? 39. 34%Na and 60. 66% Cl

What is the mass percent of the anion in calcium hydroxide? 45. 91%

The percentage of sulfur in sulfur dioxide is about 50%. What is the percentage of oxygen in this compound? About 50%

Calculate both the number of moles and the mass of a sample of chromium containing 5. 00 x 1020 atoms. 8. 30 x 10 -4 mol and 4. 32 x 10 -2 grams

Calculate the number of moles in a 57. 7 g sample of sulfur. 1. 80 mol

Which weighs more, 0. 50 mol of oxygen or 4 mol of hydrogen atoms? . 50 mol of Oxygen

Calculate the number of grams of iron that contains the same number of atoms as 2. 24 g of cobalt. 2. 12 g Fe

How many atoms are present in a 3. 57 kg sample of aluminum? 7. 97 x 1025 atoms

A single molecule of a particular compound has a mass of 4. 65 x 10 -23 g. Which of the following could be this compound? A. NO 2 B. CO C. Water D. Ammonia B.

What is the percent by mass of carbon in table sugar, C 12 H 22 O 11 42%

Calculate the molar mass for magnesium sulfate. 120. 38 g

Calculate the number of units of teflon (C 2 H 4) in 135 g. 2. 90 x 1024

Compute the mass percent of each element in the formula for penicillin F (C 14 H 2 ON 2 SO 4) C 53. 81% H 6. 453% N 8. 969% S 10. 27% O 20. 49%

A particular compound in the chemistry lab is found to contain 7. 2 x 1024 atoms of oxygen, 56. 0 g of Nitrogen, and 4. 0 mol of hydrogen. What is this compound’s empirical formula? HNO 3

What is the empirical formula of a compound that is 31. 9% potassium, 28. 9% chlorine, and 39. 2% oxygen? KCl. O 3

A compound contains 259. 2 grams of F and 40. 8 grams of C. What is the empirical formula of this compound? CF 4

A compound’s empirical formula is HO. If the formula mass is 34 grams, what is the molecular formula? H 2 O 2

Which of the following does not have the corresponding empirical formula XY 2 Z? a. b. c. d. X 2 Y 4 Z 2 XYZ X 6 Y 12 Z 6 X 3 Y 6 Z 3 B
 1 66x10 24
1 66x10 24 It's not what you say it's how you say it
It's not what you say it's how you say it What do you say after you say hello
What do you say after you say hello Ideal gas law powerpoint
Ideal gas law powerpoint You say that you love rain
You say that you love rain Moles quiz
Moles quiz The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Teori atom modern
Teori atom modern 26 protons, period 4, transition element
26 protons, period 4, transition element Mass of subatomic particles in amu
Mass of subatomic particles in amu Amu vs molar mass
Amu vs molar mass Amu orientation programme
Amu orientation programme Moles of atoms
Moles of atoms Formula mass vs molar mass
Formula mass vs molar mass Amu agenda
Amu agenda Hvad er epos
Hvad er epos Gigue amu
Gigue amu Unit of molar mass
Unit of molar mass Oaas amu-darja ääres
Oaas amu-darja ääres Kørekort d1 amu
Kørekort d1 amu Lektoraty uam
Lektoraty uam Atomic mass of copper in amu
Atomic mass of copper in amu Department of zoology amu
Department of zoology amu Amucont
Amucont Atomic mass unit
Atomic mass unit Amu chemistry
Amu chemistry Amu århus vagt
Amu århus vagt