Therapy Update Multiple Sclerosis CASSANDRA S HUGGAR PHARMD




























- Slides: 62

Therapy Update: Multiple Sclerosis CASSANDRA S. HUGGAR, PHARMD, BCPS SPINAL CORD INJURY/NEUROLOGY CLINICAL PHARMACY SPECIALIST SOUTH TEXAS VETERANS HEALTH CARE SYSTEM SEPTEMBER 16 TH, 2017

Learning Objectives Describe the four subtypes of multiple sclerosis (MS) Discuss therapeutic advancements available for the treatment of MS and place in therapy Contrast adverse effects, safety concerns, and tolerability profiles of drug therapy available 2

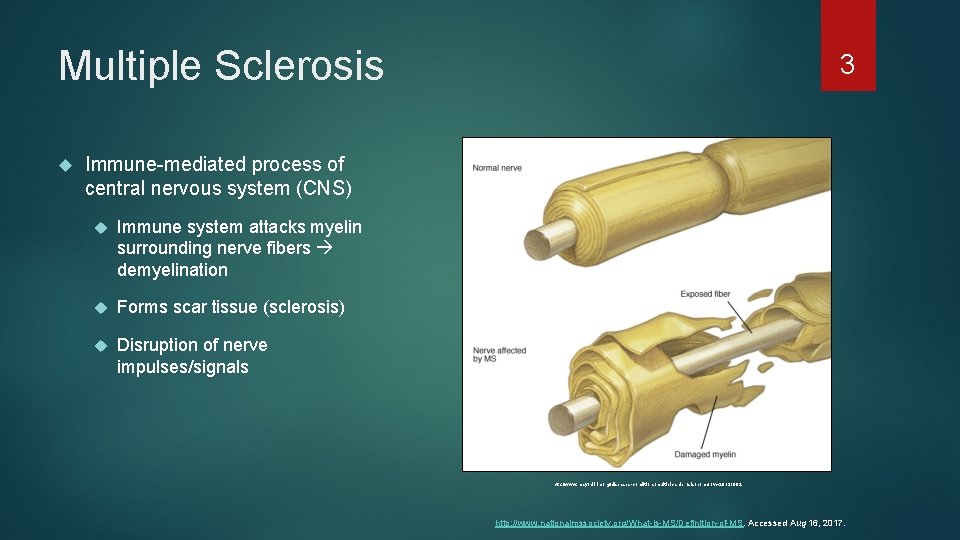
Multiple Sclerosis 3 Immune-mediated process of central nervous system (CNS) Immune system attacks myelin surrounding nerve fibers demyelination Forms scar tissue (sclerosis) Disruption of nerve impulses/signals http: //www. mayoclinic. org/diseases-conditions/multiple-sclerosis/home/ovc-20131882. http: //www. nationalmssociety. org/What-is-MS/Definition-of-MS. Accessed Aug 16, 2017.

Epidemiology 4 2. 3 million individuals worldwide 450, 000 in United States Onset of age 20 -50 2 -3 times more common in women than men More prevalent further from the equator http: //www. nationalmssociety. org/What-is-MS/Definition-of-MS. Accessed Aug 16, 2017.

Causes 5 Exact cause unknown Genetic factor First degree relatives Immunological factors Environmental factors Vitamin D deficiency Infectious factors Epstein-Barr Virus http: //www. nationalmssociety. org/What-is-MS/What-Causes-MS. Accessed Aug 22, 2017. http: //www. nationalmssociety. org/What-is-MS/What-Causes-MS/Viruses. Accessed Aug 22, 2017.

Symptoms 6 Clinical Manifestations Fatigue Numbness or tingling Weakness Vision problems Walking (gait) difficulties Dizziness/vertigo Spasticity Bladder/bowel problems Sexual problems Cognitive changes Emotional changes Depression Pain http: //www. nationalmssociety. org/Symptoms-Diagnosis/MS-Symptoms. Accessed Aug 23, 2017.

Pathophysiology Exact cause unknown Immune mediate response Immune cells infiltration from the periphery into CNS T-cells B-cells 7

8 TYPES OF MULTIPLE SCLEROSIS

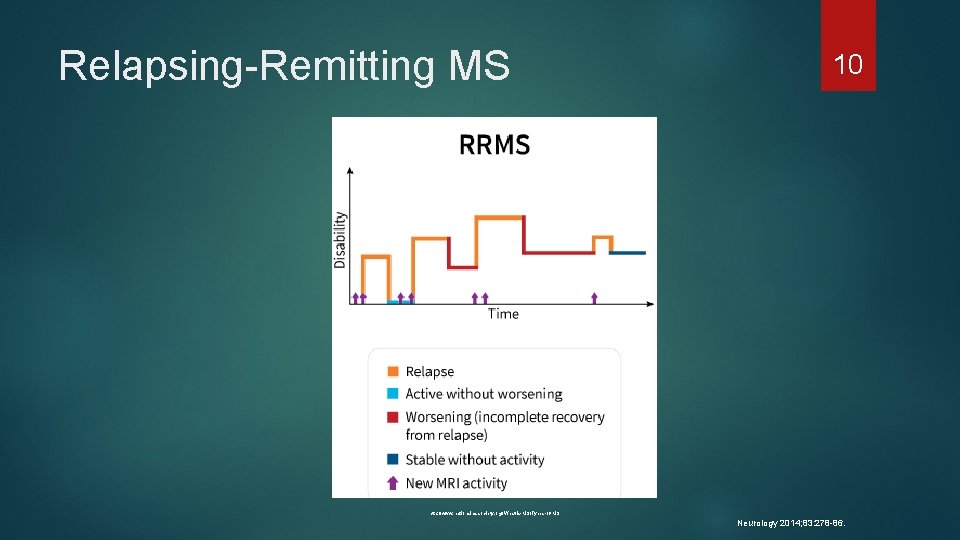
Types of Multiple Sclerosis 9 Clinically isolated syndrome (CIS) First episode Last at least 24 hours Relapsing-remitting MS (RRMS) Most common Periods of acute exacerbations “relapses” Partial or complete resolution/recovery http: //www. nationalmssociety. org/What-is-MS/Types-of-MS. Accessed August 22, 2017. Neurology 2014; 83: 278 -86.

Relapsing-Remitting MS 10 http: //www. nationalmssociety. org/What-is-MS/Types-of-MS Neurology 2014; 83: 278 -86.

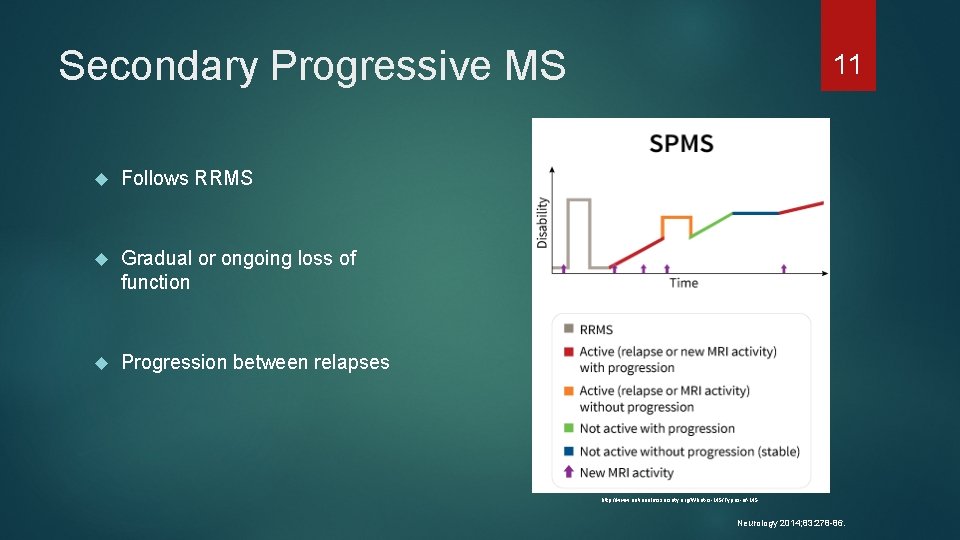
Secondary Progressive MS Follows RRMS Gradual or ongoing loss of function Progression between relapses 11 http: //www. nationalmssociety. org/What-is-MS/Types-of-MS Neurology 2014; 83: 278 -86.

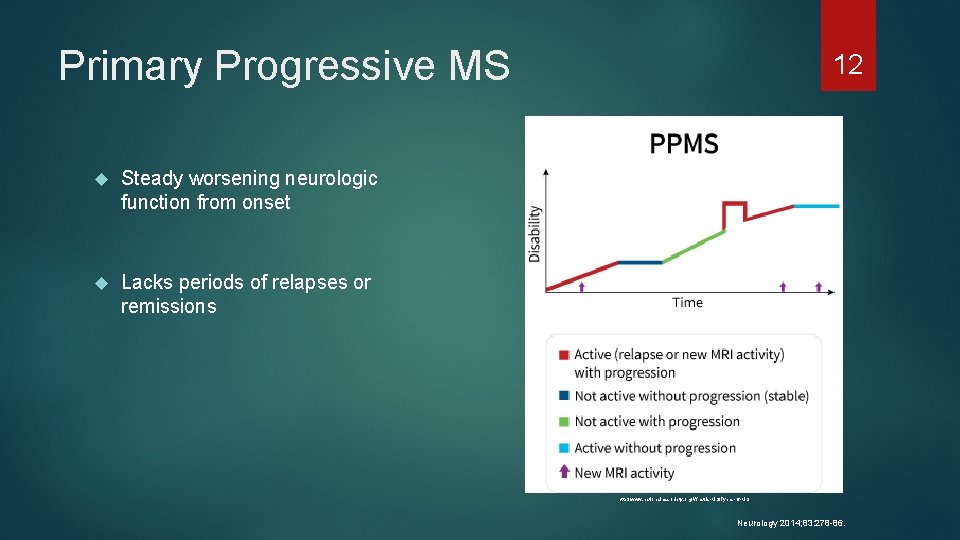
Primary Progressive MS Steady worsening neurologic function from onset Lacks periods of relapses or remissions 12 http: //www. nationalmssociety. org/What-is-MS/Types-of-MS Neurology 2014; 83: 278 -86.

Goals of Therapy Reduce attacks Slow down progression Prevent or postpone long-term disability 13

14 TREATMENT OPTIONS

15 SELF-INJECTABLE THERAPIES

Interferon Beta Avonex® Rebif® Betaseron® Extavia® Plegridy® 16

Interferon Beta Mechanism of Action FDA Indication Dose Relapse Reduction 17 • Immunomodulatory effects • RRMS • CIS (non-FDA) • Varies by product • 30 -35% relative risk reduction in annualized relapse rates Lancet 1992; 352: 1498 -504 Eur J Neurol. 2015; 23 Suppl 1: 18 -27. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

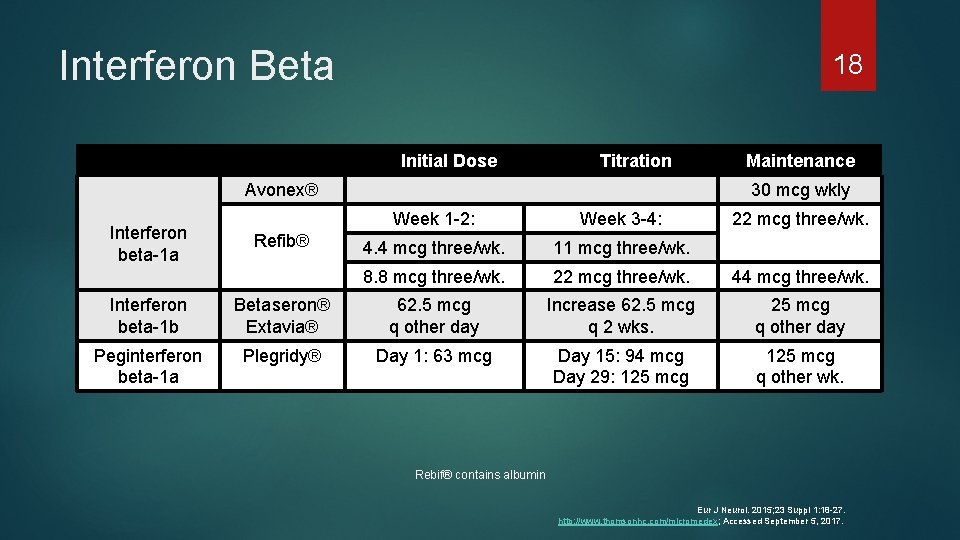
Interferon Beta 18 Initial Dose Titration Avonex® Interferon beta-1 a Refib® Maintenance 30 mcg wkly Week 1 -2: Week 3 -4: 22 mcg three/wk. 4. 4 mcg three/wk. 11 mcg three/wk. 8. 8 mcg three/wk. 22 mcg three/wk. 44 mcg three/wk. Interferon beta-1 b Betaseron® Extavia® 62. 5 mcg q other day Increase 62. 5 mcg q 2 wks. 25 mcg q other day Peginterferon beta-1 a Plegridy® Day 1: 63 mcg Day 15: 94 mcg Day 29: 125 mcg q other wk. Rebif® contains albumin Eur J Neurol. 2015; 23 Suppl 1: 18 -27. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

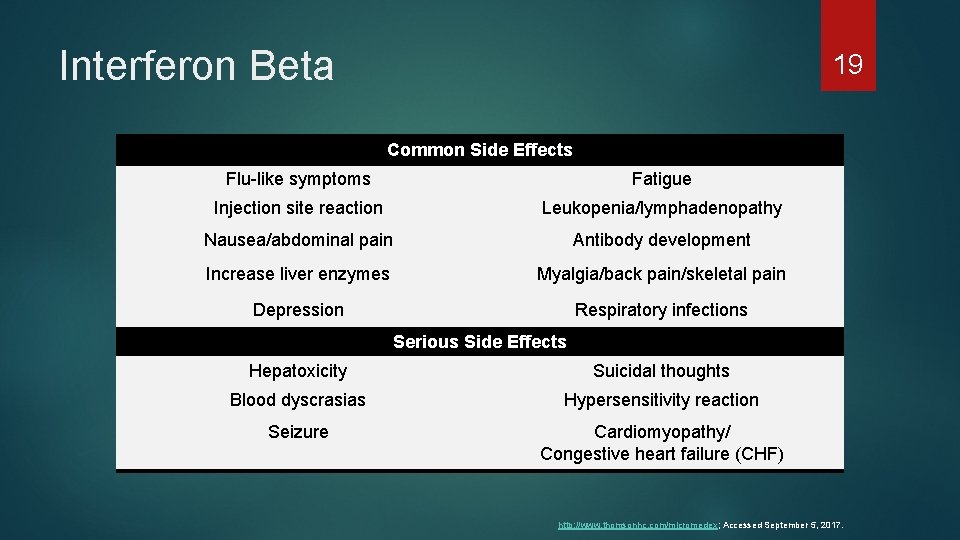
Interferon Beta 19 Common Side Effects Flu-like symptoms Fatigue Injection site reaction Leukopenia/lymphadenopathy Nausea/abdominal pain Antibody development Increase liver enzymes Myalgia/back pain/skeletal pain Depression Respiratory infections Serious Side Effects Hepatoxicity Suicidal thoughts Blood dyscrasias Hypersensitivity reaction Seizure Cardiomyopathy/ Congestive heart failure (CHF) http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

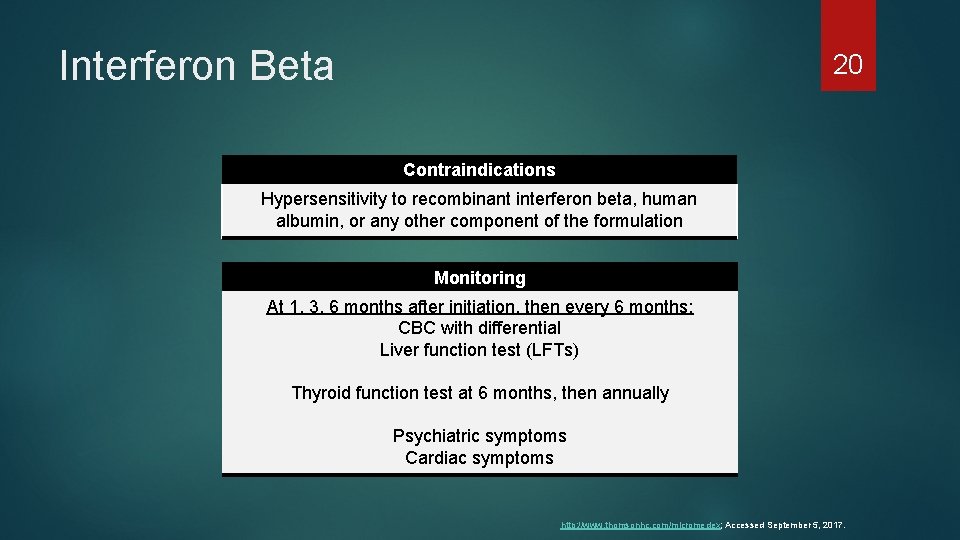
Interferon Beta 20 Contraindications Hypersensitivity to recombinant interferon beta, human albumin, or any other component of the formulation Monitoring At 1, 3, 6 months after initiation, then every 6 months: CBC with differential Liver function test (LFTs) Thyroid function test at 6 months, then annually Psychiatric symptoms Cardiac symptoms http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

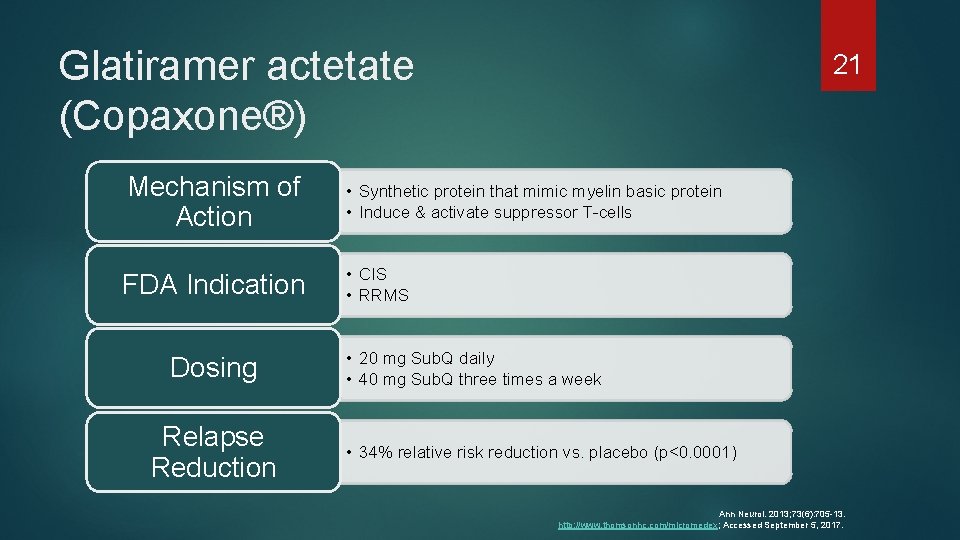
Glatiramer actetate (Copaxone®) 21 Mechanism of Action • Synthetic protein that mimic myelin basic protein • Induce & activate suppressor T-cells FDA Indication • CIS • RRMS Dosing Relapse Reduction • 20 mg Sub. Q daily • 40 mg Sub. Q three times a week • 34% relative risk reduction vs. placebo (p<0. 0001) Ann Neurol. 2013; 73(6): 705 -13. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

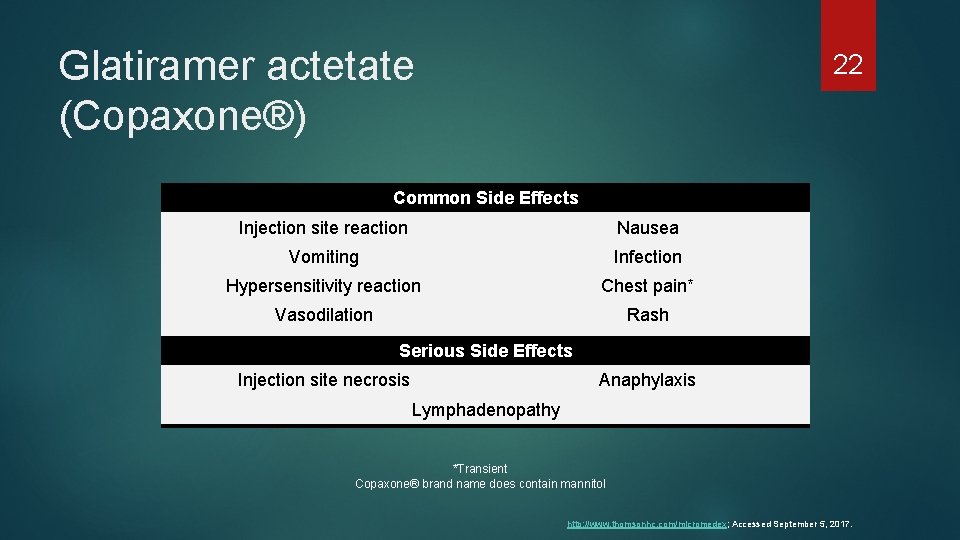
Glatiramer actetate (Copaxone®) 22 Common Side Effects Injection site reaction Nausea Vomiting Infection Hypersensitivity reaction Chest pain* Vasodilation Rash Serious Side Effects Injection site necrosis Anaphylaxis Lymphadenopathy *Transient Copaxone® brand name does contain mannitol http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

23 ORAL THERAPIES

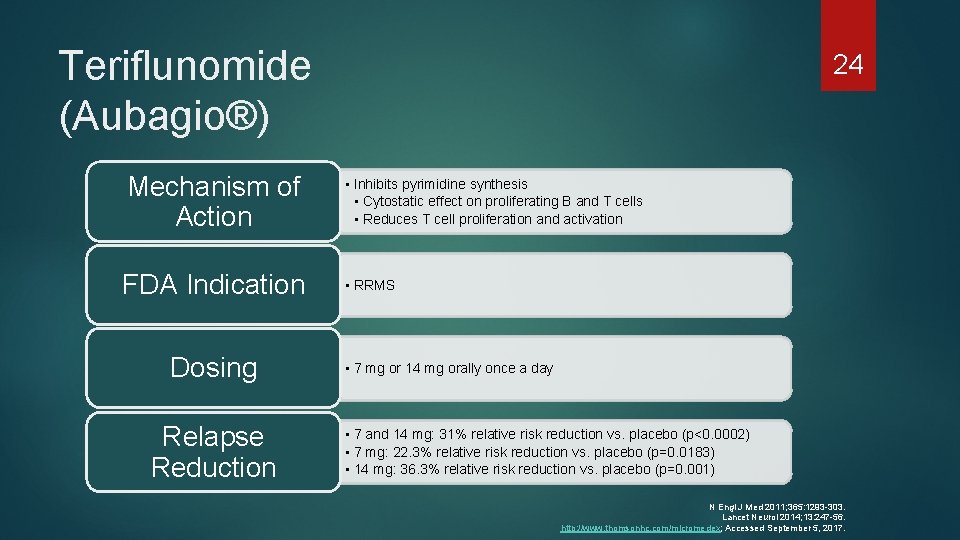
Teriflunomide (Aubagio®) Mechanism of Action FDA Indication Dosing Relapse Reduction 24 • Inhibits pyrimidine synthesis • Cytostatic effect on proliferating B and T cells • Reduces T cell proliferation and activation • RRMS • 7 mg or 14 mg orally once a day • 7 and 14 mg: 31% relative risk reduction vs. placebo (p<0. 0002) • 7 mg: 22. 3% relative risk reduction vs. placebo (p=0. 0183) • 14 mg: 36. 3% relative risk reduction vs. placebo (p=0. 001) N Engl J Med 2011; 365: 1293 -303. Lancet Neurol 2014; 13: 247 -56. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

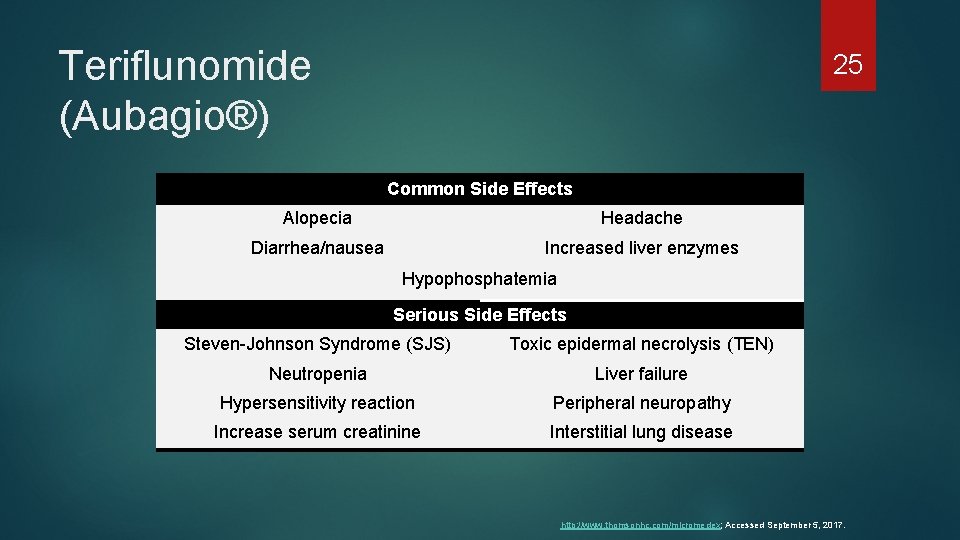
Teriflunomide (Aubagio®) 25 Common Side Effects Alopecia Headache Diarrhea/nausea Increased liver enzymes Hypophosphatemia Serious Side Effects Steven-Johnson Syndrome (SJS) Toxic epidermal necrolysis (TEN) Neutropenia Liver failure Hypersensitivity reaction Peripheral neuropathy Increase serum creatinine Interstitial lung disease http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

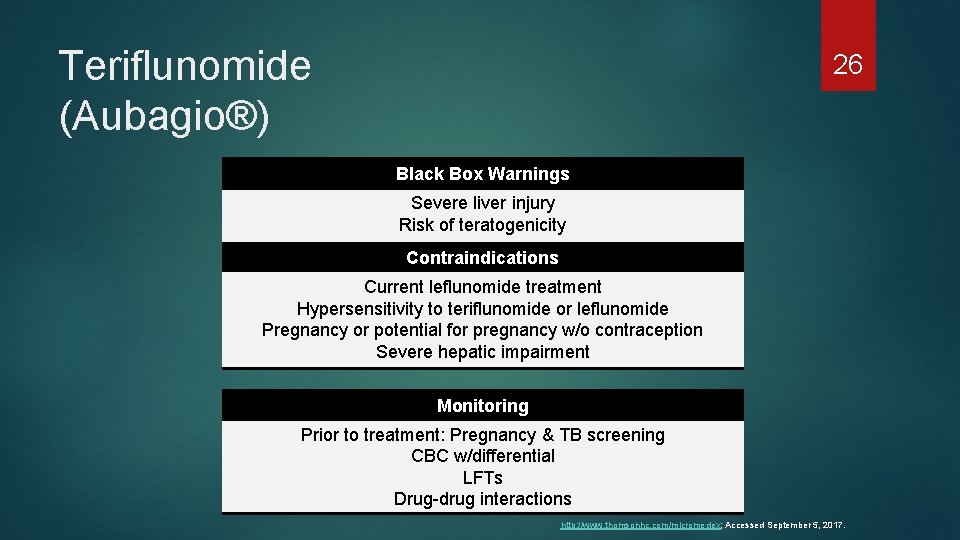
Teriflunomide (Aubagio®) 26 Black Box Warnings Severe liver injury Risk of teratogenicity Contraindications Current leflunomide treatment Hypersensitivity to teriflunomide or leflunomide Pregnancy or potential for pregnancy w/o contraception Severe hepatic impairment Monitoring Prior to treatment: Pregnancy & TB screening CBC w/differential LFTs Drug-drug interactions http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Teriflunomide (Aubagio®) Pregnancy 27 Avoid pregnancy until undetectable levels (<0. 02 mg/L) verified Males Found in semen Remains in semen up to 2 yrs. Enhanced drug elimination Cholestyramine or activated charcoal powder for 11 days http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Fingolimod (Gilyena®) 28 Mechanism of Action • Sphingosine 1 -phosphate (S 1 P) receptor agonist • Sequesters & blocks lymphocyte migration to CNS FDA Indication • RRMS Dosing Relapse Reduction • 0. 5 mg orally once a day • 54% relative risk reduction vs. placebo (p<0. 001) • 51% relative risk reduction vs. interferon (p<0. 001) N Eng J Med 2010; 362: 387 -401. N Eng J Med 2010; 362: 402 -15. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

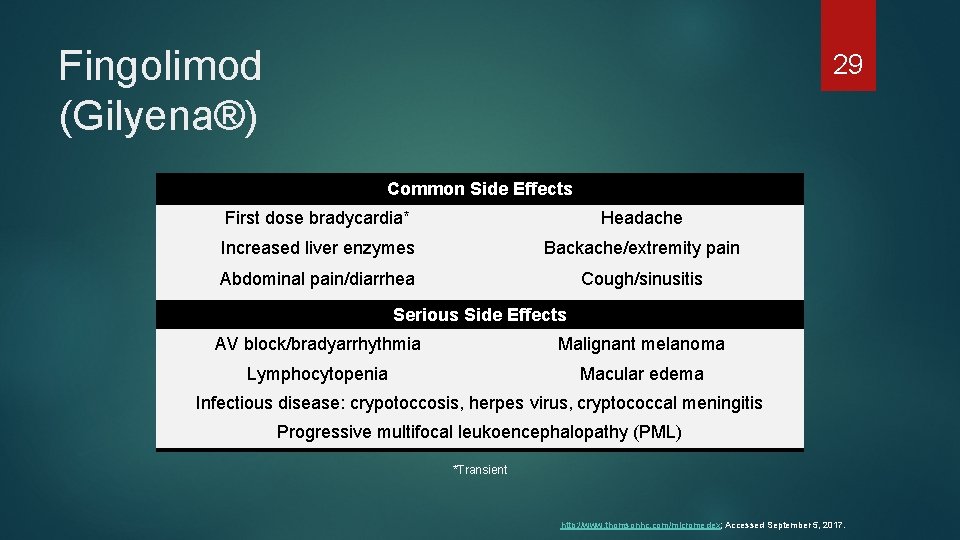
Fingolimod (Gilyena®) 29 Common Side Effects First dose bradycardia* Headache Increased liver enzymes Backache/extremity pain Abdominal pain/diarrhea Cough/sinusitis Serious Side Effects AV block/bradyarrhythmia Malignant melanoma Lymphocytopenia Macular edema Infectious disease: crypotoccosis, herpes virus, cryptococcal meningitis Progressive multifocal leukoencephalopathy (PML) *Transient http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

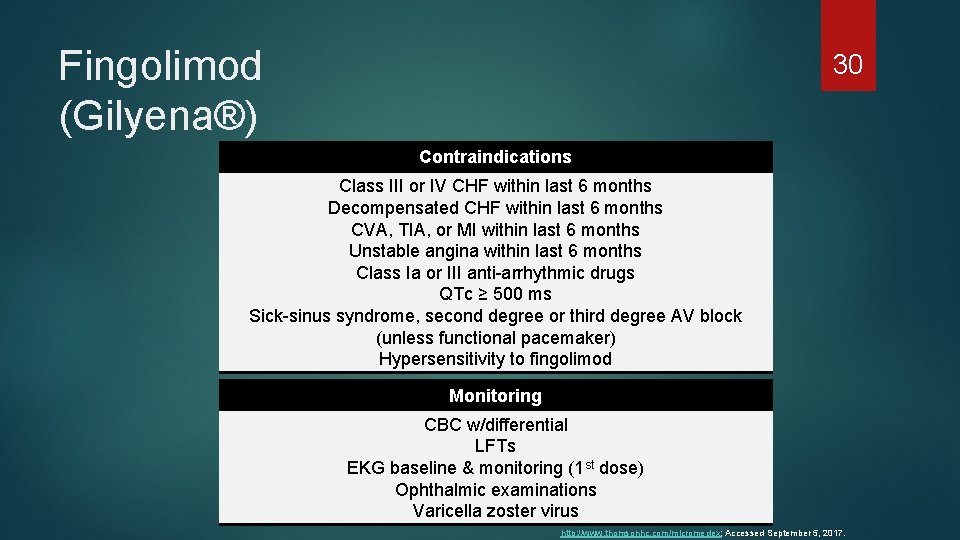
Fingolimod (Gilyena®) 30 Contraindications Class III or IV CHF within last 6 months Decompensated CHF within last 6 months CVA, TIA, or MI within last 6 months Unstable angina within last 6 months Class Ia or III anti-arrhythmic drugs QTc ≥ 500 ms Sick-sinus syndrome, second degree or third degree AV block (unless functional pacemaker) Hypersensitivity to fingolimod Monitoring CBC w/differential LFTs EKG baseline & monitoring (1 st dose) Ophthalmic examinations Varicella zoster virus http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Fingolimod (Gilyena®) 31 Pregnancy Avoid pregnancy Fetal malformations Elimination Up to 2 months after discontinuation http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

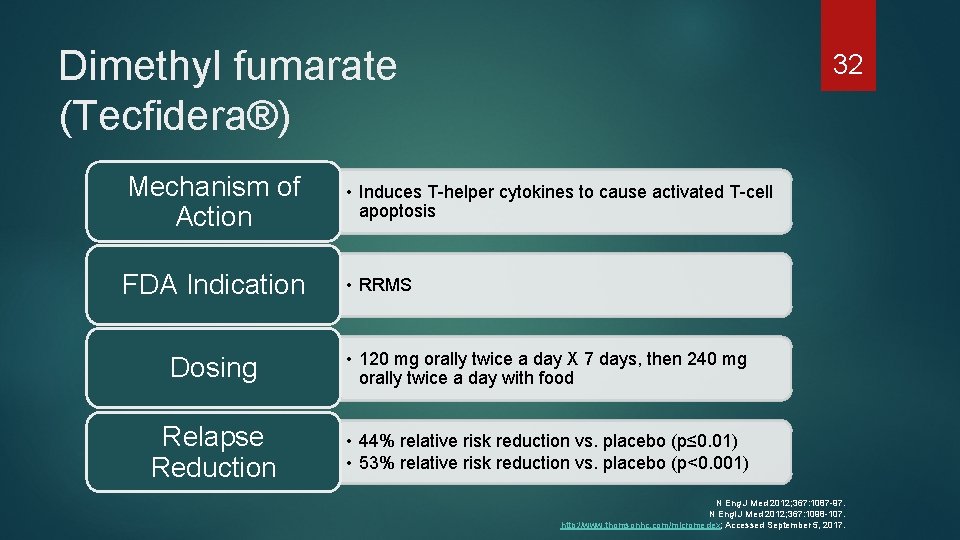
Dimethyl fumarate (Tecfidera®) 32 Mechanism of Action • Induces T-helper cytokines to cause activated T-cell apoptosis FDA Indication • RRMS Dosing • 120 mg orally twice a day X 7 days, then 240 mg orally twice a day with food Relapse Reduction • 44% relative risk reduction vs. placebo (p≤ 0. 01) • 53% relative risk reduction vs. placebo (p<0. 001) N Eng J Med 2012; 367: 1087 -97. N Engl J Med 2012; 367: 1098 -107. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

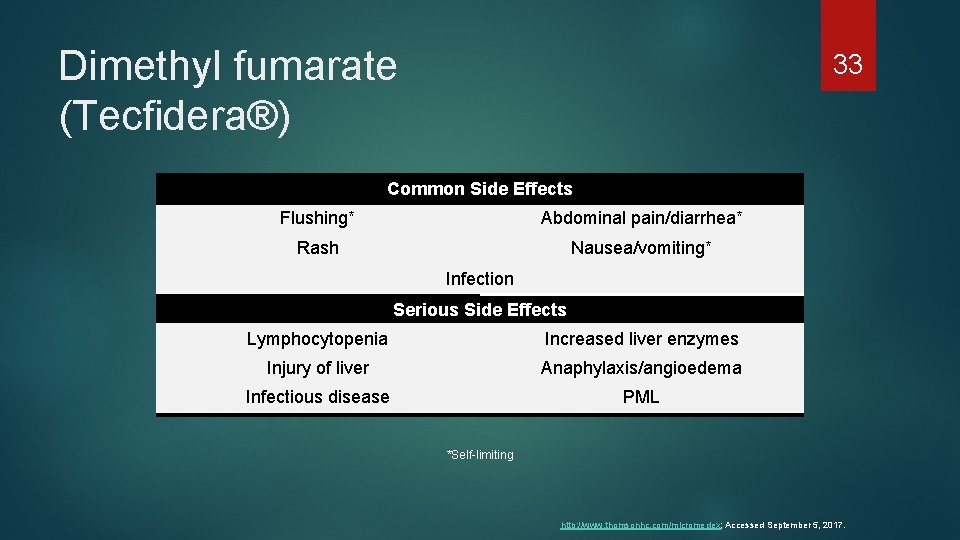
Dimethyl fumarate (Tecfidera®) 33 Common Side Effects Flushing* Abdominal pain/diarrhea* Rash Nausea/vomiting* Infection Serious Side Effects Lymphocytopenia Increased liver enzymes Injury of liver Anaphylaxis/angioedema Infectious disease PML *Self-limiting http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

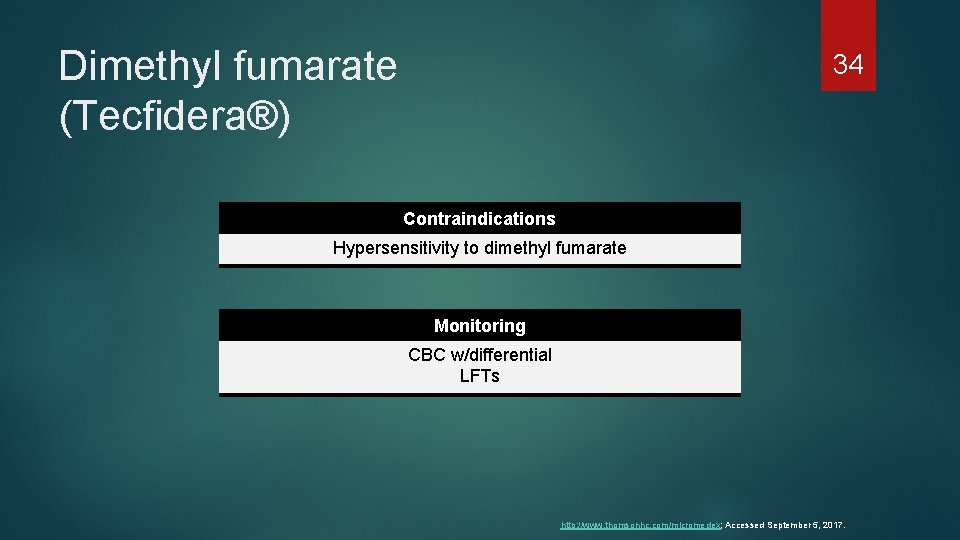
Dimethyl fumarate (Tecfidera®) 34 Contraindications Hypersensitivity to dimethyl fumarate Monitoring CBC w/differential LFTs http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

35 INFUSIBLE THERAPIES

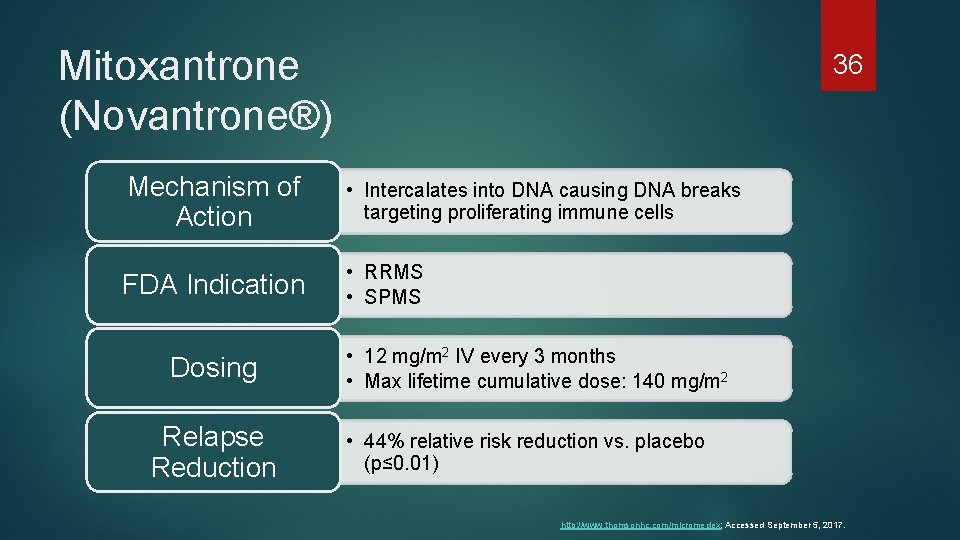
Mitoxantrone (Novantrone®) 36 Mechanism of Action • Intercalates into DNA causing DNA breaks targeting proliferating immune cells FDA Indication • RRMS • SPMS Dosing Relapse Reduction • 12 mg/m 2 IV every 3 months • Max lifetime cumulative dose: 140 mg/m 2 • 44% relative risk reduction vs. placebo (p≤ 0. 01) http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Mitoxantrone (Novantrone®) 37 Common Side Effects Alopecia Diarrhea/nausea/vomiting Menstrual disturbances Edema Cardiac disease/Cardiac arrhythmia Inflammatory disease of mucous membranes Neutropenia Leukopenia Anemia Thrombocytopenia Increased liver enzymes Headache UTI Infection http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

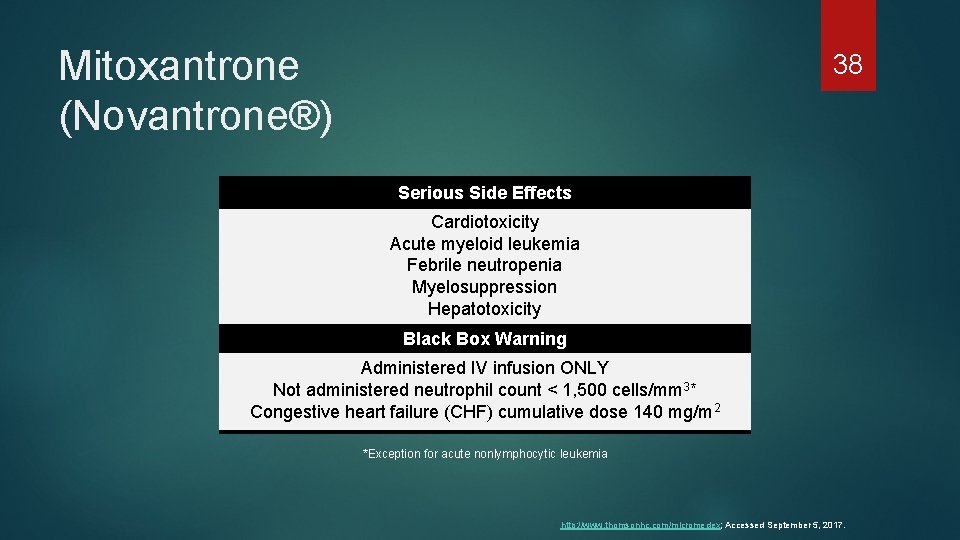
Mitoxantrone (Novantrone®) 38 Serious Side Effects Cardiotoxicity Acute myeloid leukemia Febrile neutropenia Myelosuppression Hepatotoxicity Black Box Warning Administered IV infusion ONLY Not administered neutrophil count < 1, 500 cells/mm 3* Congestive heart failure (CHF) cumulative dose 140 mg/m 2 *Exception for acute nonlymphocytic leukemia http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

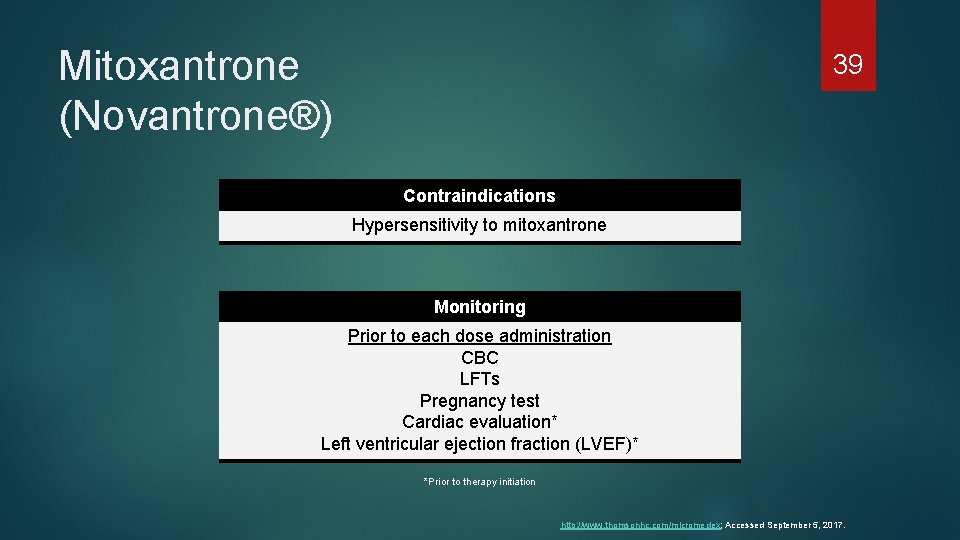
Mitoxantrone (Novantrone®) 39 Contraindications Hypersensitivity to mitoxantrone Monitoring Prior to each dose administration CBC LFTs Pregnancy test Cardiac evaluation* Left ventricular ejection fraction (LVEF)* *Prior to therapy initiation http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

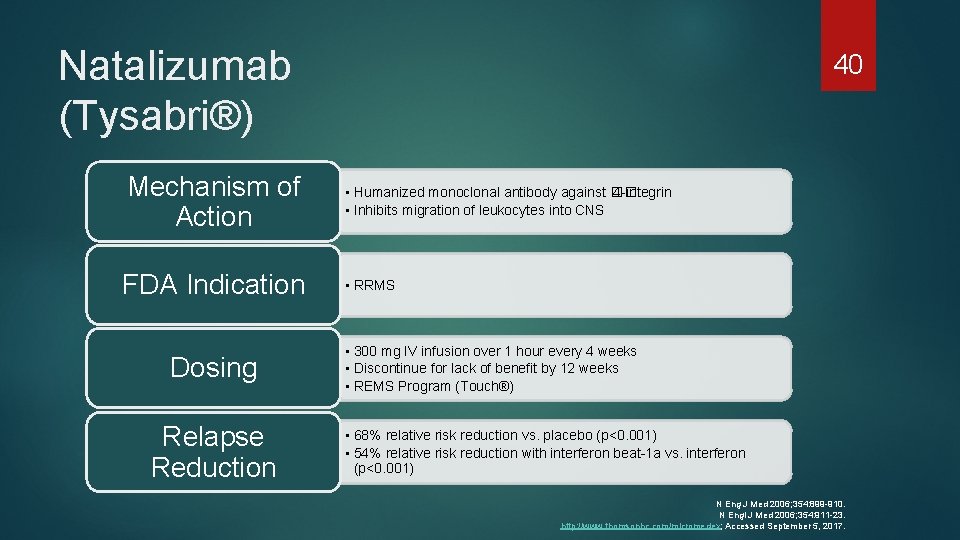
Natalizumab (Tysabri®) Mechanism of Action FDA Indication Dosing Relapse Reduction 40 • Humanized monoclonal antibody against �� 4 -integrin • Inhibits migration of leukocytes into CNS • RRMS • 300 mg IV infusion over 1 hour every 4 weeks • Discontinue for lack of benefit by 12 weeks • REMS Program (Touch®) • 68% relative risk reduction vs. placebo (p<0. 001) • 54% relative risk reduction with interferon beat-1 a vs. interferon (p<0. 001) N Eng J Med 2006; 354: 899 -910. N Engl J Med 2006; 354: 911 -23. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

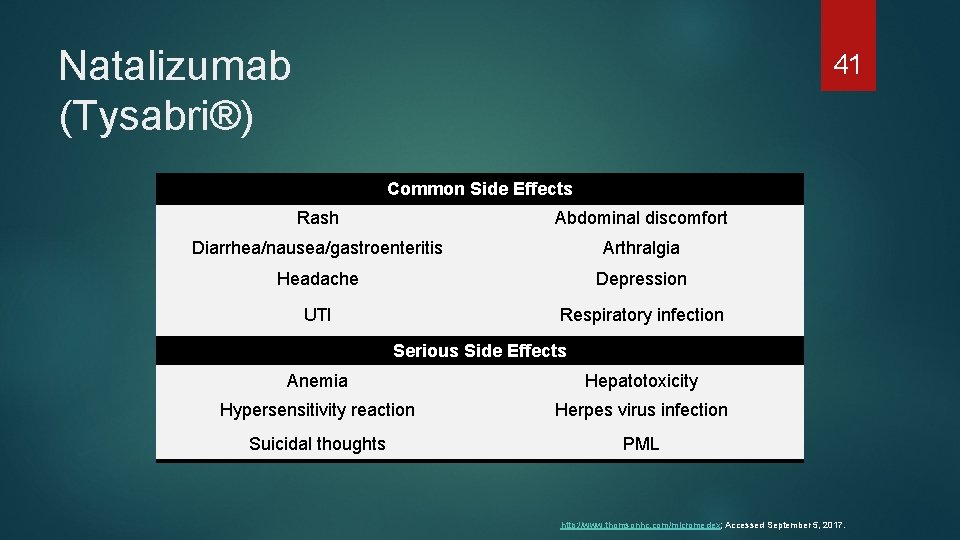
Natalizumab (Tysabri®) 41 Common Side Effects Rash Abdominal discomfort Diarrhea/nausea/gastroenteritis Arthralgia Headache Depression UTI Respiratory infection Serious Side Effects Anemia Hepatotoxicity Hypersensitivity reaction Herpes virus infection Suicidal thoughts PML http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

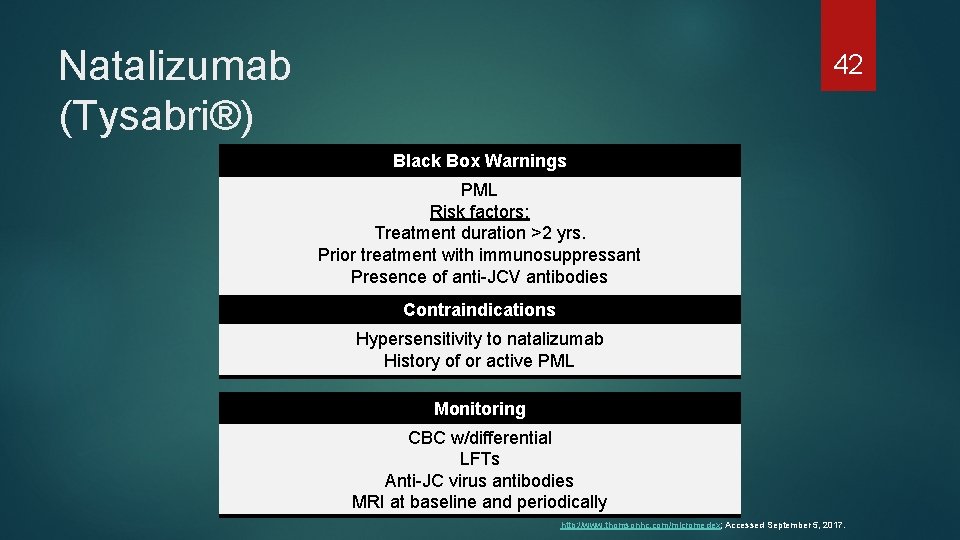
Natalizumab (Tysabri®) 42 Black Box Warnings PML Risk factors: Treatment duration >2 yrs. Prior treatment with immunosuppressant Presence of anti-JCV antibodies Contraindications Hypersensitivity to natalizumab History of or active PML Monitoring CBC w/differential LFTs Anti-JC virus antibodies MRI at baseline and periodically http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

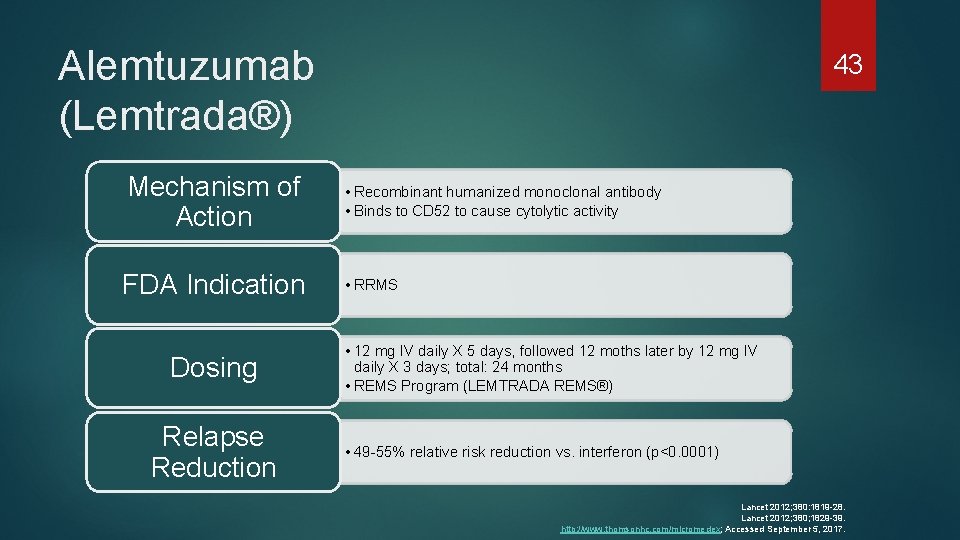
Alemtuzumab (Lemtrada®) Mechanism of Action FDA Indication Dosing Relapse Reduction 43 • Recombinant humanized monoclonal antibody • Binds to CD 52 to cause cytolytic activity • RRMS • 12 mg IV daily X 5 days, followed 12 moths later by 12 mg IV daily X 3 days; total: 24 months • REMS Program (LEMTRADA REMS®) • 49 -55% relative risk reduction vs. interferon (p<0. 0001) Lancet 2012; 380: 1819 -28. Lancet 2012; 380; 1829 -39. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

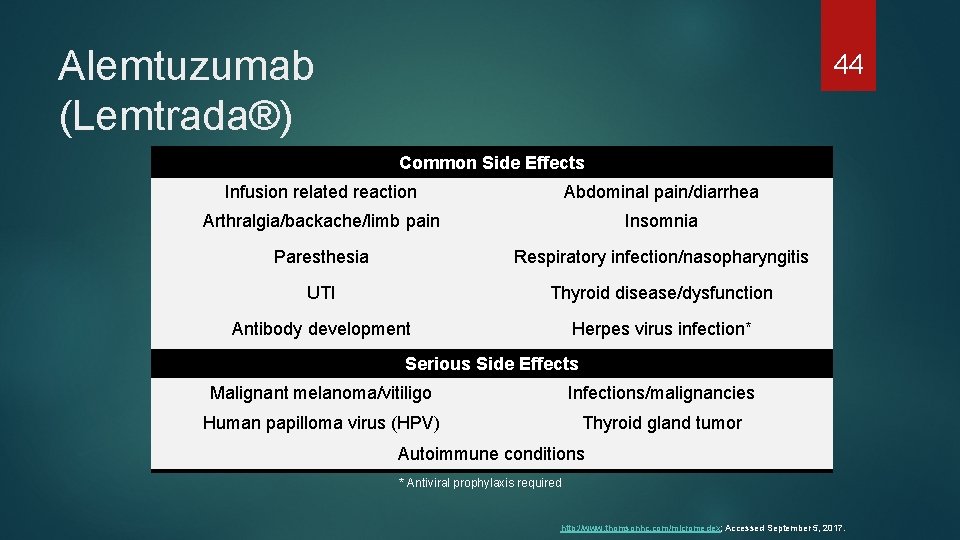
Alemtuzumab (Lemtrada®) 44 Common Side Effects Infusion related reaction Abdominal pain/diarrhea Arthralgia/backache/limb pain Insomnia Paresthesia Respiratory infection/nasopharyngitis UTI Thyroid disease/dysfunction Antibody development Herpes virus infection* Serious Side Effects Malignant melanoma/vitiligo Infections/malignancies Human papilloma virus (HPV) Thyroid gland tumor Autoimmune conditions * Antiviral prophylaxis required http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

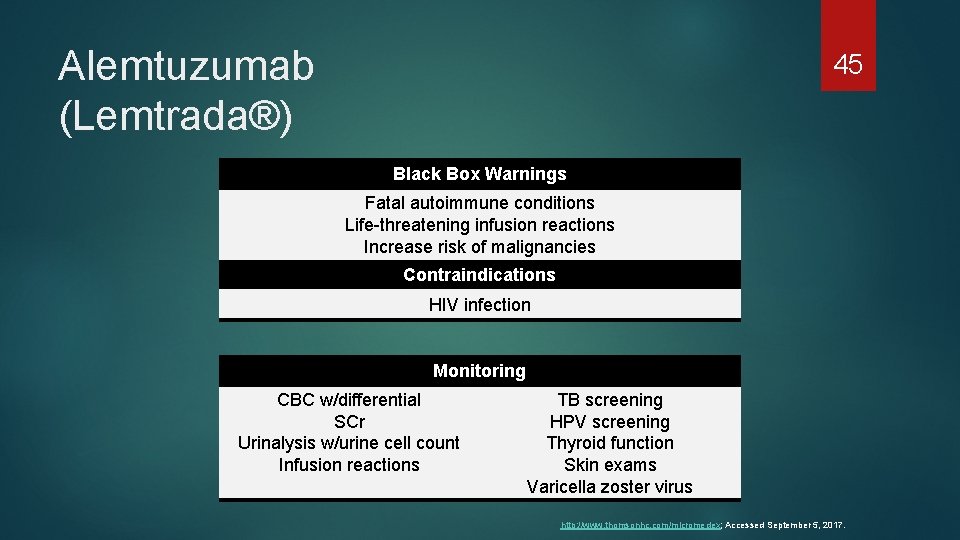
Alemtuzumab (Lemtrada®) 45 Black Box Warnings Fatal autoimmune conditions Life-threatening infusion reactions Increase risk of malignancies Contraindications HIV infection Monitoring CBC w/differential SCr Urinalysis w/urine cell count Infusion reactions TB screening HPV screening Thyroid function Skin exams Varicella zoster virus http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

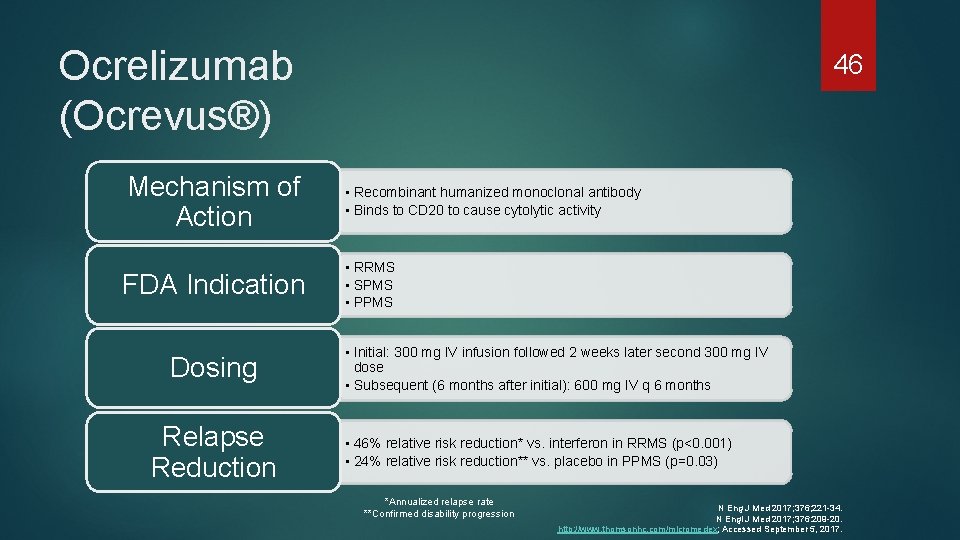
Ocrelizumab (Ocrevus®) Mechanism of Action FDA Indication Dosing Relapse Reduction 46 • Recombinant humanized monoclonal antibody • Binds to CD 20 to cause cytolytic activity • RRMS • SPMS • PPMS • Initial: 300 mg IV infusion followed 2 weeks later second 300 mg IV dose • Subsequent (6 months after initial): 600 mg IV q 6 months • 46% relative risk reduction* vs. interferon in RRMS (p<0. 001) • 24% relative risk reduction** vs. placebo in PPMS (p=0. 03) *Annualized relapse rate **Confirmed disability progression N Eng J Med 2017; 376; 221 -34. N Engl J Med 2017; 376: 209 -20. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Ocrelizumab (Ocrevus®) 47 Common Side Effects Infection of skin and/or subcutaneous tissue Respiratory infection Infusion reaction Serious Side Effects Herpes virus infection Malignant neoplasm of breast http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Ocrelizumab (Ocrevus®) 48 Contraindications Active hepatitis B infection History of life-threatening infusion reaction to ocrelizumab Monitoring Hep B screening prior to initiation Sign and symptoms of active infection Infusion reactions http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

49 OFF-LABEL USE

Rituximab (Rituxan®) Mechanism of Action Non-FDA Indication Dosing Efficacy 50 • Chimeric monoclonal antibody • Binds to CD 20 on B-cells to cause cytolytic activity • RRMS • PPMS • RRMS: 1, 000 mg IV infusion on days 1 and 15 for 24 wks. • PPMS: 1, 000 mg IV infusion on days 1 and 15 every 24 wks. • 43% relative risk reduction* vs. placebo in RRMS (p=0. 08) • No significant difference in disease progression with rituximab vs. placebo in PPMS (30. 2% vs. 38. 5%; p=0. 1442) *Annualized relapse rate at 48 weeks N Eng J Med 2008: 358: 676 -88. Ann Neurol 2009; 460 -71. http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Rituximab (Rituxan®) 51 Common Side Effects Infusion related reaction Peripheral edema/weight gain Diarrhea/nausea Blood dyscrasias Antibody development UTI Respiratory tract infection Serious Side Effects CV complication/ Hypotension Stevens-Johns syndrome/ Toxic epidermal necrolysis Elevated liver enzymes PML Nephrotoxicity Reactivation of hepatitis B Angioedema Infectious disease http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

Rituximab (Rituxan®) 52 Black Box Warnings Fatal infusion reactions Severe mucocutaneous reactions Hepatic B virus reactivation PML Monitoring Hep B screening prior to initiation CBC prior to each course Renal function Cardiac monitoring Infusion reactions http: //www. thomsonhc. com/micromedex; Accessed September 5, 2017.

53 EFFICACY & SAFETY

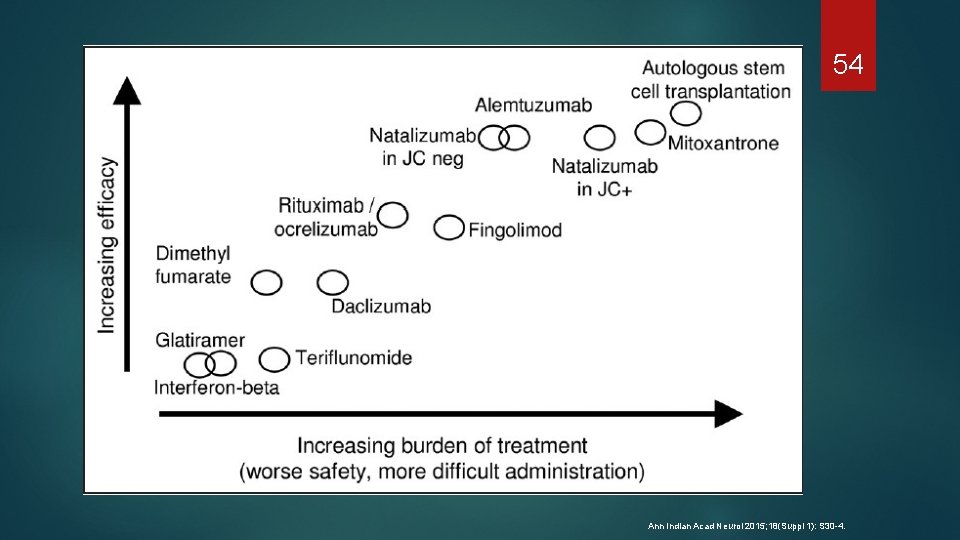
54 Ann Indian Acad Neurol 2015; 18(Suppl 1): S 30 -4.

55 PIPELINE THERAPY

In The Pipeline Siponimod Selective S 1 P receptor modulator than fingolimod SPMS Ozanimod & Ponesimod Selective S 1 P receptor modulator than fingolimod RRMS Minocycline CIS 56

In The Pipeline Laquinimod Oral therapy Monoclonal antibody �� 4 -integrin Ofatumumab Fully humanized antibody targeting CD 20 May reduce infusion reaction and possible antibody formation 57

58 QUESTIONS?

CE Question #1 True/False: Ocrelizumab (Ocrevus®) is the only Food and Drug Administration (FDA) approved treatment for primary progressive multiple sclerosis (MS) 59

CE Question #2 Which of the following oral medications for the treatment of MS should be monitored with first dose administration? A. Fingolimod (Gilyena®) B. Dimethyl fumarate (Tecfidera®) C. Alemtuzumab (Lemtrada®) D. Teriflunomide (Aubagio®) 60

CE Question #3 Which of the following are associated risk factors of natalizumabassociated progressive multifocal leukoencephalopathy (PML)? Select all that are correct. A. Duration of natalizumab treatment B. Prior immunosuppressant use C. Anti-JC virus antibody status D. All the above 61

CE Question #4 ZM is a 45 y/o M with relapse remitting MS X 7 years. Pt’s PMH significant for HTN, HLD, and 1 st degree AV block. Pt is to be discharged on dimethyl fumarate 120 mg twice a day for 7 days, then increase to 240 mg twice a day. What monitoring parameters should be scheduled for ZM prior to discharge? A. CBC w/differential at baseline, within 6 months of initiation and then every 6 -12 months thereafter B. Basic metabolic panel (BMP) at 3 months C. Yearly eye examination D. Cardiovascular evaluation 62