Rapid HIV Testing and Its Role in Advancing





















- Slides: 47

Rapid HIV Testing and Its Role in Advancing HIV Prevention: 2004 Update Bernard M. Branson, M. D. Chief, Lab Determinants and Diagnostics Section Centers for Disease Control and Prevention

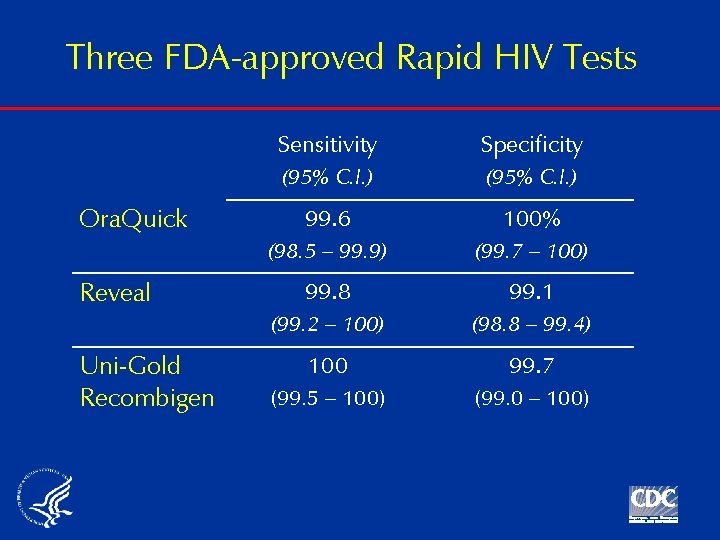
Three FDA-approved Rapid HIV Tests Ora. Quick Reveal Uni-Gold Recombigen Sensitivity Specificity (95% C. I. ) 99. 6 100% (98. 5 – 99. 9) (99. 7 – 100) 99. 8 99. 1 (99. 2 – 100) (98. 8 – 99. 4) 100 99. 7 (99. 5 – 100) (99. 0 – 100)

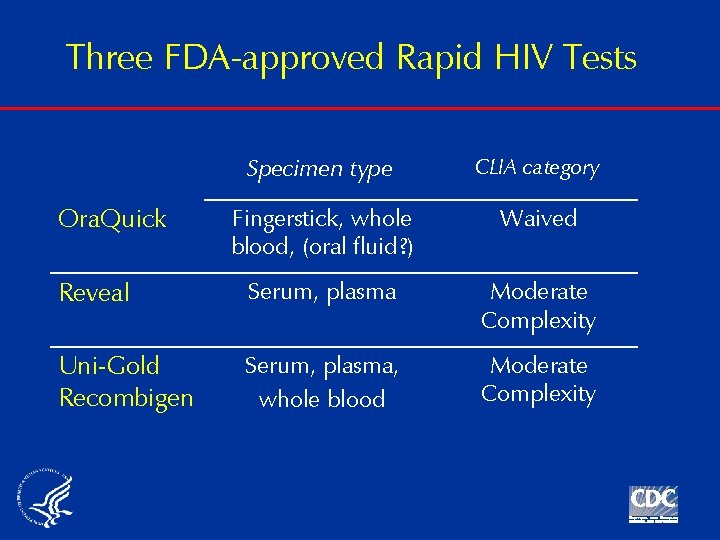
Three FDA-approved Rapid HIV Tests Specimen type CLIA category Fingerstick, whole blood, (oral fluid? ) Waived Reveal Serum, plasma Moderate Complexity Uni-Gold Recombigen Serum, plasma, whole blood Moderate Complexity Ora. Quick

Ora. Quick: Fingerstick, whole blood

Obtain finger stick specimen…

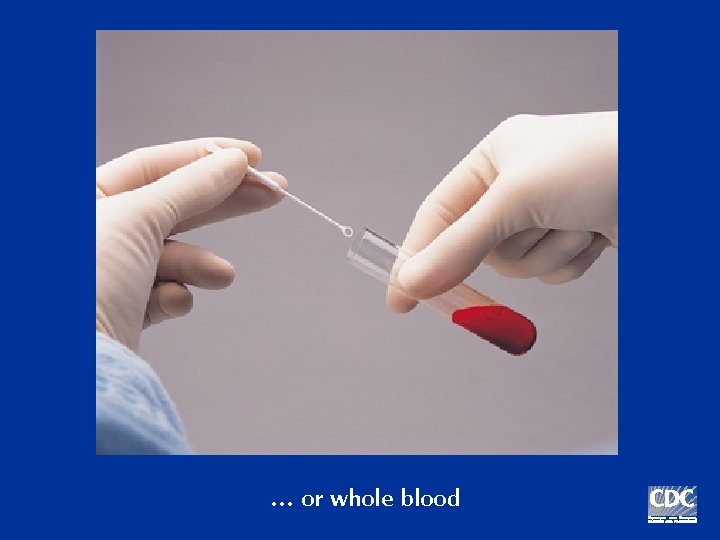
… or whole blood

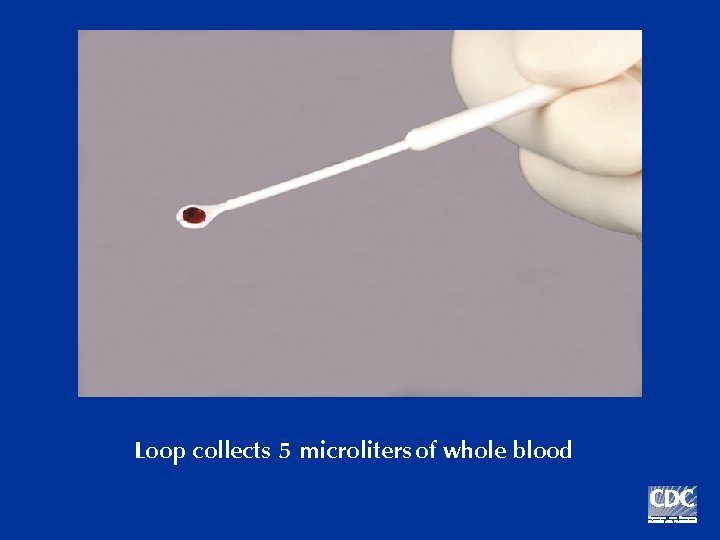
Loop collects 5 microliters of whole blood

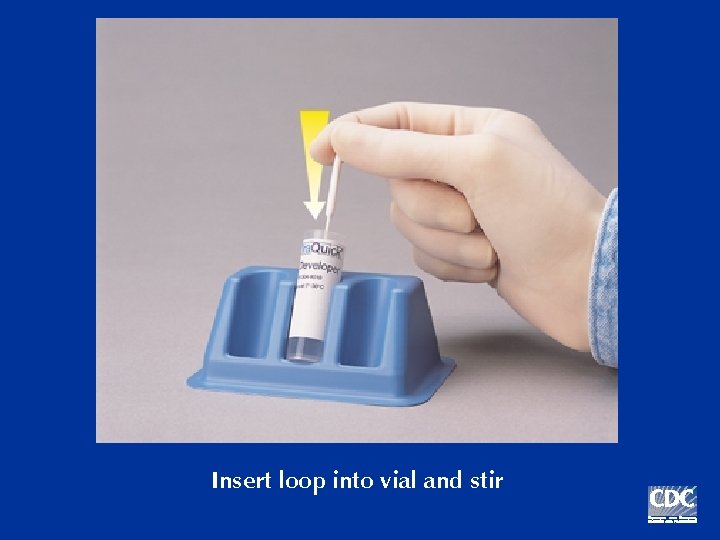
Insert loop into vial and stir

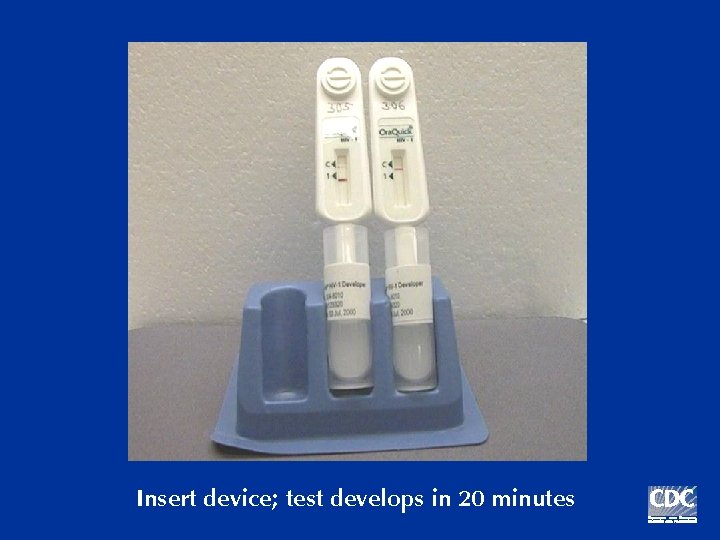
Insert device; test develops in 20 minutes

Reactive Control Positive HIV-1 C C T T Positive Negative Read results in 20 – 40 minutes

Requirements for Ora. Quick Testing n Sold only to “clinical laboratories” n To perform CLIA-waived tests, entities must: 1) Enroll in CLIA program 2) Obtain a Certificate of Waiver 3) Pay a biennial fee 4) Follow manufacturers’ instructions 5) Meet state requirements

Requirements for Ora. Quick Testing n Have an adequate quality assurance program n Assurance that operators will receive and use instructional materials n QA guidelines for Ora. Quick testing and sample forms: www. cdc. gov/hiv/rapid_testing

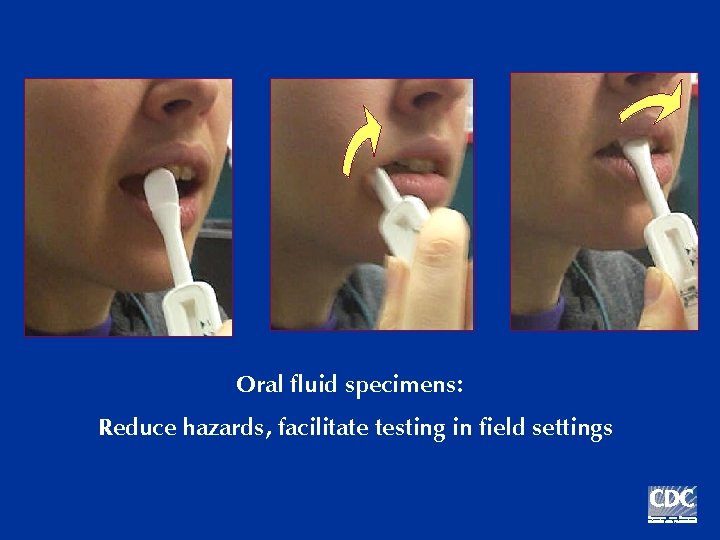
Oral fluid specimens: Reduce hazards, facilitate testing in field settings

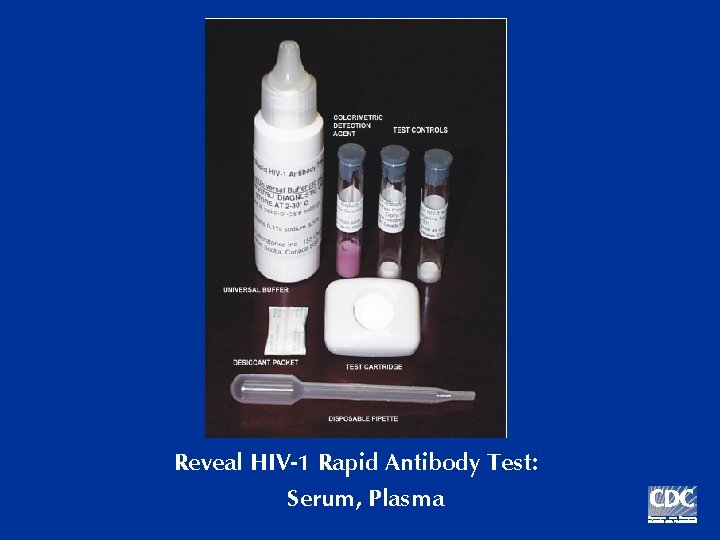
Reveal HIV-1 Rapid Antibody Test: Serum, Plasma

Centrifuge to obtain serum or plasma

Add 20 drops of buffer to reconstitute conjugate. (Refrigerate to store)

Add 3 drops buffer to moisten membrane

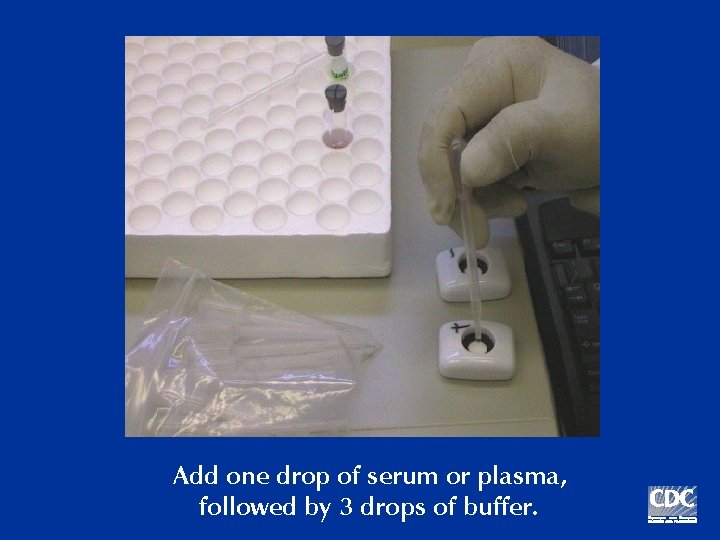
Add one drop of serum or plasma, followed by 3 drops of buffer.

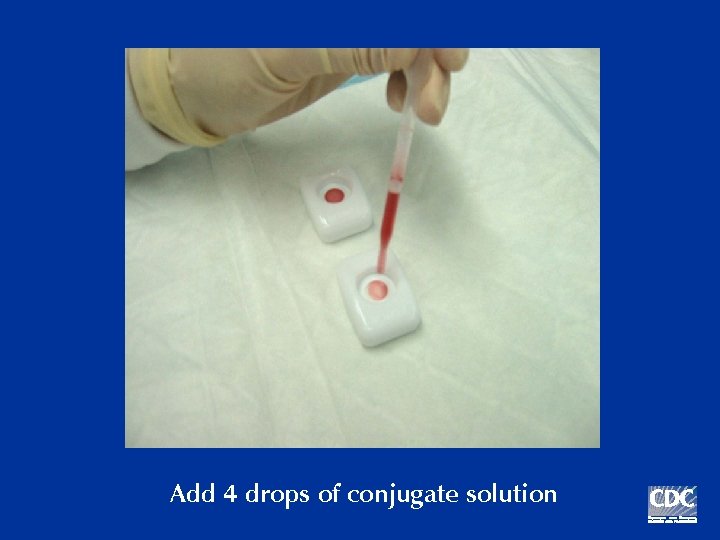
Add 4 drops of conjugate solution

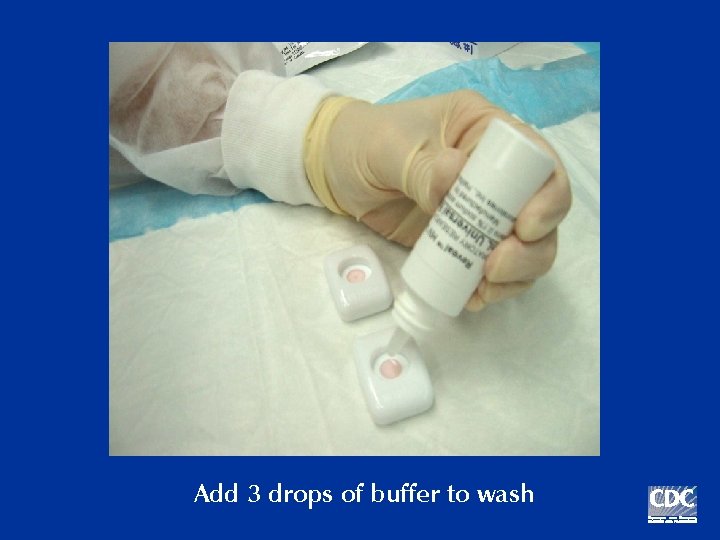
Add 3 drops of buffer to wash

Positive Negative Read results immediately

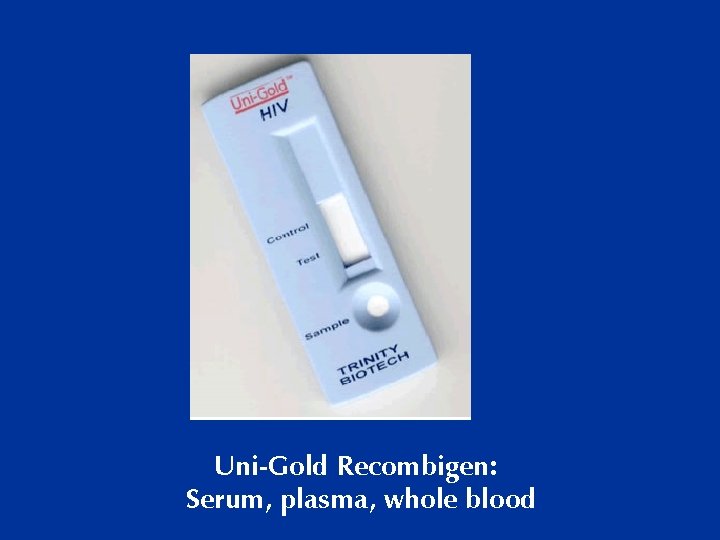
Uni-Gold Recombigen: Serum, plasma, whole blood

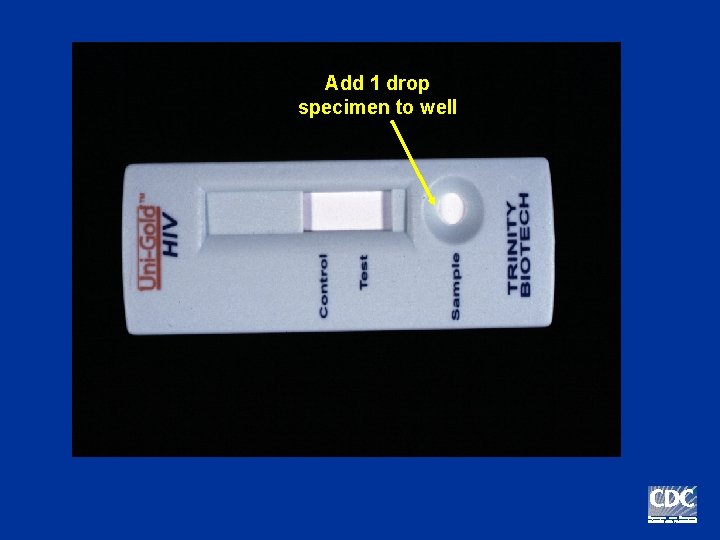
Add 1 drop specimen to well

Add 4 drops of wash solution

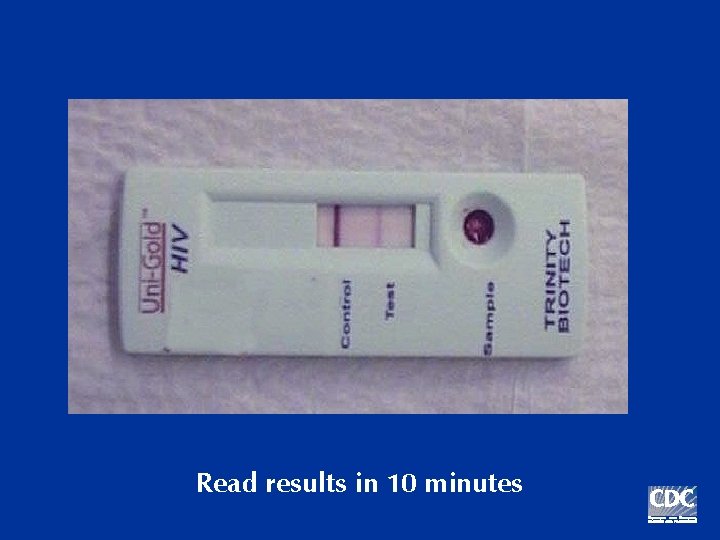
Read results in 10 minutes

Point-of-Care Testing n To expand testing in non-clinical settings: – Fingerstick or oral fluid specimen – One-step – Easy to interpret – Internal control

The Need for Training n n n Blood & body fluid precautions Obtaining the specimen (finger stick or blood draw) Performing the test Providing test results and counseling Quality assurance OSHA requirements

Remember the tradeoffs… n Good News: More HIV-positive people receive their test results. n Bad News: Some people will receive a falsepositive result before confirmatory testing.

Reports from the 2003 HIV Prevention Conference n Promising news with rapid HIV tests for – – Routine screening in medical settings – Increasing receipt of results at CT sites – Screening in labor and delivery – Outreach testing

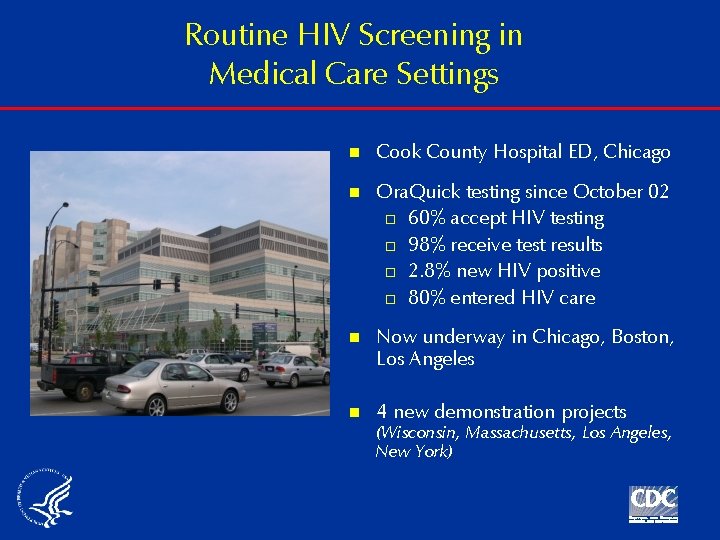
Routine HIV Screening in Medical Care Settings n Cook County Hospital ED, Chicago n Ora. Quick testing since October 02 o 60% accept HIV testing o 98% receive test results o 2. 8% new HIV positive o 80% entered HIV care n Now underway in Chicago, Boston, Los Angeles n 4 new demonstration projects (Wisconsin, Massachusetts, Los Angeles, New York)

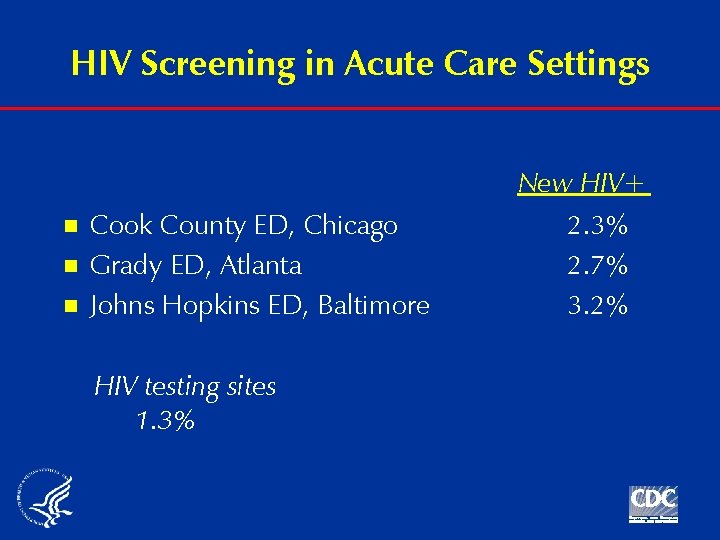
HIV Screening in Acute Care Settings n n n Cook County ED, Chicago Grady ED, Atlanta Johns Hopkins ED, Baltimore HIV testing sites 1. 3% New HIV+ 2. 3% 2. 7% 3. 2%

HIV Screening with Ora. Quick in Labor and Delivery: the MIRIAD Study n Testing of pregnant women in labor for whom no HIV test results are available; 12 hospitals in 5 cities: Atlanta, Chicago, Miami, New Orleans, New York n To date o 4597 women screened o 34 new HIV infections identified o 2 false positive Ora. Quick tests, no false negatives o 8 false-positive EIAs

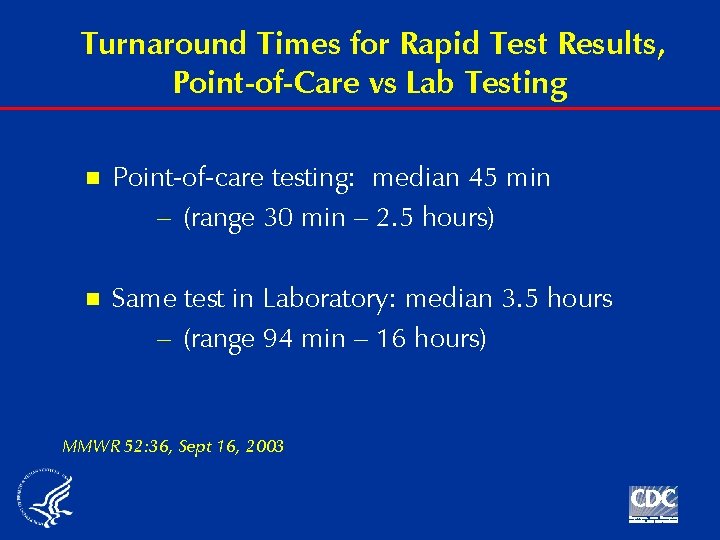
Turnaround Times for Rapid Test Results, Point-of-Care vs Lab Testing n Point-of-care testing: median 45 min – (range 30 min – 2. 5 hours) n Same test in Laboratory: median 3. 5 hours – (range 94 min – 16 hours) MMWR 52: 36, Sept 16, 2003

Ora. Quick Outreach to High-risk Persons of Color n n n On-site testing at sites throughout the community Group pretest counseling. Individual testing and post-test counseling. Patrick Keenan MD University of Minnesota Medical School Department of Family Practice and Community Health

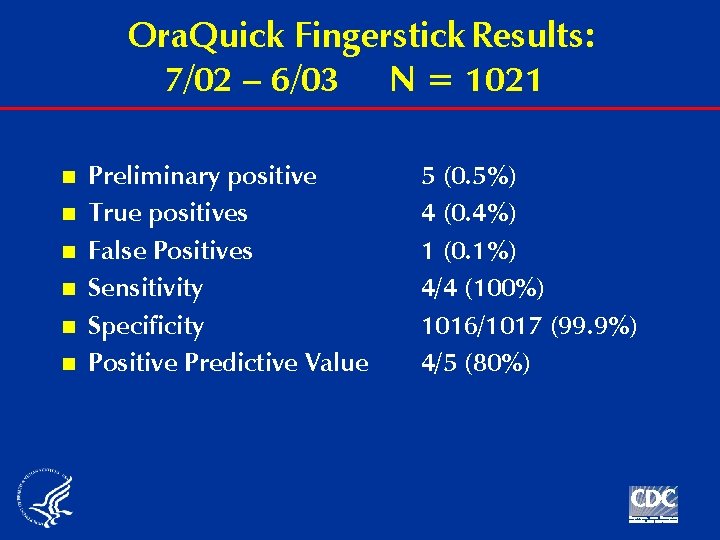
Ora. Quick Fingerstick Results: 7/02 – 6/03 N = 1021 n n n Preliminary positive True positives False Positives Sensitivity Specificity Positive Predictive Value 5 (0. 5%) 4 (0. 4%) 1 (0. 1%) 4/4 (100%) 1016/1017 (99. 9%) 4/5 (80%)

Results n 99. 7% of clients received their test results and post-test counseling. n The average time between fingerstick and learning test result was 28 minutes.

Client Survey Results n “I would rather have my finger stuck than have blood drawn from my vein” Agree or strongly agree = 95% Disagree or strongly disagree = 5%

Post-Marketing Surveillance n 14 states in 2003, expansion in 2004 as more project areas implement rapid testing n (Note: Supplement to Program Announcement) n Monitoring: Ø Changes in utilization of testing Ø Acceptance (choice of tests) Ø Client and counselor satisfaction Ø Follow-up on false-positives Ø Adverse events

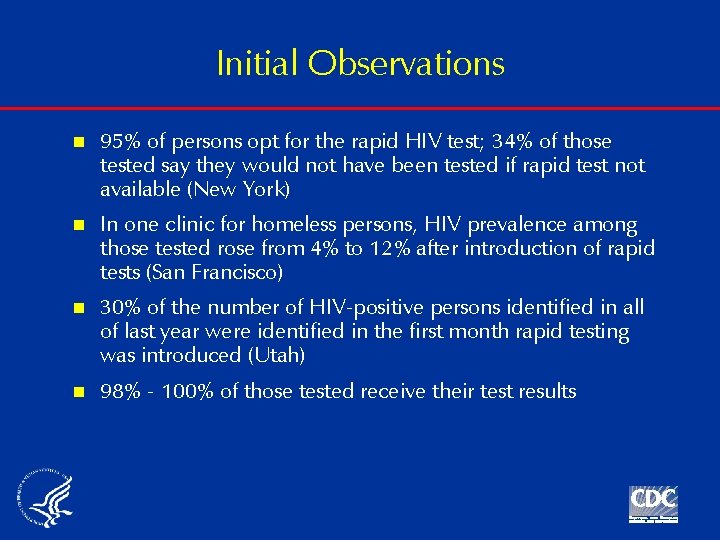
Initial Observations n 95% of persons opt for the rapid HIV test; 34% of those tested say they would not have been tested if rapid test not available (New York) n In one clinic for homeless persons, HIV prevalence among those tested rose from 4% to 12% after introduction of rapid tests (San Francisco) n 30% of the number of HIV-positive persons identified in all of last year were identified in the first month rapid testing was introduced (Utah) n 98% - 100% of those tested receive their test results

Post-Marketing Surveillance n In New York State test sites: 30% increase in persons tested Ø 85% increase in MSM Ø 42% increase in IDU Ø 96% increase in persons with hx of STD diagnosis n Counselors’ Ø Ø confidence in their overall role in rapid testing rose from 54% to 100% after first 12 weeks of testing scores on proficiency specimens at 12 weeks were 100%

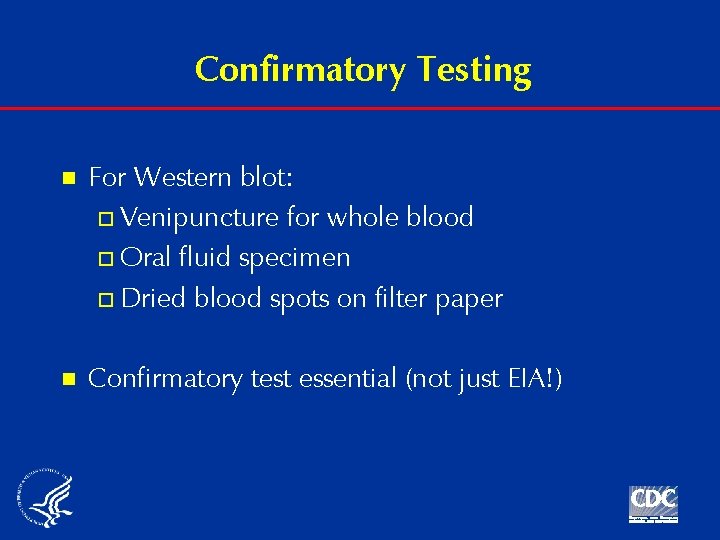
Confirmatory Testing n For Western blot: o Venipuncture for whole blood o Oral fluid specimen o Dried blood spots on filter paper n Confirmatory test essential (not just EIA!)

Additional Resources General and technical information (updated frequently): www. cdc. gov/hiv/rapid_testing


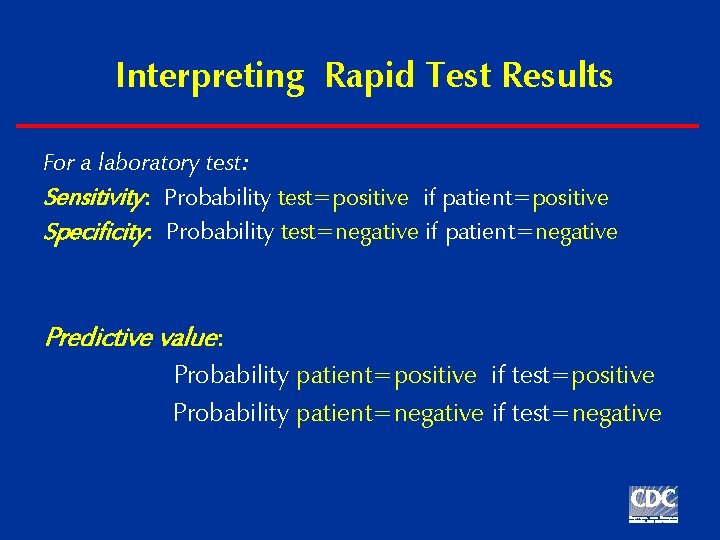
Interpreting Rapid Test Results For a laboratory test: Sensitivity: Probability test=positive if patient=positive Specificity: Probability test=negative if patient=negative Predictive value: Probability patient=positive if test=positive Probability patient=negative if test=negative

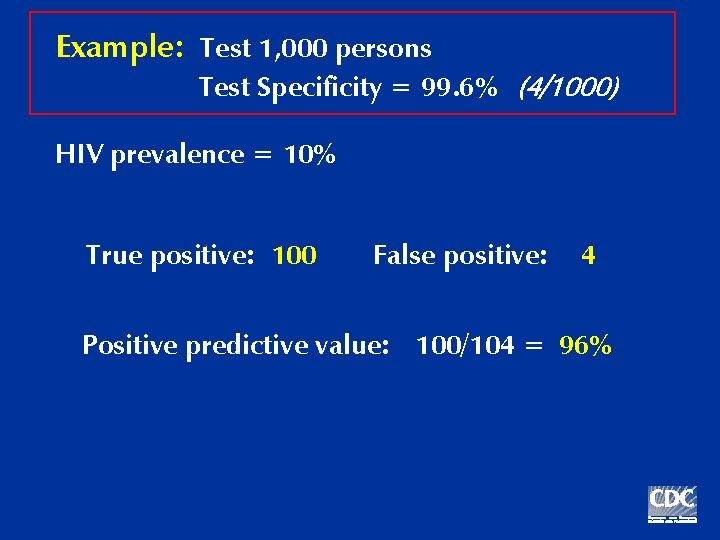
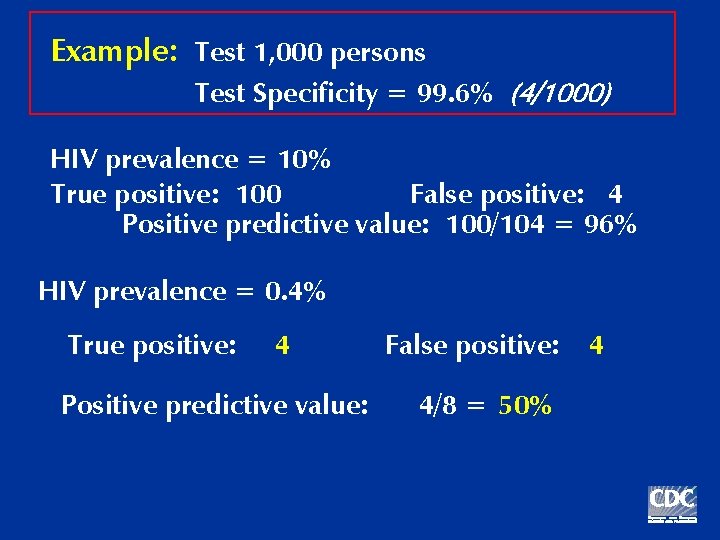
Example: Test 1, 000 persons Test Specificity = 99. 6% (4/1000) HIV prevalence = 10% True positive: 100 False positive: 4 Positive predictive value: 100/104 = 96%

Example: Test 1, 000 persons Test Specificity = 99. 6% (4/1000) HIV prevalence = 10% True positive: 100 False positive: 4 Positive predictive value: 100/104 = 96% HIV prevalence = 0. 4% True positive: 4 Positive predictive value: False positive: 4/8 = 50% 4

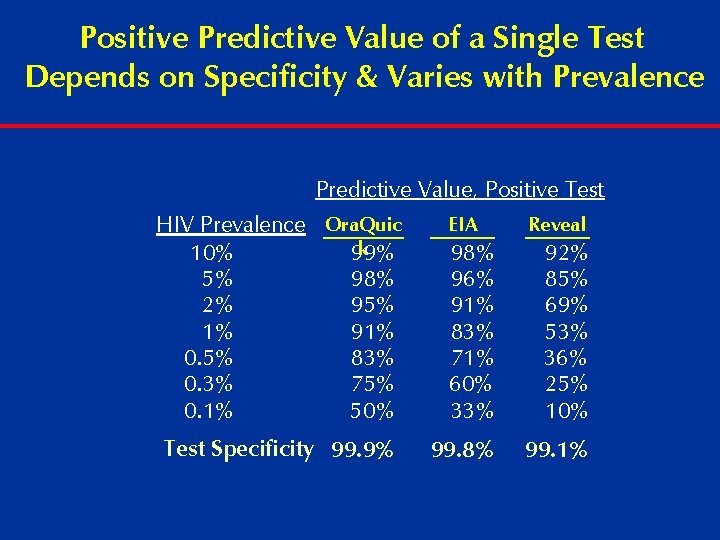
Positive Predictive Value of a Single Test Depends on Specificity & Varies with Prevalence Predictive Value, Positive Test HIV Prevalence Ora. Quic k 10% 99% 5% 98% 2% 95% 1% 91% 0. 5% 83% 0. 3% 75% 0. 1% 50% EIA 98% 96% 91% 83% 71% 60% 33% 92% 85% 69% 53% 36% 25% 10% Test Specificity 99. 9% 99. 8% 99. 1% Reveal