HIV Medication Update NEHA SHETH PANDIT PHARMD AAHIVP














- Slides: 42

HIV Medication Update NEHA SHETH PANDIT, PHARMD, AAHIVP, BCPS UNIVERSITY OF MARYLAND SCHOOL OF PHARMACY ASSOCIATE PROFESSOR, HIV/INFECTIOUS DISEASES SEPTEMBER 2018

Disclosure DR. NEHA SHETH PANDIT, PHARM. D. , AAHIVP, BCPS HAS NOTHING TO DISCLOSE.

Objectives Identify previous and current guidelines for HIV treatment Describe clinical uses of recently marketed anti- retrovirals Describe the potential influence new anti- retrovirals will have on HIV management

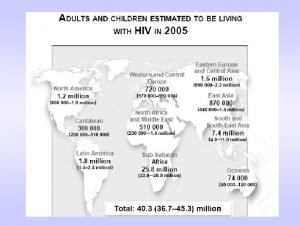
Epidemiology Since the beginning of the epidemic 77. 3 million people have been infected In 2017, 36. 9 million people were living with HIV worldwide In 2015, 1. 1 million people were living with HIV in the US In 2016, 30, 430 people were living with HIV in Maryland In 2015, 12, 473 people were living with HIV in Baltimore City In 2001 there were 3. 4 million new infections In 2016 there were 1. 8 million new infections worldwide In 2015 there were 38, 500 new infections in the US In 2016, there were 1, 118 new infections in the Maryland In 2015, there were 353 new infections in Baltimore City http: //www. unaids. org/en/resources/fact-sheet http: //www. cdc. gov/hiv/statistics/overview/ataglance. html http: //phpa. dhmh. maryland. gov/OIDEOR/CHSE/Site. Assets/Pages/statistics/Maryland-HIV-Fact-Sheet. pdf http: //phpa. dhmh. maryland. gov/OIDEOR/CHSE/Shared%20 Documents/World-AIDS-Day. pdf

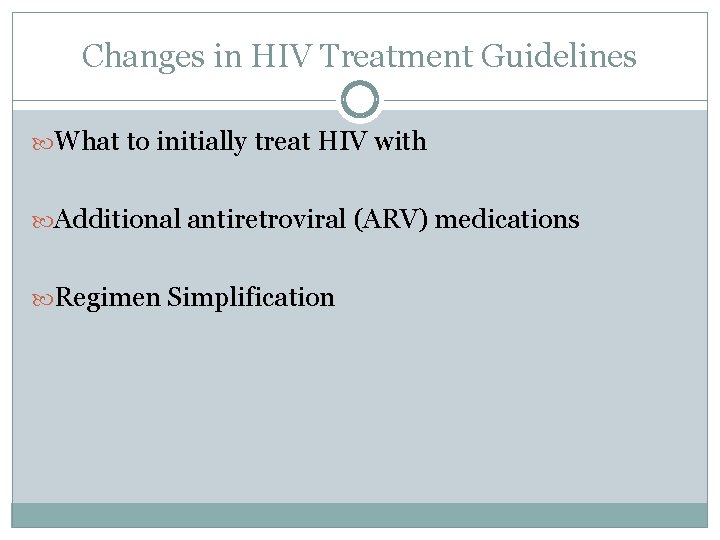
Changes in HIV Treatment Guidelines What to initially treat HIV with Additional antiretroviral (ARV) medications Regimen Simplification

1991: First Approved HIV medication 1998: Symptomatic, CD 4< 500, VL >10, 000 When to treat HIV 2001: Symptomatic, CD 4< 350, VL >30, 000 2002: Symptomatic, CD 4< 350, VL >55, 000 2004: Symptomatic, CD 4< 350, VL >100, 000 2007: Symptomatic, CD 4< 350 2012: All HIV infected patients Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

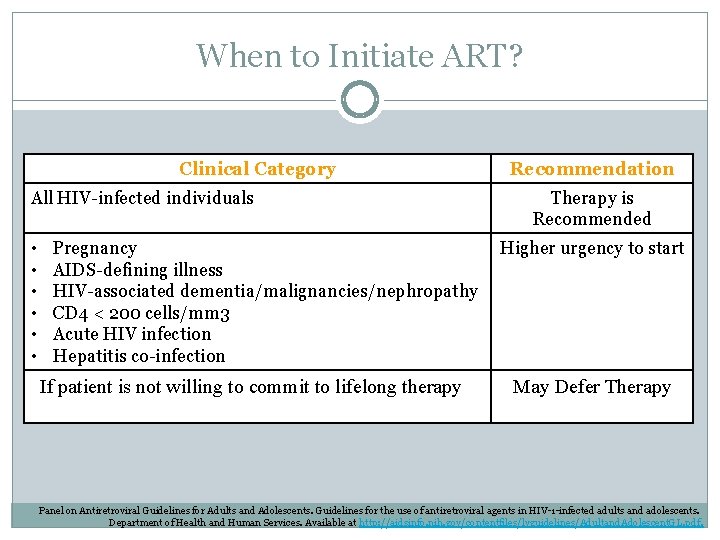
When to Initiate ART? Clinical Category All HIV-infected individuals • • • Pregnancy AIDS-defining illness HIV-associated dementia/malignancies/nephropathy CD 4 < 200 cells/mm 3 Acute HIV infection Hepatitis co-infection If patient is not willing to commit to lifelong therapy Recommendation Therapy is Recommended Higher urgency to start May Defer Therapy Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

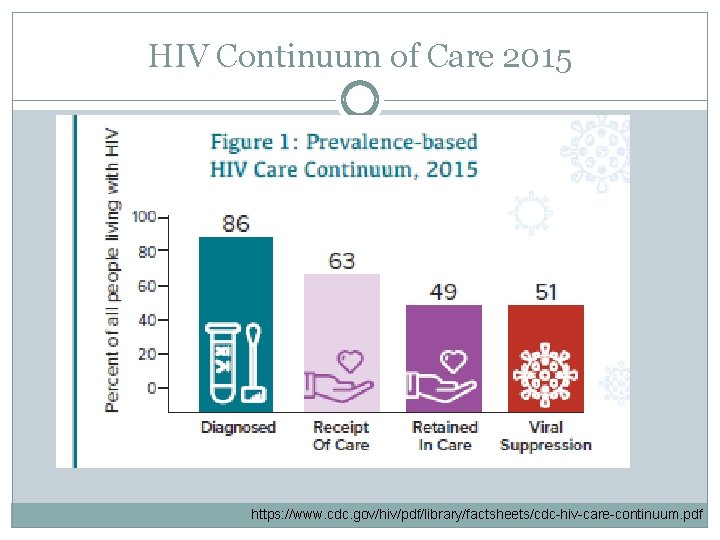
HIV Continuum of Care 2015 https: //www. cdc. gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum. pdf

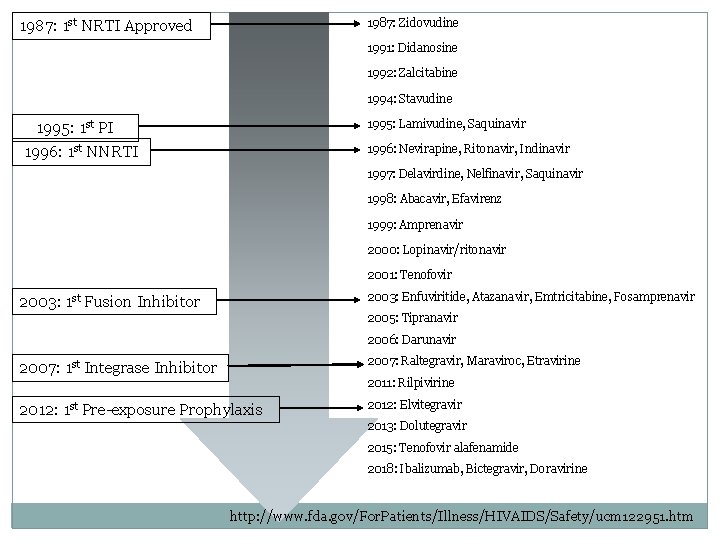
1987: Zidovudine 1987: 1 st NRTI Approved 1991: Didanosine 1992: Zalcitabine 1994: Stavudine 1995: Lamivudine, Saquinavir 1995: 1 st PI 1996: Nevirapine, Ritonavir, Indinavir 1996: 1 st NNRTI 1997: Delavirdine, Nelfinavir, Saquinavir 1998: Abacavir, Efavirenz 1999: Amprenavir 2000: Lopinavir/ritonavir 2001: Tenofovir 2003: Enfuviritide, Atazanavir, Emtricitabine, Fosamprenavir 2003: 1 st Fusion Inhibitor 2005: Tipranavir 2006: Darunavir 2007: 1 st Integrase Inhibitor 2007: Raltegravir, Maraviroc, Etravirine 2012: 1 st Pre-exposure Prophylaxis 2012: Elvitegravir 2011: Rilpivirine 2013: Dolutegravir 2015: Tenofovir alafenamide 2018: Ibalizumab, Bictegravir, Doravirine http: //www. fda. gov/For. Patients/Illness/HIVAIDS/Safety/ucm 122951. htm

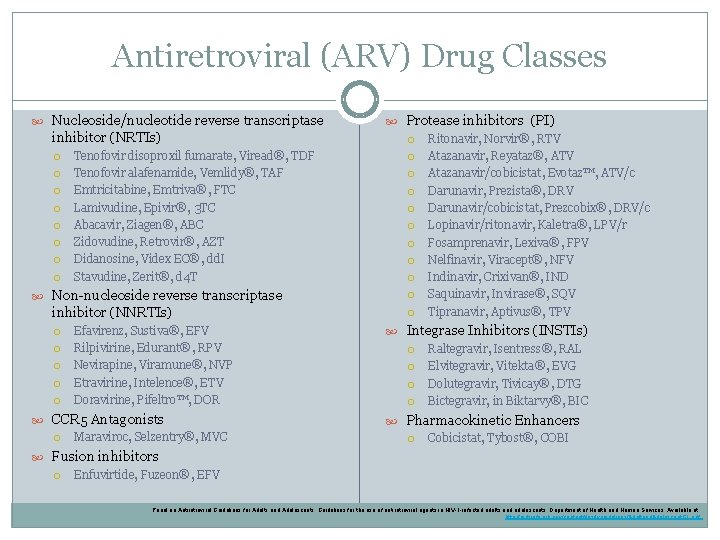
Antiretroviral (ARV) Drug Classes Nucleoside/nucleotide reverse transcriptase inhibitor (NRTIs) Tenofovir disoproxil fumarate, Viread®, TDF Tenofovir alafenamide, Vemlidy®, TAF Emtricitabine, Emtriva®, FTC Lamivudine, Epivir®, 3 TC Abacavir, Ziagen®, ABC Zidovudine, Retrovir®, AZT Didanosine, Videx EC®, dd. I Stavudine, Zerit®, d 4 T Non-nucleoside reverse transcriptase inhibitor (NNRTIs) Efavirenz, Sustiva®, EFV Rilpivirine, Edurant®, RPV Nevirapine, Viramune®, NVP Etravirine, Intelence®, ETV Doravirine, Pifeltro™, DOR CCR 5 Antagonists Maraviroc, Selzentry®, MVC Protease inhibitors (PI) Ritonavir, Norvir®, RTV Atazanavir, Reyataz®, ATV Atazanavir/cobicistat, Evotaz™, ATV/c Darunavir, Prezista®, DRV Darunavir/cobicistat, Prezcobix®, DRV/c Lopinavir/ritonavir, Kaletra®, LPV/r Fosamprenavir, Lexiva®, FPV Nelfinavir, Viracept®, NFV Indinavir, Crixivan®, IND Saquinavir, Invirase®, SQV Tipranavir, Aptivus®, TPV Integrase Inhibitors (INSTIs) Raltegravir, Isentress®, RAL Elvitegravir, Vitekta®, EVG Dolutegravir, Tivicay®, DTG Bictegravir, in Biktarvy®, BIC Pharmacokinetic Enhancers Cobicistat, Tybost®, COBI Fusion inhibitors Enfuvirtide, Fuzeon®, EFV Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

Combination Products Combivir® Zidovudine 300 mg + Lamivudine 150 mg po bid Trizivir® Zidovudine 300 mg + Lamivudine 150 mg + Abacavir 300 mg po bid Epzicom® Lamivudine 300 mg + Abacavir 600 mg po daily Truvada® TDF + Emtricitabine 200 mg po daily Descovy® TAF + Emtricitabine 200 mg po daily Cimduo TDF + Lamivudine 300 mg po daily Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

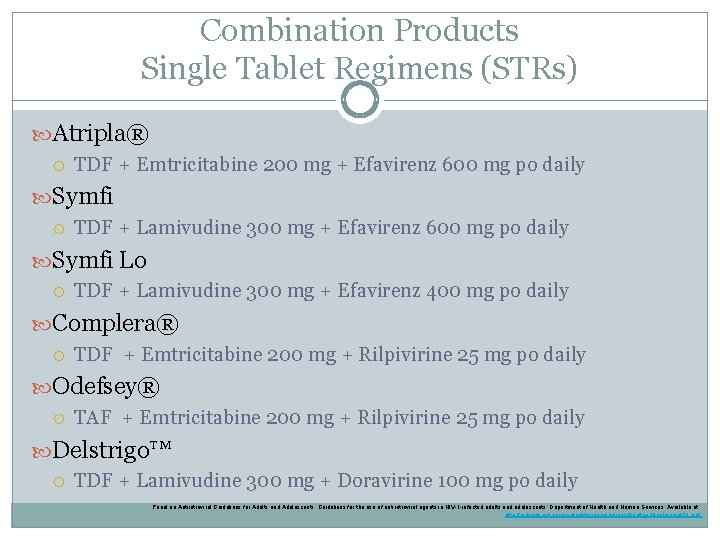
Combination Products Single Tablet Regimens (STRs) Atripla® TDF + Emtricitabine 200 mg + Efavirenz 600 mg po daily Symfi TDF + Lamivudine 300 mg + Efavirenz 600 mg po daily Symfi Lo TDF + Lamivudine 300 mg + Efavirenz 400 mg po daily Complera® TDF + Emtricitabine 200 mg + Rilpivirine 25 mg po daily Odefsey® TAF + Emtricitabine 200 mg + Rilpivirine 25 mg po daily Delstrigo™ TDF + Lamivudine 300 mg + Doravirine 100 mg po daily Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

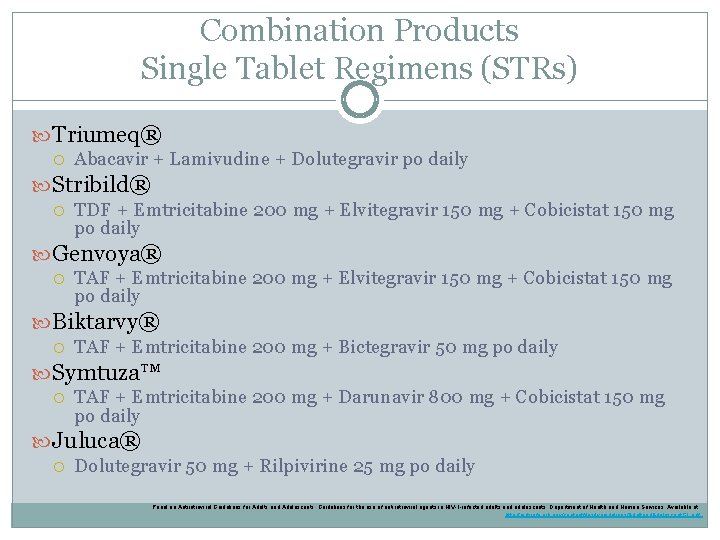
Combination Products Single Tablet Regimens (STRs) Triumeq® Abacavir + Lamivudine + Dolutegravir po daily Stribild® TDF + Emtricitabine 200 mg + Elvitegravir 150 mg + Cobicistat 150 mg po daily Genvoya® TAF + Emtricitabine 200 mg + Elvitegravir 150 mg + Cobicistat 150 mg po daily Biktarvy® TAF + Emtricitabine 200 mg + Bictegravir 50 mg po daily Symtuza™ TAF + Emtricitabine 200 mg + Darunavir 800 mg + Cobicistat 150 mg po daily Juluca® Dolutegravir 50 mg + Rilpivirine 25 mg po daily Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

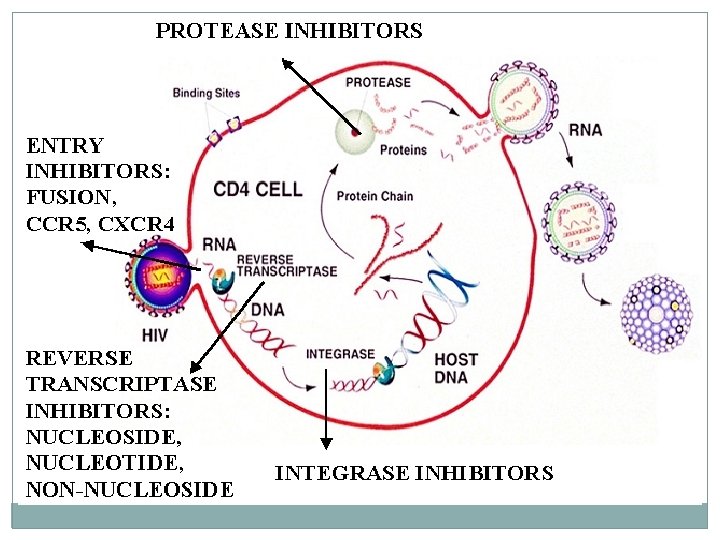
Pathophysiology

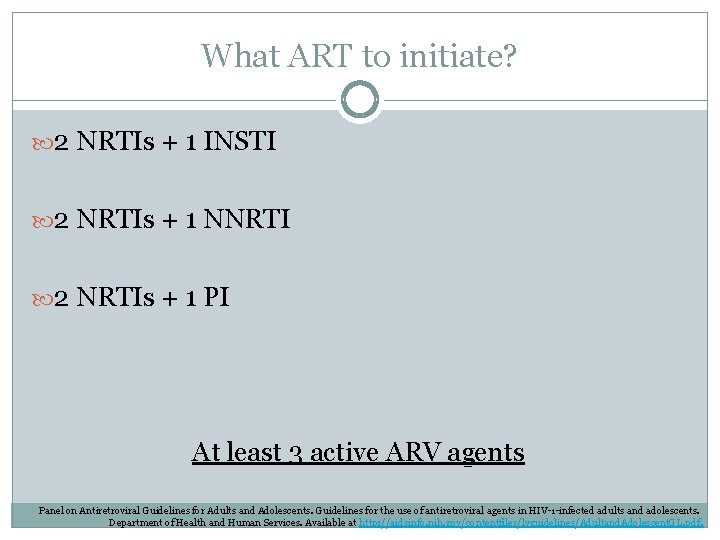
What ART to initiate? 2 NRTIs + 1 INSTI 2 NRTIs + 1 NNRTI 2 NRTIs + 1 PI At least 3 active ARV agents Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf.

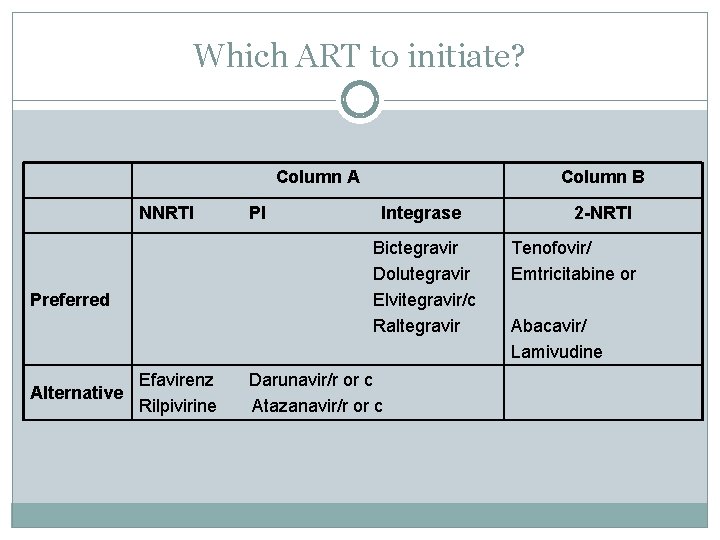
Which ART to initiate? Column A NNRTI Integrase Bictegravir Dolutegravir Elvitegravir/c Raltegravir Preferred Alternative PI Column B Efavirenz Rilpivirine Darunavir/r or c Atazanavir/r or c 2 -NRTI Tenofovir/ Emtricitabine or Abacavir/ Lamivudine

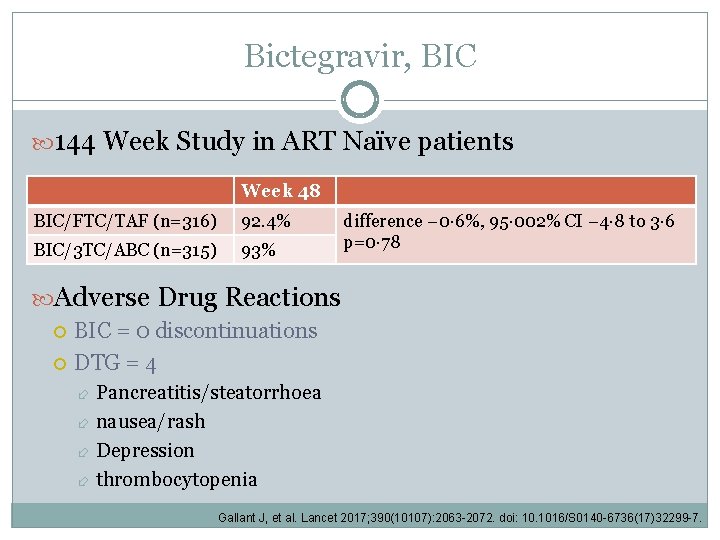
Bictegravir, BIC 144 Week Study in ART Naïve patients Week 48 BIC/FTC/TAF (n=316) 92. 4% BIC/3 TC/ABC (n=315) 93% difference − 0· 6%, 95· 002% CI − 4· 8 to 3· 6 p=0· 78 Adverse Drug Reactions BIC = 0 discontinuations DTG = 4 Pancreatitis/steatorrhoea nausea/rash Depression thrombocytopenia Gallant J, et al. Lancet 2017; 390(10107): 2063 -2072. doi: 10. 1016/S 0140 -6736(17)32299 -7.

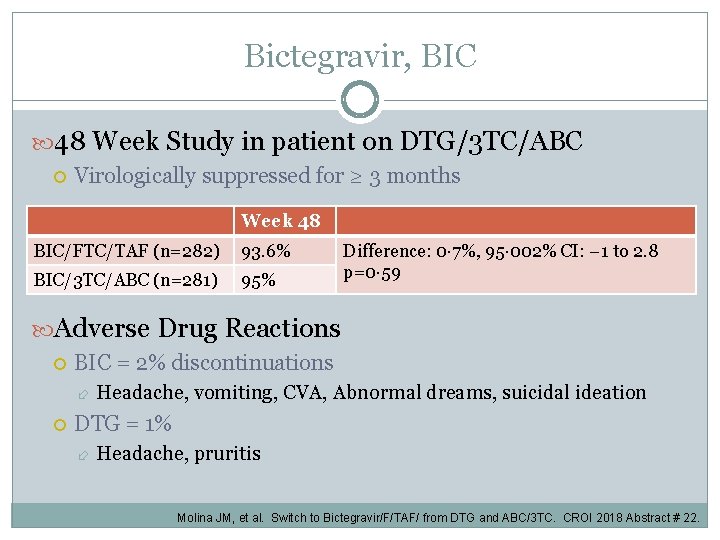
Bictegravir, BIC 48 Week Study in patient on DTG/3 TC/ABC Virologically suppressed for ≥ 3 months Week 48 BIC/FTC/TAF (n=282) 93. 6% BIC/3 TC/ABC (n=281) 95% Difference: 0· 7%, 95· 002% CI: − 1 to 2. 8 p=0· 59 Adverse Drug Reactions BIC = 2% discontinuations Headache, vomiting, CVA, Abnormal dreams, suicidal ideation DTG = 1% Headache, pruritis Molina JM, et al. Switch to Bictegravir/F/TAF/ from DTG and ABC/3 TC. CROI 2018 Abstract # 22.

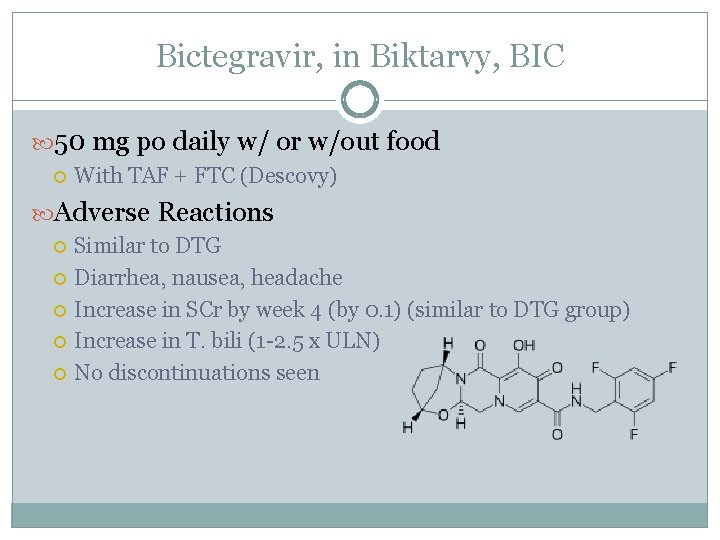
Bictegravir, in Biktarvy, BIC 50 mg po daily w/ or w/out food With TAF + FTC (Descovy) Adverse Reactions Similar to DTG Diarrhea, nausea, headache Increase in SCr by week 4 (by 0. 1) (similar to DTG group) Increase in T. bili (1 -2. 5 x ULN) No discontinuations seen

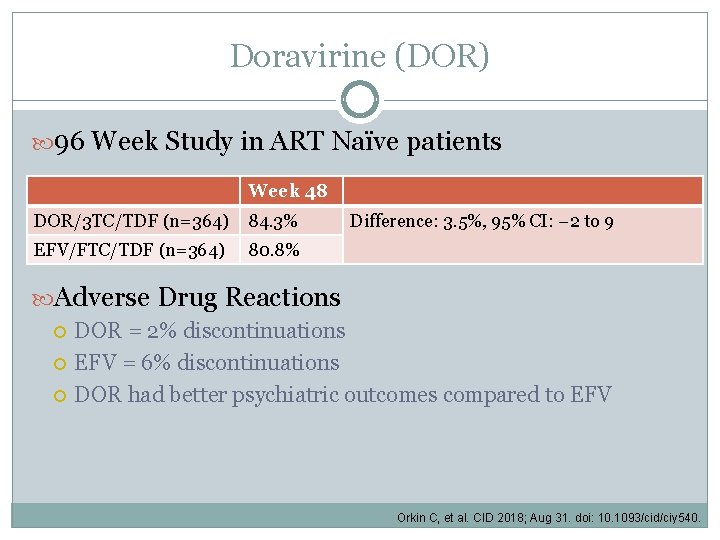
Doravirine (DOR) 96 Week Study in ART Naïve patients Week 48 DOR/3 TC/TDF (n=364) 84. 3% EFV/FTC/TDF (n=364) 80. 8% Difference: 3. 5%, 95% CI: − 2 to 9 Adverse Drug Reactions DOR = 2% discontinuations EFV = 6% discontinuations DOR had better psychiatric outcomes compared to EFV Orkin C, et al. CID 2018; Aug 31. doi: 10. 1093/cid/ciy 540.

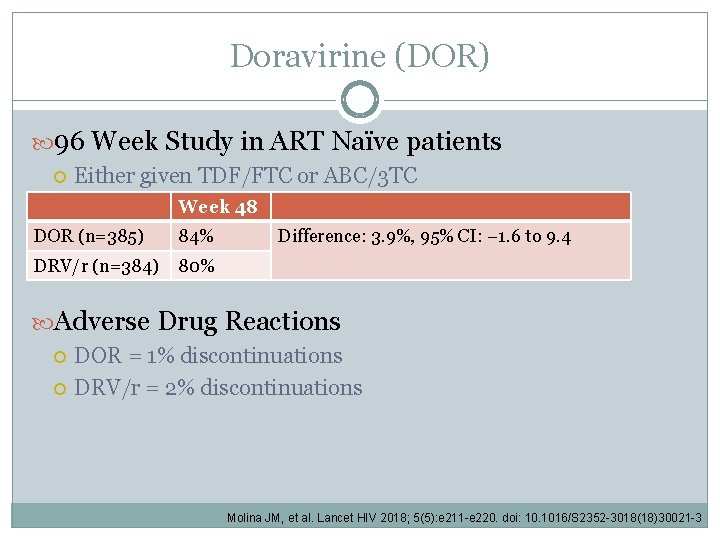
Doravirine (DOR) 96 Week Study in ART Naïve patients Either given TDF/FTC or ABC/3 TC Week 48 DOR (n=385) 84% DRV/r (n=384) 80% Difference: 3. 9%, 95% CI: − 1. 6 to 9. 4 Adverse Drug Reactions DOR = 1% discontinuations DRV/r = 2% discontinuations Molina JM, et al. Lancet HIV 2018; 5(5): e 211 -e 220. doi: 10. 1016/S 2352 -3018(18)30021 -3

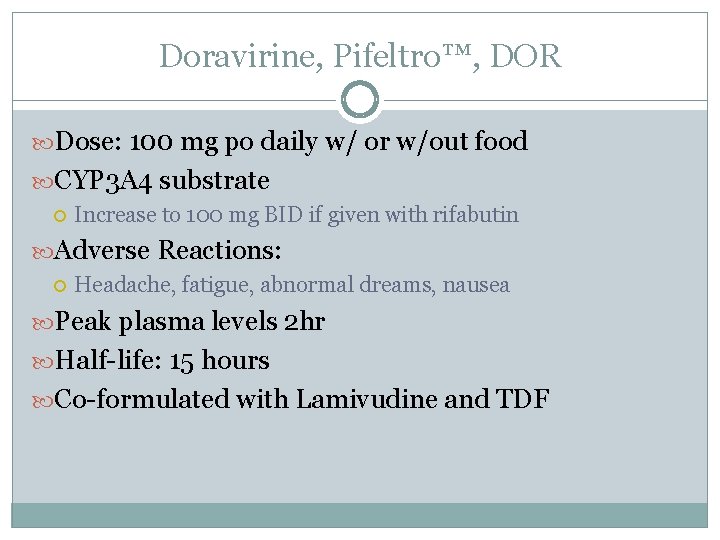
Doravirine, Pifeltro™, DOR Dose: 100 mg po daily w/ or w/out food CYP 3 A 4 substrate Increase to 100 mg BID if given with rifabutin Adverse Reactions: Headache, fatigue, abnormal dreams, nausea Peak plasma levels 2 hr Half-life: 15 hours Co-formulated with Lamivudine and TDF

Regimen Simplification/De-esclation Changing an effective regimen to a ‘simpler’ regimen Ensure patient is adequately suppressed Within Class: Efavirenz to Rilpivirine TDF to TAF Raltegravir to Elvitegravir/Dolutegravir/Bictegravir RTV boosted PI to coformulated COBI boosted PI Less pill burden

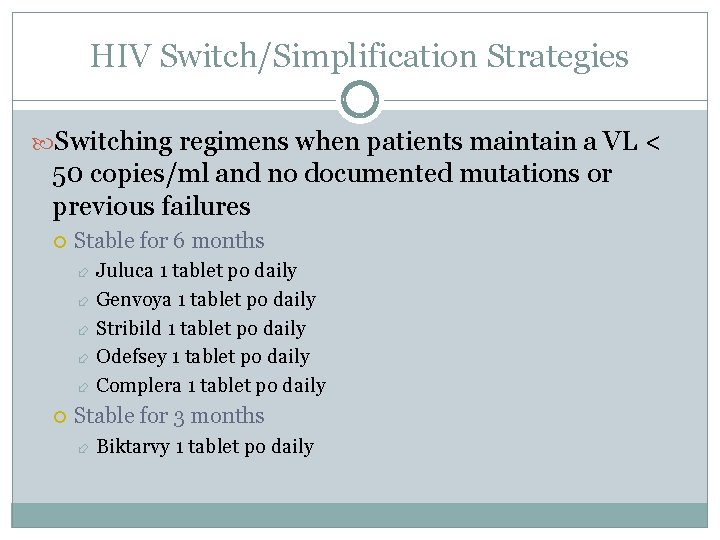
HIV Switch/Simplification Strategies Switching regimens when patients maintain a VL < 50 copies/ml and no documented mutations or previous failures Stable for 6 months Juluca 1 tablet po daily Genvoya 1 tablet po daily Stribild 1 tablet po daily Odefsey 1 tablet po daily Complera 1 tablet po daily Stable for 3 months Biktarvy 1 tablet po daily

Why Dolutegravir? Long plasma half-life = 15. 3 hours High barrier to resistance Low incidence of severe toxicities Minimal drug-drug interactions

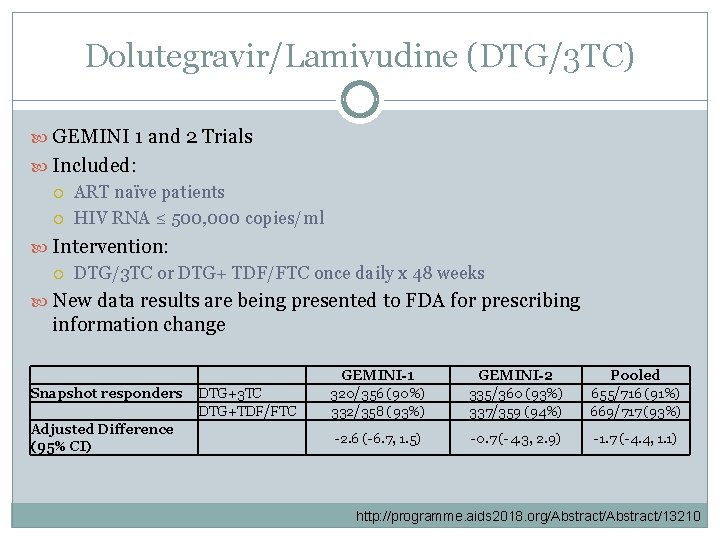
Dolutegravir/Lamivudine (DTG/3 TC) GEMINI 1 and 2 Trials Included: ART naïve patients HIV RNA ≤ 500, 000 copies/ml Intervention: DTG/3 TC or DTG+ TDF/FTC once daily x 48 weeks New data results are being presented to FDA for prescribing information change Snapshot responders Adjusted Difference (95% CI) DTG+3 TC DTG+TDF/FTC GEMINI-1 320/356 (90%) 332/358 (93%) GEMINI-2 335/360 (93%) 337/359 (94%) Pooled 655/716 (91%) 669/717 (93%) -2. 6 (-6. 7, 1. 5) -0. 7 (-4. 3, 2. 9) -1. 7 (-4. 4, 1. 1) http: //programme. aids 2018. org/Abstract/13210

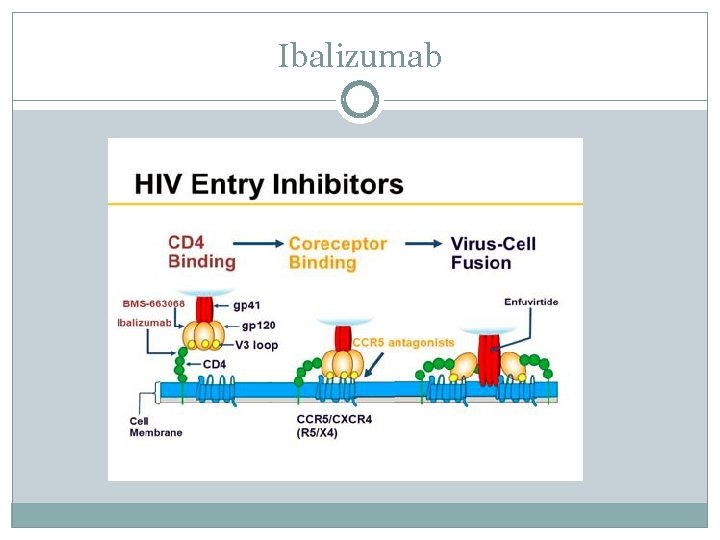
Ibalizumab

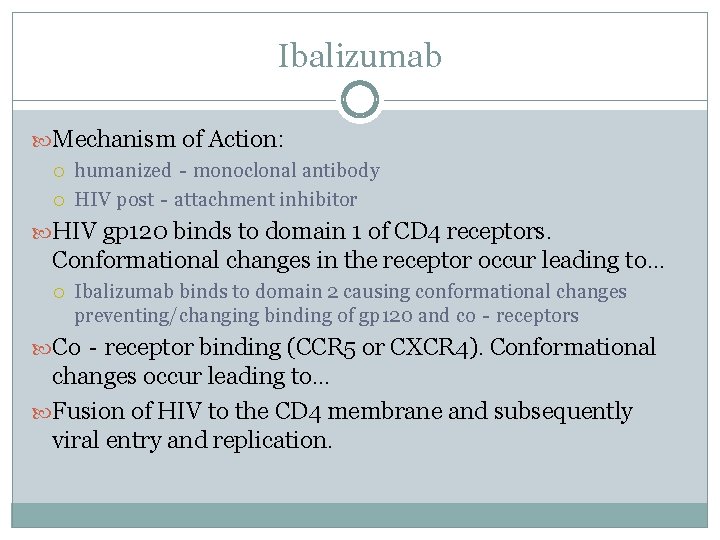
Ibalizumab Mechanism of Action: humanized‐monoclonal antibody HIV post‐attachment inhibitor HIV gp 120 binds to domain 1 of CD 4 receptors. Conformational changes in the receptor occur leading to… Ibalizumab binds to domain 2 causing conformational changes preventing/changing binding of gp 120 and co‐receptors Co‐receptor binding (CCR 5 or CXCR 4). Conformational changes occur leading to… Fusion of HIV to the CD 4 membrane and subsequently viral entry and replication.

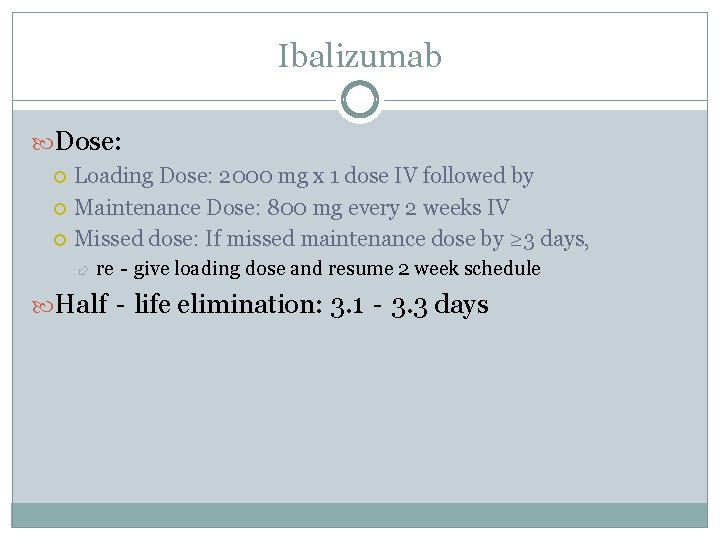
Ibalizumab Dose: Loading Dose: 2000 mg x 1 dose IV followed by Maintenance Dose: 800 mg every 2 weeks IV Missed dose: If missed maintenance dose by ≥ 3 days, re‐give loading dose and resume 2 week schedule Half‐life elimination: 3. 1‐ 3. 3 days

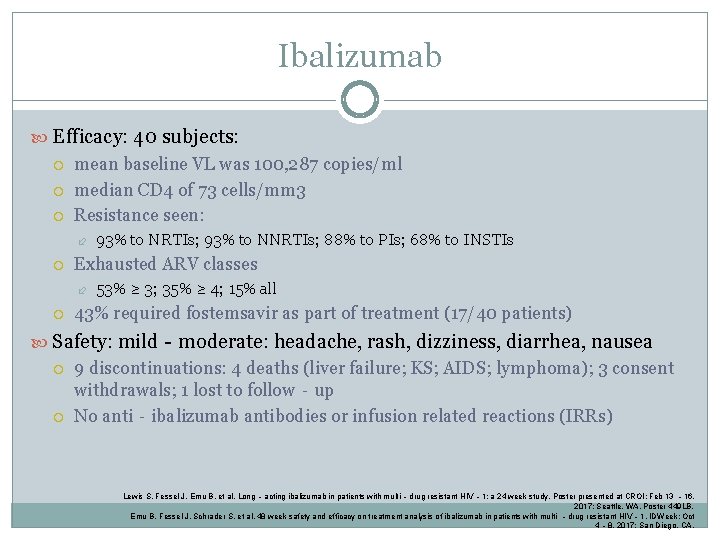
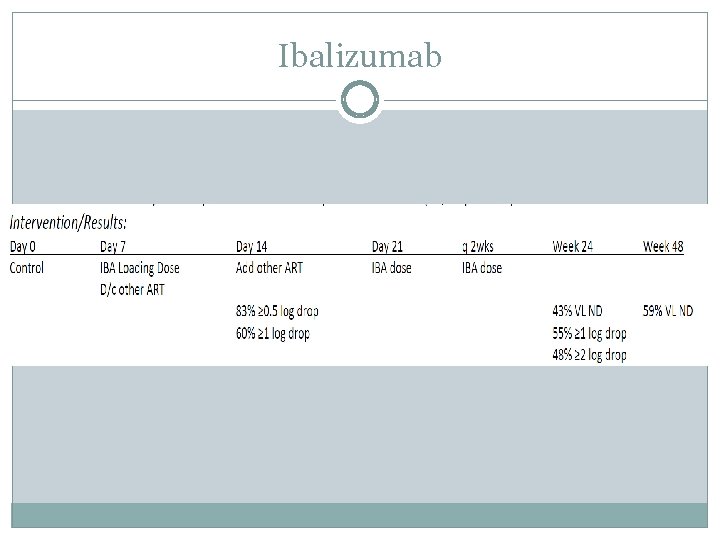
Ibalizumab Efficacy: 40 subjects: mean baseline VL was 100, 287 copies/ml median CD 4 of 73 cells/mm 3 Resistance seen: Exhausted ARV classes 93% to NRTIs; 93% to NNRTIs; 88% to PIs; 68% to INSTIs 53% ≥ 3; 35% ≥ 4; 15% all 43% required fostemsavir as part of treatment (17/40 patients) Safety: mild‐moderate: headache, rash, dizziness, diarrhea, nausea 9 discontinuations: 4 deaths (liver failure; KS; AIDS; lymphoma); 3 consent withdrawals; 1 lost to follow‐up No anti‐ibalizumab antibodies or infusion related reactions (IRRs) Lewis S, Fessel J, Emu B, et al. Long ‐acting ibalizumab in patients with multi‐drug resistant HIV‐ 1: a 24 week study. Poster presented at CROI: Feb 13 ‐ 16, 2017; Seattle, WA. Poster 449 LB. Emu B, Fessel J, Schrader S, et al. 48 week safety and efficacy on treatment analysis of ibalizumab in patients with multi ‐drug resistant HIV‐ 1. IDWeek; Oct 4‐ 8, 2017; San Diego, CA.

Ibalizumab

Fostemsavir Prodrug of temsavir (BMS-626529) Binds to the HIV gp 120 protein, preventing HIV and CD 4 T -lymphocyte attachment 272 patients resistant to ≥ 2 classes of ART Fostemsavir 600 mg BID x 8 day +failing regimen, then Optimized background therapy 10% had no fully active ART in OBR Day 8: 0. 79 vs 0. 17 log viral reduction (fostemsavir vs. placebo) Will likely apply for FDA approval in 2019 Pialoux G et al. Phase 3 study of fostemsavir in heavily treatmentexperienced HIV-1 -infected participants: BRIGHTE week 24 subgroup analysis in randomized cohort subjects. 22 nd International ADIS Conference (AIDS 2018), 23– 27 July 2018, Amsterdam. Poster abstract THPEB 045.

HIV Pipeline Medications Integrase Inhibitor Cabotegravir oral and LA Cabotegravir/Rilpivirine LA Fusion Inhibitor Albuvirtide Monoclonal Antibodies PRO 140 UB-421

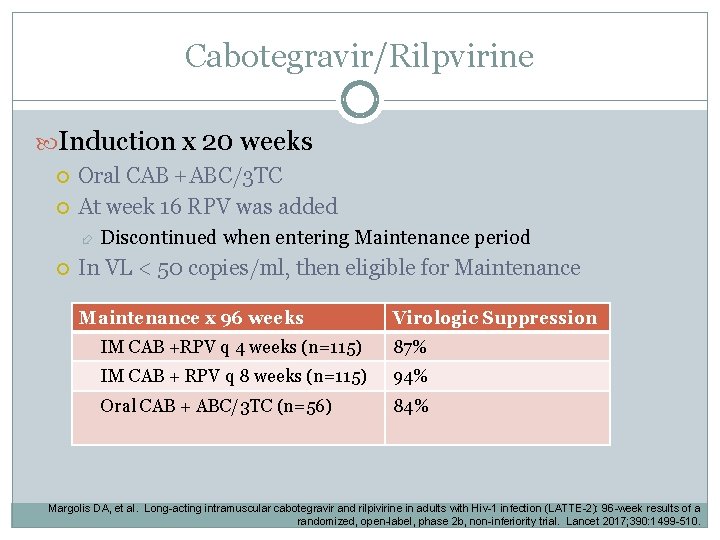
Cabotegravir/Rilpvirine Induction x 20 weeks Oral CAB +ABC/3 TC At week 16 RPV was added Discontinued when entering Maintenance period In VL < 50 copies/ml, then eligible for Maintenance x 96 weeks Virologic Suppression IM CAB +RPV q 4 weeks (n=115) 87% IM CAB + RPV q 8 weeks (n=115) 94% Oral CAB + ABC/3 TC (n=56) 84% Margolis DA, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with Hiv-1 infection (LATTE-2): 96 -week results of a randomized, open-label, phase 2 b, non-inferiority trial. Lancet 2017; 390: 1499 -510.

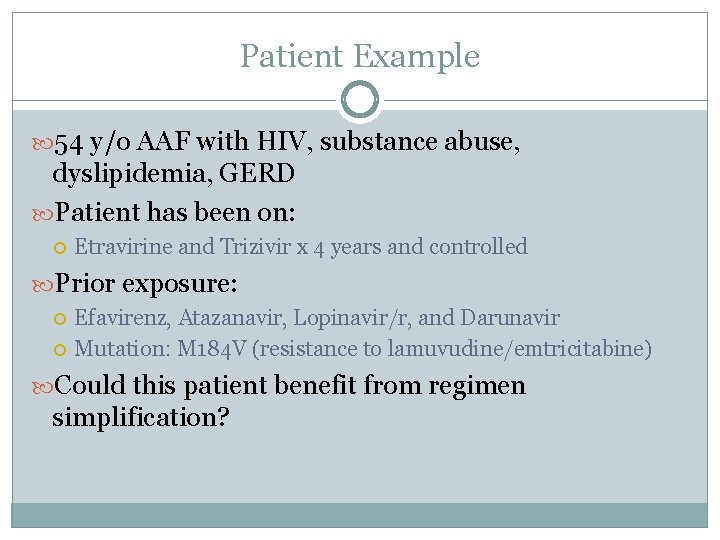
Patient Example 54 y/o AAF with HIV, substance abuse, dyslipidemia, GERD Patient has been on: Etravirine and Trizivir x 4 years and controlled Prior exposure: Efavirenz, Atazanavir, Lopinavir/r, and Darunavir Mutation: M 184 V (resistance to lamuvudine/emtricitabine) Could this patient benefit from regimen simplification?

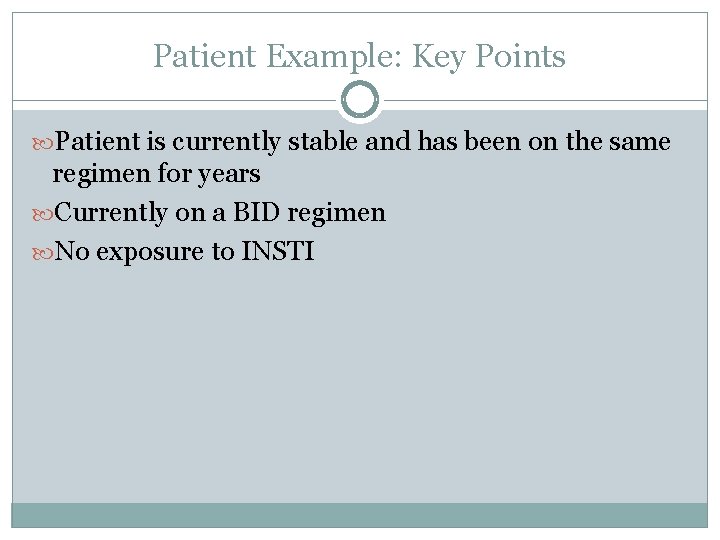
Patient Example: Key Points Patient is currently stable and has been on the same regimen for years Currently on a BID regimen No exposure to INSTI

Objectives Identify previous and current guidelines for HIV treatment All HIV infected patients should be offered HIV treatment Changes in initial combination regimens recommended Discussion of regimen simplification

Objectives Describe clinical uses of recently marketed anti-retrovirals Ibalizumab Treatment simplification

Objectives Describe the potential influence new anti-retrovirals will have on HIV management Long-acting medications New Entry Inhibitors Multi-drug resistance

References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. UNAIDS Global HIV & AIDS Statistics – 2018 fact Sheet: http: //www. unaids. org/en/resources/fact-sheet CDC HIV in the United States: At a Glance: http: //www. cdc. gov/hiv/statistics/overview/ataglance. html MDH: HIV in Maryland, 2014: August 2016: http: //phpa. dhmh. maryland. gov/OIDEOR/CHSE/Site. Assets/Pages/statistics/Maryland-HIV-Fact-Sheet. pdf MDH: World AIDS Day 2013: http: //phpa. dhmh. maryland. gov/OIDEOR/CHSE/Shared%20 Documents/World-AIDSDay. pdf Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services. Available at http: //aidsinfo. nih. gov/contentfiles/lvguidelines/Adultand. Adolescent. GL. pdf. CDC Understanding the HIV Care Continuum: https: //www. cdc. gov/hiv/pdf/library/factsheets/cdc-hiv-carecontinuum. pdf FDA What’s New at the FDA in HIV/AIDS: http: //www. fda. gov/For. Patients/Illness/HIVAIDS/Safety/ucm 122951. htm Gallant J, et al. Lancet 2017; 390(10107): 2063 -2072. Molina JM, et al. CROI 2018 Abstract # 22. Orkin C, et al. CID 2018; Aug 31. Molina JM, et al. Lancet HIV 2018; 5(5): e 211 -e 220. Cahn P, et al. AIDS 2018. TUAB 0106 LB Lewis S, et al. CROI 2017. Poster 449 LB. Emu B, et al. IDWeek 2017. Pialoux G et al. AIDS 2018. Abstract THPEB 045. Margolis DA, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with Hiv-1 infection (LATTE-2): 96 week results of a randomized, open-label, phase 2 b, non-inferiority trial. Lancet 2017; 390: 1499 -510.

HIV Medication Update NEHA SHETH PANDIT, PHARMD, AAHIVP, BCPS UNIVERSITY OF MARYLAND SCHOOL OF PHARMACY ASSOCIATE PROFESSOR, HIV/INFECTIOUS DISEASES SEPTEMBER 2018

CE Access Code HIVmedupdate 42 PARTICIPANTS HAVE UNTIL NOVEMBER 6, 2018 TO EARN 1. 0 CONTACT HOUR OF CONTINUING PHARMACY EDUCATION (CPE) CREDIT FOR THIS ACTIVITY BY FULL SESSION ATTENDANCE/PARTICIPATION AND SUCCESSFUL COMPLETION OF THE ONLINE ACTIVITY EVALUATION AND POST-ASSESSMENT TEST. ACCESS TO THE EVALUATION AND TEST IS THROUGH USE OF THE CE ACCESS CODE AS DISPLAYED ON THIS SLIDE. AFTER NOVEMBER 6 TH, 2018 NO CE CREDIT WILL BE AVAILABLE FOR THIS PROGRAM. CREDITS WILL BE TRANSFERRED ELECTRONICALLY TO THE CPE MONITOR SYSTEM.
 Rutgers pharmacy fellowship
Rutgers pharmacy fellowship Elizabeth farrington pharmd
Elizabeth farrington pharmd Dr rahul pandit
Dr rahul pandit Backup and recovery techniques
Backup and recovery techniques Pavlov'un öğrenme modeli
Pavlov'un öğrenme modeli Howard sheth modeli
Howard sheth modeli Engel kollat et blackwell
Engel kollat et blackwell Assael modeli
Assael modeli Abdulla sheth
Abdulla sheth Neha pahuja
Neha pahuja Neha urkude
Neha urkude Hospital waste management introduction
Hospital waste management introduction Victoria ding
Victoria ding Neha surapaneni
Neha surapaneni Neha premkumar
Neha premkumar Haivn
Haivn Hiv
Hiv Chii chinonzi hiv
Chii chinonzi hiv Aids symptoms
Aids symptoms Hiv
Hiv Virus hiv
Virus hiv Ott a messze földön árván hontalan
Ott a messze földön árván hontalan Tes klamidia di prodia
Tes klamidia di prodia Procentowe ryzyko zakażenia hiv
Procentowe ryzyko zakażenia hiv Hiv lifecycle
Hiv lifecycle Ciclo vitale hiv
Ciclo vitale hiv Hiv treatments
Hiv treatments Hiv
Hiv Hiv test
Hiv test Hiv
Hiv Murder by hiv
Murder by hiv Hiv stays alive in dried blood
Hiv stays alive in dried blood Steps of index testing
Steps of index testing Aids is caused by
Aids is caused by Herpes syntom
Herpes syntom Stadium hiv
Stadium hiv Vertical resposta
Vertical resposta Hiv test window period
Hiv test window period Hora no brasil agora
Hora no brasil agora Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Hiv diagnostics conference
Hiv diagnostics conference Hiv
Hiv Ephi ethiopia
Ephi ethiopia