Rutgers Pharmaceutical Industry Fellowship Program Ernest Mario School






















- Slides: 32

Rutgers Pharmaceutical Industry Fellowship Program Ernest Mario School of Pharmacy Rutgers, The State University of New Jersey

Agenda and Objectives Provide an overview of the Rutgers Pharmaceutical Industry Fellowship (RPIF) program Review the fellowship application process Explore the opportunities for Pharm. Ds in the pharmaceutical industry Resources and Additional Information Q&A session

What is an Industry Fellowship? Header “Arial Black”

Fellowship vs. Residency Attribute Fellowship Residency Impact on patient care Global scale Individual cases Use of clinical knowledge Varies depending on functional area Direct patient care General structure 1 -2 year experience in core function ± rotations 1 year general practice ± 1 year specialty Practice setting Corporate Inpatient/Outpatient Scholarly activities Teaching Research Publications Salary Competitive Stipend Competitive stipend

Rutgers Pharmaceutical Industry Fellowship (RPIF) Program Joseph A. Barone, Pharm. D, FCCP Dean and Professor II Ernest Mario School of Pharmacy Michael Toscani, Pharm. D Research Professor, Past Fellowship Director Institute for Pharmaceutical Industry Fellowships Lesley Fierro, Pharm. D Fellowship Director Institute for Pharmaceutical Industry Fellowships

RPIF Program Mission Opportunity to accelerate your career path in the industry through a variety of specialty training programs Established history with a large network of leading companies and distinguished alumni Investment in the development of the fellows and supports the advancement of pharmacists in the industry Unique professional and leadership opportunities offered through both Rutgers and partner companies

RPIF Program Outcomes Established in This year, there are 1984 >200 fellows across 18 partner companies with 2 fellows Over 885 alumni leaders found in a wide range of partner companies worldwide • Pharmaceutical and Biopharmaceutical industries • Biotechnology companies • Medical Communications companies • Government agencies (i. e. FDA, CDC) • Healthcare Insurers and Payers • Advertising agencies • RPIF program has greatly expanded pharmacists’ role in RPIF program has greatly the industry expanded pharmacists’ role in the industry

Header “Arial Black” RPIF Program Curriculum Scholarly Activities Teaching

Fellowship Components Header “Arial Black” Industry Component • Hands on experience in specialty function and/or disease area • Guidance from preceptors, mentors, and alumni • Professional development through conferences, workshops, and more • Internal and External rotation opportunities Header “Arial Black” Rutgers Component • Professional Development Series • Teaching opportunities at Ernest Mario School of Pharmacy • Research collaboration with Rutgers faculty for publications/posters • Leadership opportunities as committee chairs and leads

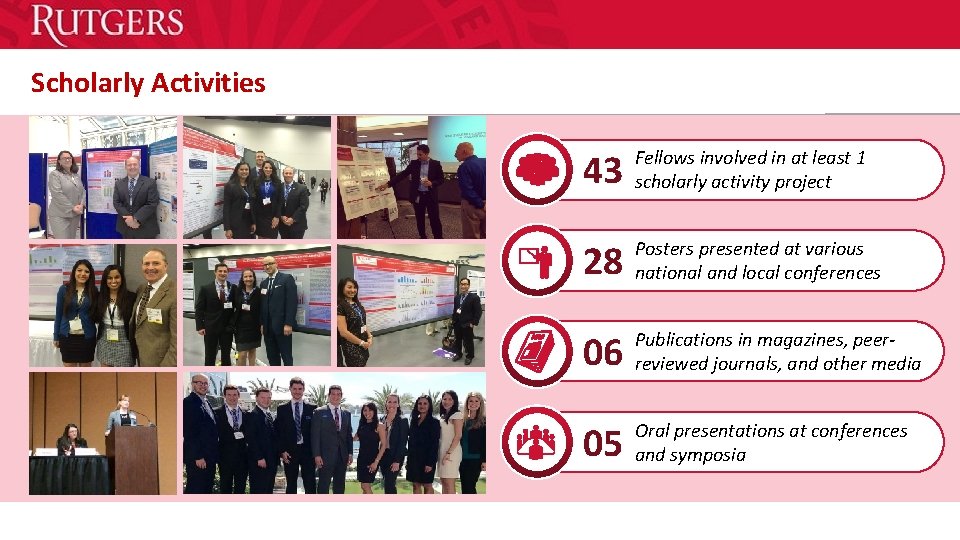
Scholarly Activities 43 Fellows involved in at least 1 Header “Arial Black” scholarly activity project 28 Posters presented at various Header “Arial Black” national and local conferences 06 Publications in magazines, peer. Header “Arial Black” reviewed journals, and other media 05 Oral presentations at conferences Header “Arial Black” and symposia

Partner Companies for 2021 -2022

Preparation Header “Arial Black”

Candidate Eligibility Obtain a Doctor of Pharmacy (Pharm. D) and/or Doctorate of Philosophy (Ph. D) degree by July 1, 2021 from an ACPE accredited pharmacy program Attend formal virtual interview process at the ASHP Midyear Clinical Meeting and sign up for Personal Placement Services (PPS)

Requirements for Interviewing ✔ RPIF Application portal opens September 1 st – Access from RPIF website – http: //pharmafellows. rutgers. edu – Submit all required materials ASAP – CV, letter of intent, 3 letters of recommendation ✔ Interview requests will be open from October 23 rd to November 6 th ✔ Candidates must submit their CV/LOI by October 23 rd in order to be offered a 1 st round interview – LOR deadline Dec 1 st

Part 1: Virtual Live Information Session Registration opens Tuesday, September 1 st for FIND Part 1 Explore opportunities for Pharm. Ds in pharmaceutical industry Rutgers Pharmaceutical Industry Fellowship FIND INFORMATIONAL DAY RPIF Program Overview Fellowship application process Midyear Preparation Thursday, September 24 th from 6 PM - 9 PM EST Q&A session

Part 2: Fellowship Information & Networking Sessions “Helping you find your pathway to the industry!” When: October 8 th-16 th Location: Virtual Description: • Educational overview of different functional areas within industry • Q & A Panel session with fellows in different areas • Networking sessions - spanning the course of one week with partner companies, fellows and preceptors from various positions – great way to learn more about each position, meet some of the people you might be working with to get a better idea of which positions you want to apply to

Interview Preparation Tips Register for Midyear Clinical meeting and PPS early Research positions offered in advance Dress professionally (business professional attire) Send thank you emails! Arrive on time for virtual interviews Relax and be yourself! Make sure to interview in a quiet, well-lit room with appropriate camera angle Attend outreaches and webinars! Have a strong Internet connection and check that mic and camera work

Opportunities for Pharm. Ds in the Header “Arial Black” Pharmaceutical and Biopharmaceutical Industry

OPTION 1 a Opportunities for Pharm. Ds in the Industry Research & Development Medical Affairs Commercial • Clinical Pharmacology • Medical Strategy • Marketing • Pre-Clinical • Medical Information • Market Research • Early Phase Development • MSL • Advocacy & Policy • Late Phase Development • Publications • Market Access • Medical Education • Business Development • Clinical Operations • HEOR Regulatory Affairs • Advertising & Promotions • Regulatory Strategy • Drug Safety • Risk Management

Cross Functional Interactions Business Analytics Commercial Research and Development Medical Affairs Clinical R & D Marketing Manufacturing Market Research Market Access Regulatory Sciences

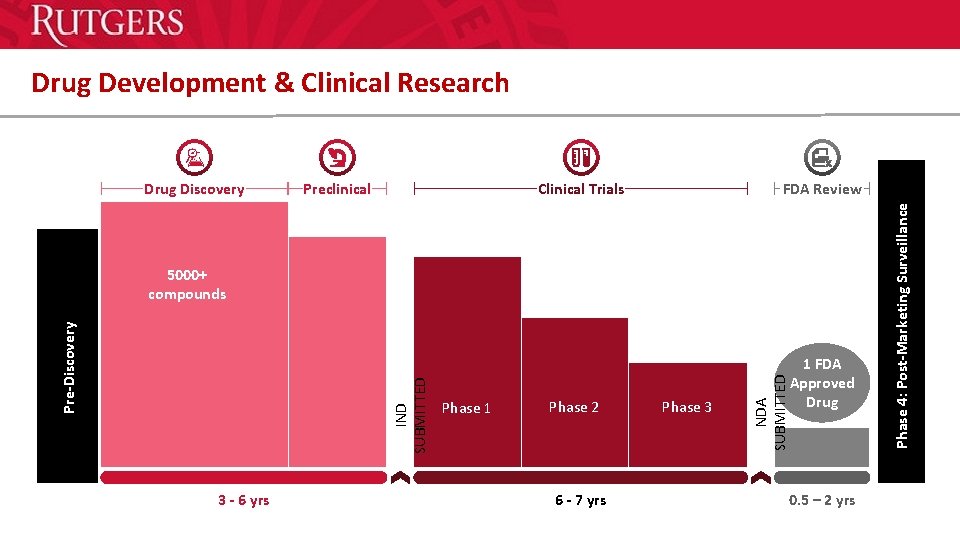
Drug Development & Clinical Research Preclinical Clinical Trials FDA Review 3 - 6 yrs Phase 1 Phase 2 6 - 7 yrs Phase 3 1 FDA Approved Drug NDA SUBMITTED IND SUBMITTED Pre-Discovery 5000+ compounds 0. 5 – 2 yrs Phase 4: Post-Marketing Surveillance Drug Discovery

Medical Affairs & Strategy Liaison Medical Education Medical Brand Strategy Medical Comm. / Pubs 1. 2. http: //www. ziphermed. com/ Understanding Pharma Series Course on Medical Affairs. Medical Information

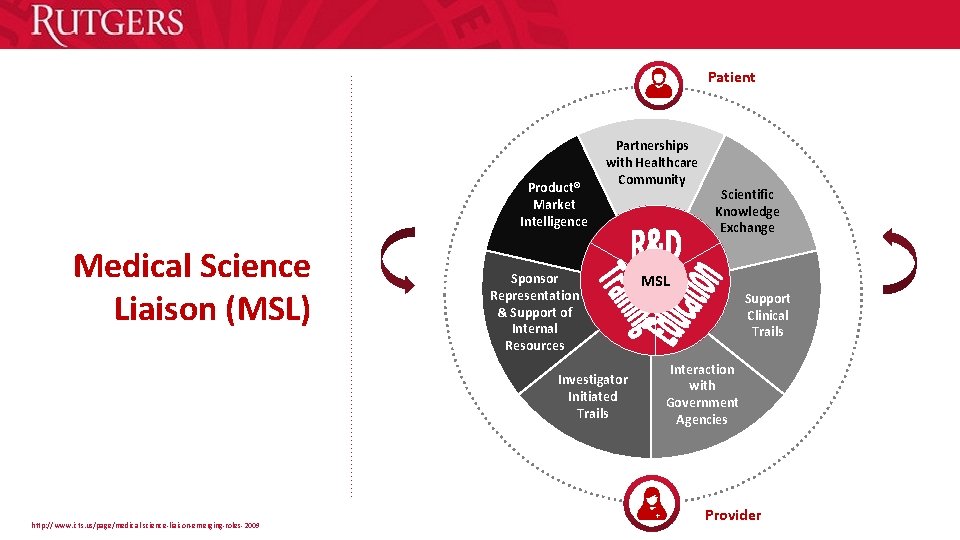
Patient Product® Market Intelligence Medical Science Liaison (MSL) Partnerships with Healthcare Community Sponsor Representation & Support of Internal Resources Investigator Initiated Trails http: //www. icts. us/page/medical-science-liaison-emerging-roles-2009 Scientific Knowledge Exchange MSL Support Clinical Trails Interaction with Government Agencies Provider

Health Economics and Outcomes Research (HEOR) http: //www. aoic. net/focus/heor. html

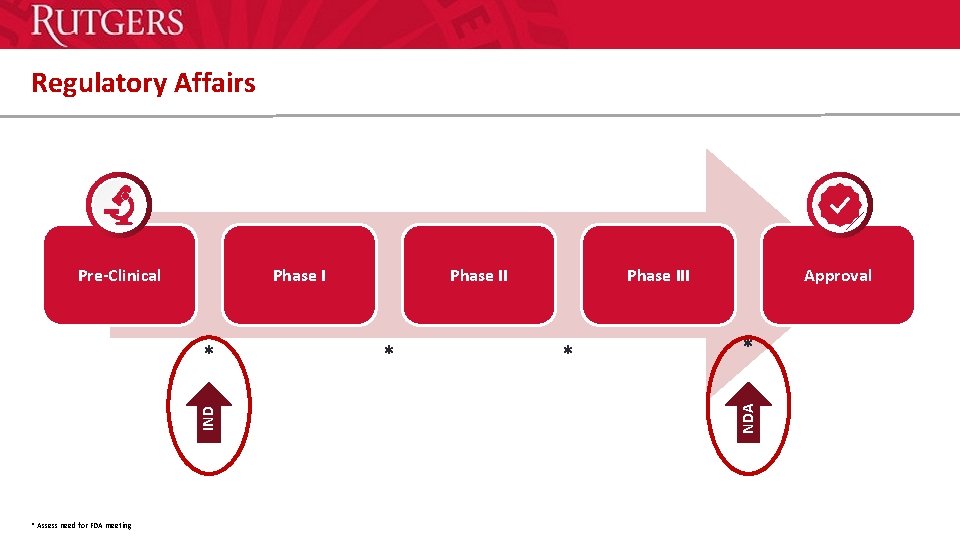
Regulatory Affairs Phase I IND * * Assess need for FDA meeting Phase II * Phase III * Approval * NDA Pre-Clinical

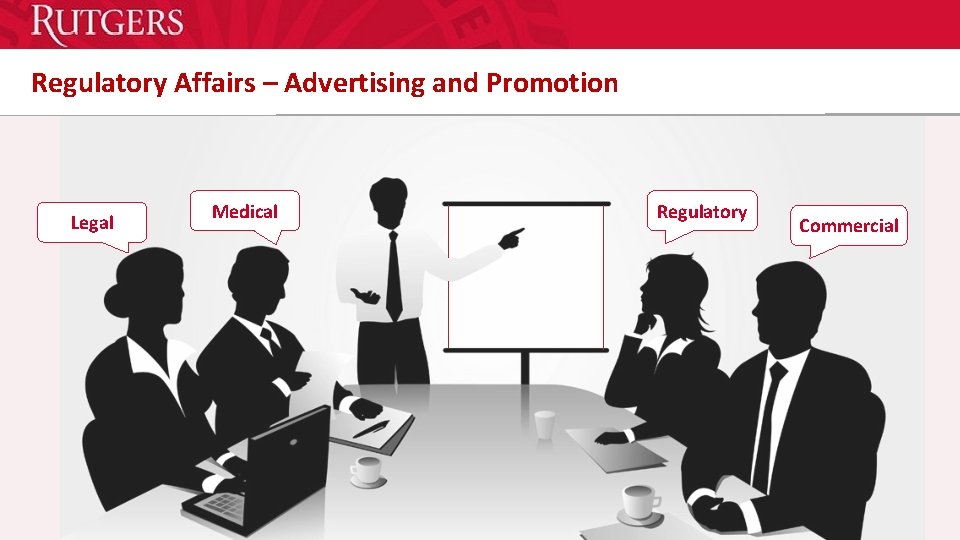
Regulatory Affairs – Advertising and Promotion Legal Medical Regulatory Commercial

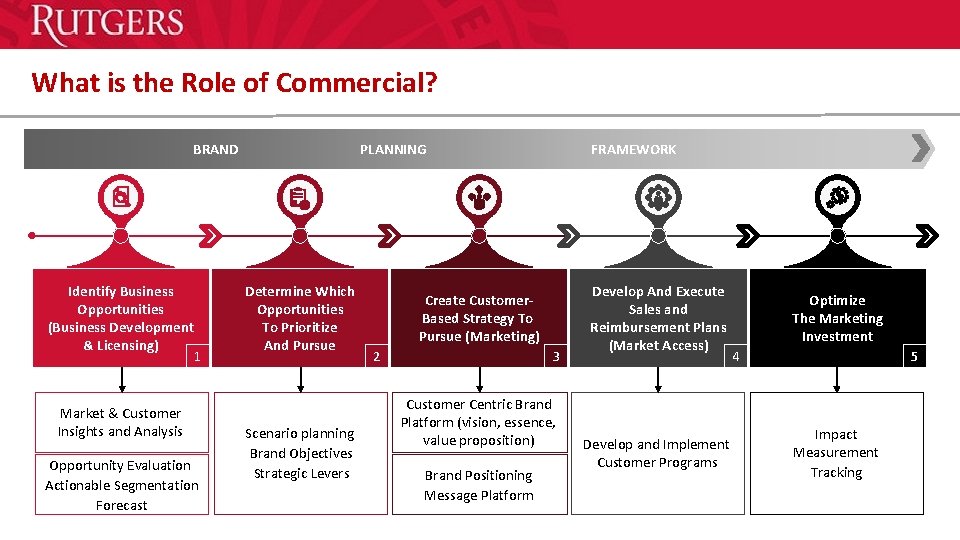
What is the Role of Commercial? BRAND Identify Business Opportunities (Business Development & Licensing) 1 Market & Customer Insights and Analysis Opportunity Evaluation Actionable Segmentation Forecast PLANNING Determine Which Opportunities To Prioritize And Pursue Scenario planning Brand Objectives Strategic Levers FRAMEWORK Create Customer- Based Strategy To Pursue (Marketing) 2 3 Customer Centric Brand Platform (vision, essence, value proposition) Brand Positioning Message Platform Develop And Execute Sales and Reimbursement Plans (Market Access) Optimize The Marketing Investment 4 Develop and Implement Customer Programs 5 Impact Measurement Tracking

Summary RPIF program offers fellowships to help jump-start your career in industry by offering the following: Challenging and supportive environment Networking and mentorship opportunities Opportunities for research, publications, teaching, and leadership Wide range of avenues and career paths for Pharm. Ds in the pharmaceutical and biopharmaceutical industry


Resources and Helpful Links • Visit our website – http: //Pharma. Fellows. rutgers. edu • Join our Facebook group – http: //Facebook. com/Rutgers. Fellowship • Join our “Rutgers Fellowship Student Interest Group” Linked. In Page – http: //Tinyurl. com/4 xn 2 kbh

OPTION 1 @Rutgers. Fellow | #RPIF | Rutgers Fellow 31

SURVEY TIME https: //rutgers. ca 1. qualtrics. com/jfe/form/SV_9 GODPpi. Ezh. UGdkp