HIV Testing and Management in the era of








- Slides: 34


HIV Testing and Management in the era of Pr. EP HIV testing: updates and challenges Bernard M Branson MD @Twitter. Handle Share your thoughts on this presentation with #IAS 2019

Background and Disclosure of Financial Relationships • Dr. Branson previously served as Associate Director for Laboratory Diagnostics in CDC’s Division of HIV/AIDS Prevention until October 2014. • Dr. Branson currently serves as consultant to the Gilead Sciences FOCUS Program and Chembio Diagnostic Systems, Inc.

4 Use of Brand Names • This presentation will refer to individual HIV tests by brand name for the purposes of identification and clarity. • No endorsement of any specific test is intended.

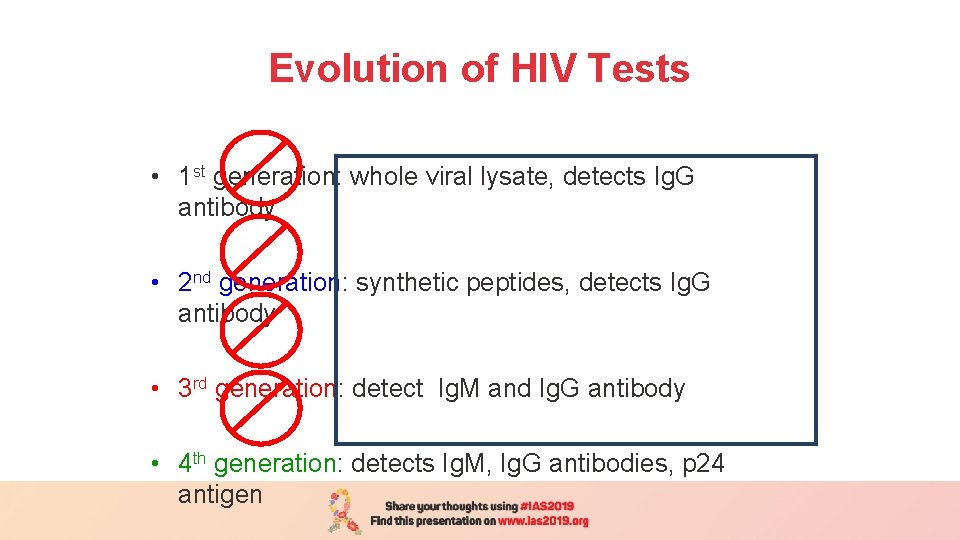
Evolution of HIV Tests • 1 st generation: whole viral lysate, detects Ig. G antibody • 2 nd generation: synthetic peptides, detects Ig. G antibody • 3 rd generation: detect Ig. M and Ig. G antibody • 4 th generation: detects Ig. M, Ig. G antibodies, p 24 antigen

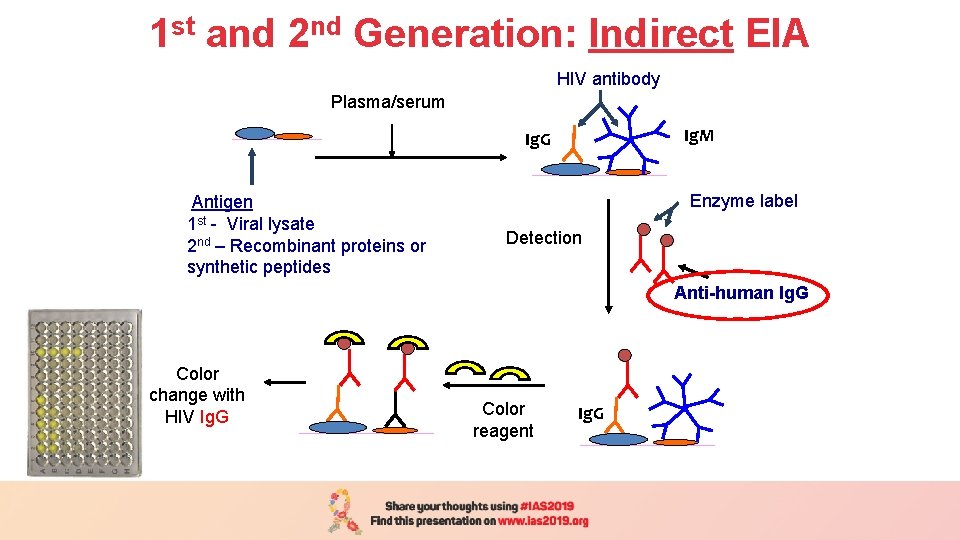
1 st and 2 nd Generation: Indirect EIA Plasma/serum (1 h/37 o C) Plasma/serum HIV antibody Ig. M Ig. G Antigen 1 st - Viral lysate 2 nd – Recombinant proteins or synthetic peptides Enzyme label Detection Anti-human Ig. G Color change with HIV Ig. G Color reagent Ig. G

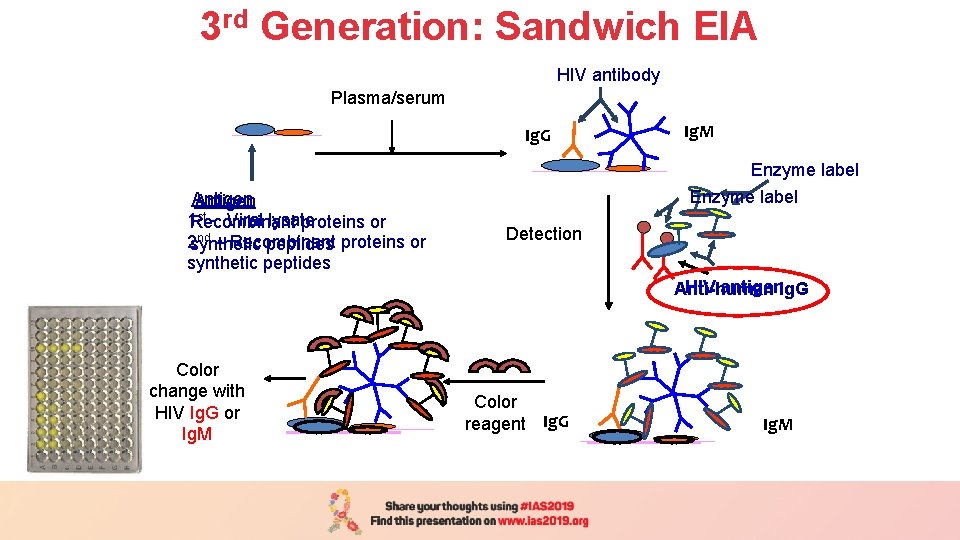
3 rd Generation: Sandwich EIA Plasma/serum (1 h/37 o C) Plasma/serum HIV antibody Ig. G Antigen st - Viral lysate 1 Recombinant proteins or nd 2 synthetic – Recombinant peptides proteins or synthetic peptides Ig. M Enzyme label Detection HIV antigen. Ig. G Anti-human Color change with HIV Ig. G or Ig. M Color reagent Ig. G Ig. M

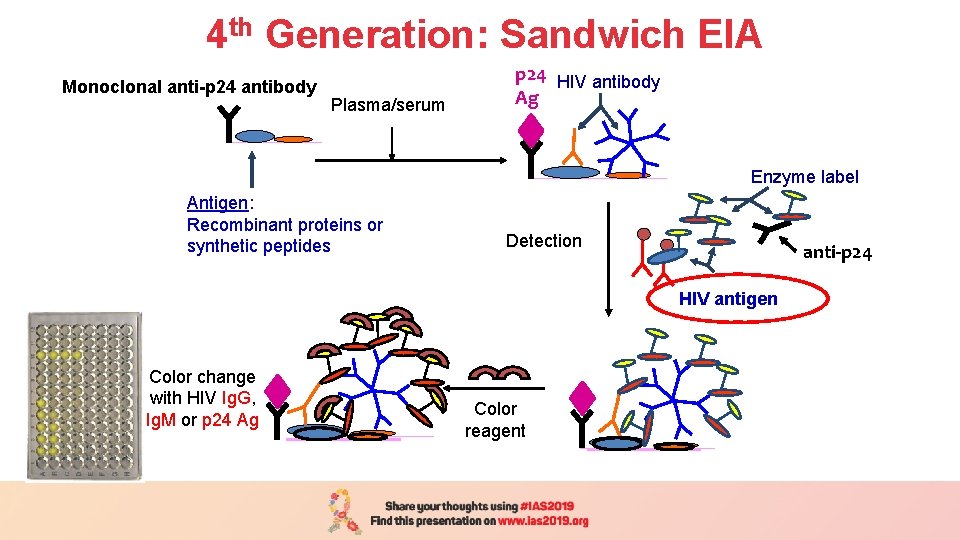
4 th Generation: Sandwich EIA Plasma/serum Monoclonal anti-p 24 antibody (1 h/37 o C) Plasma/serum p 24 HIV antibody Ag Enzyme label Antigen: Recombinant proteins or synthetic peptides Detection anti-p 24 HIV antigen Color change with HIV Ig. G, Ig. M or p 24 Ag Color reagent

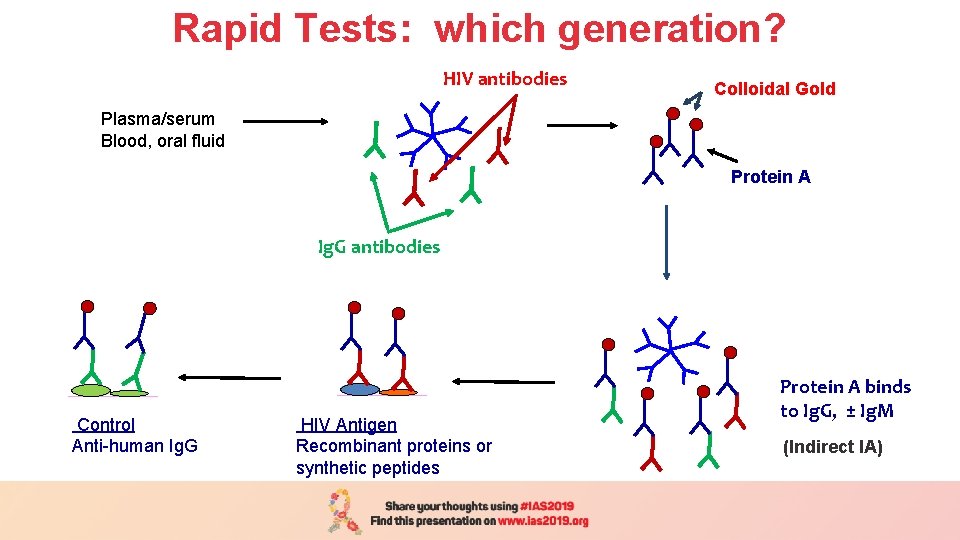
Rapid Tests: which generation? HIV antibodies Plasma/serum Blood, oral fluid Plasma/serum Colloidal Gold (1 h/37 o C) Protein A Ig. G antibodies Control Anti-human Ig. G HIV Antigen Recombinant proteins or synthetic peptides Protein A binds to Ig. G, ± Ig. M (Indirect IA)

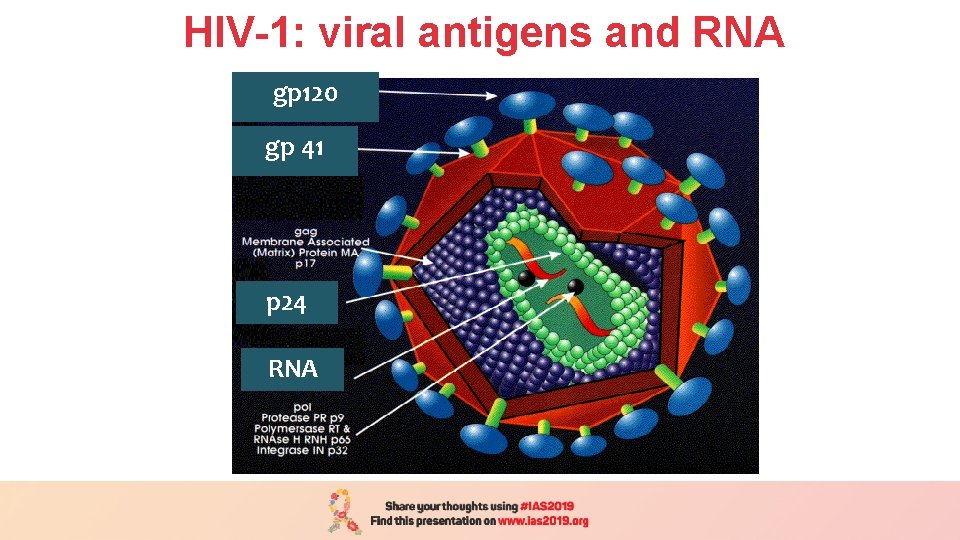
HIV-1: viral antigens and RNA gp 120 gp 41 p 24 RNA

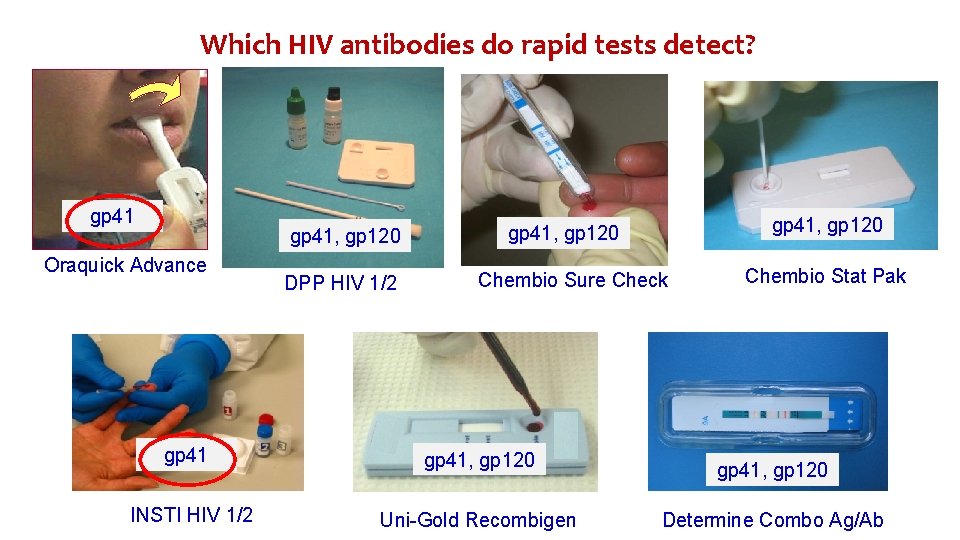
Which HIV antibodies do rapid tests detect? gp 41, gp 120 Oraquick Advance DPP HIV 1/2 gp 41, gp 120 Chembio Sure Check gp 41, gp 120 INSTI HIV 1/2 Uni-Gold Recombigen Chembio Stat Pak gp 41, gp 120 Determine Combo Ag/Ab

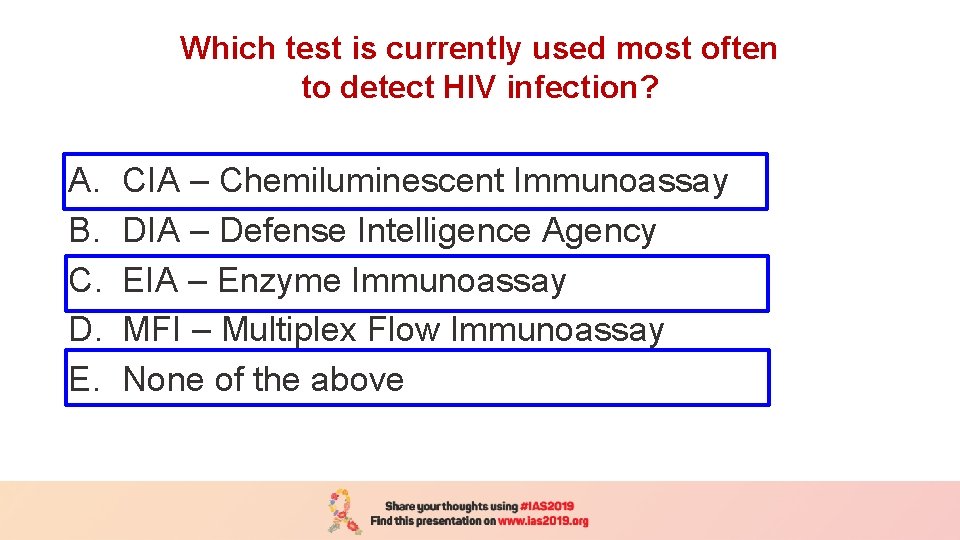
Which test is currently used most often to detect HIV infection? A. B. C. D. E. CIA – Chemiluminescent Immunoassay DIA – Defense Intelligence Agency EIA – Enzyme Immunoassay MFI – Multiplex Flow Immunoassay None of the above

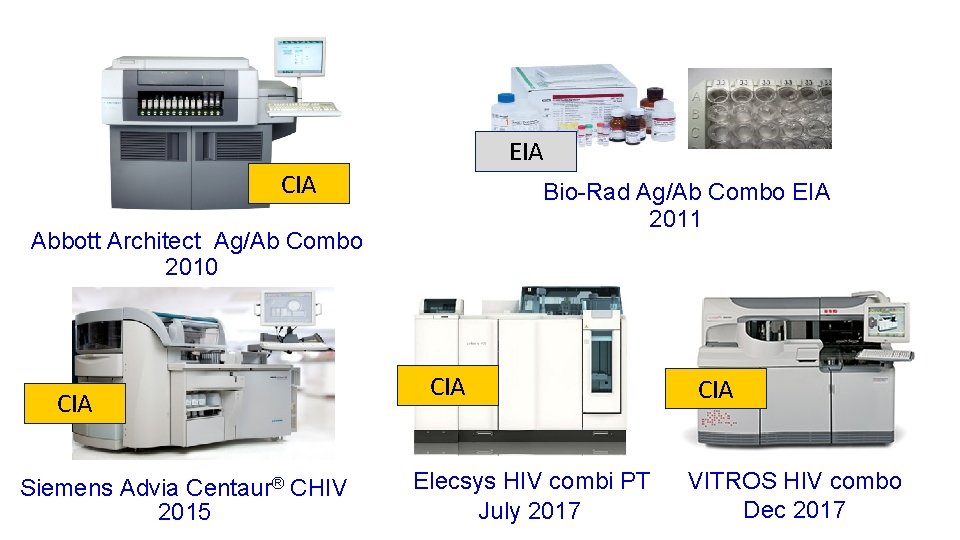
EIA CIA Bio-Rad Ag/Ab Combo EIA 2011 Abbott Architect Ag/Ab Combo 2010 CIA Siemens Advia Centaur® CHIV 2015 CIA Elecsys HIV combi PT July 2017 CIA VITROS HIV combo Dec 2017

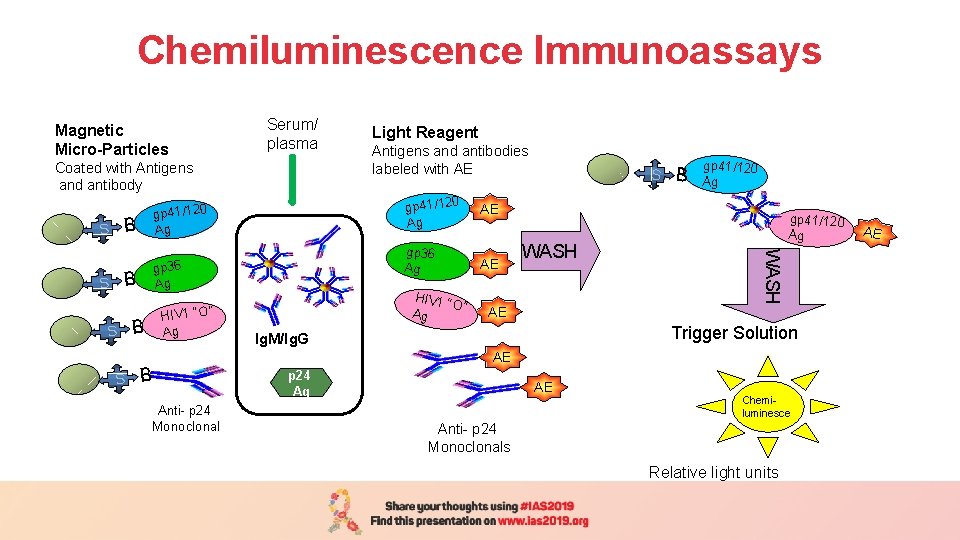
Chemiluminescence Immunoassays Magnetic Micro-Particles Serum/ plasma Coated with Antigens and antibody gp 41/120 Ag B S B B S S S B Ag B B Αnti- p 24 Monoclonal gp 41/120 Ag AE gp 36 Ag AE HIV 1 “ O” Ag HIV 1 “O” B Antigens and antibodies labeled with AE S WASH AE B gp 41/120 Ag WASH gp 36 Ag Light Reagent Trigger Solution Ig. M/Ig. G AE p 24 Ag AE Chemiluminesce Αnti- p 24 Monoclonals Relative light units AE

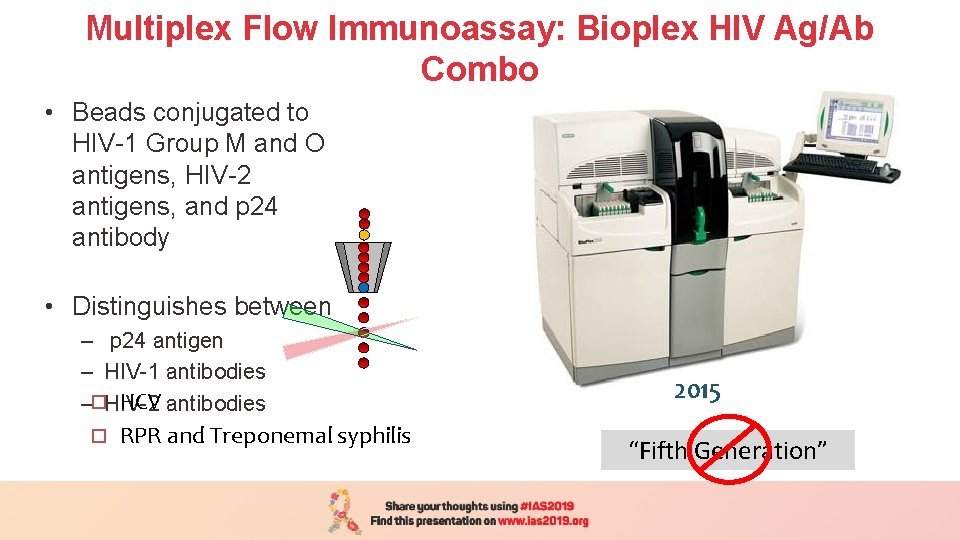
Multiplex Flow Immunoassay: Bioplex HIV Ag/Ab Combo • Beads conjugated to HIV-1 Group M and O antigens, HIV-2 antigens, and p 24 antibody • Distinguishes between – p 24 antigen – HIV-1 antibodies HCV antibodies –¨HIV-2 ¨ RPR and Treponemal syphilis 2015 “Fifth Generation”

New terminology • Instrumented lab test • Single-use rapid test 16

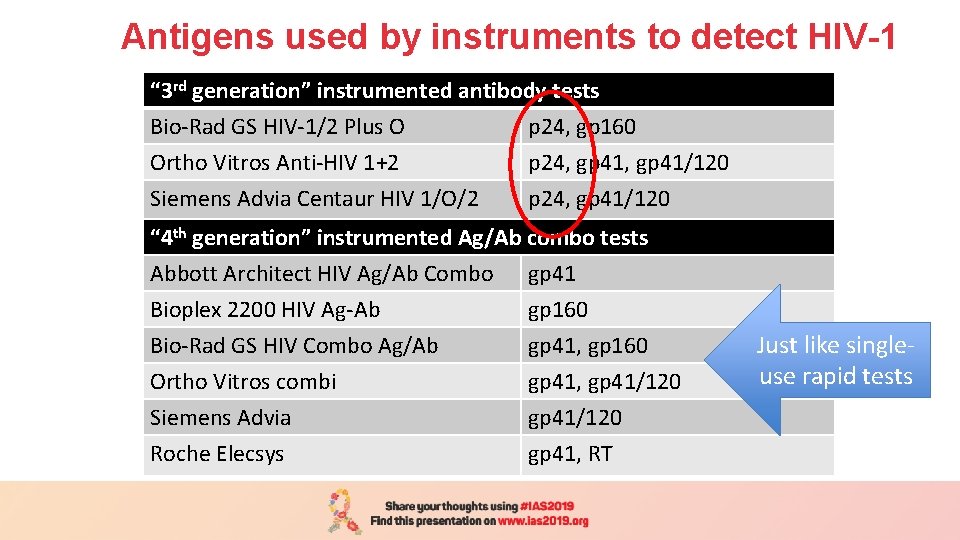
Antigens used by instruments to detect HIV-1 “ 3 rd generation” instrumented antibody tests Bio-Rad GS HIV-1/2 Plus O p 24, gp 160 Ortho Vitros Anti-HIV 1+2 p 24, gp 41/120 Siemens Advia Centaur HIV 1/O/2 p 24, gp 41/120 “ 4 th generation” instrumented Ag/Ab combo tests Abbott Architect HIV Ag/Ab Combo gp 41 Bioplex 2200 HIV Ag-Ab Bio-Rad GS HIV Combo Ag/Ab Ortho Vitros combi Siemens Advia Roche Elecsys gp 160 gp 41, gp 41/120 gp 41, RT Just like singleuse rapid tests

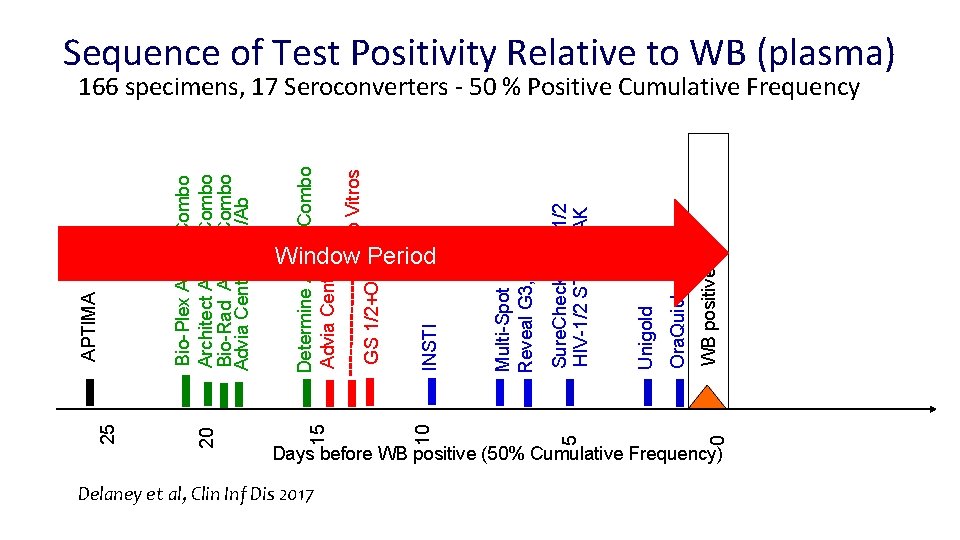
20 25 Days before WB positive (50% Cumulative Frequency) Delaney et al, Clin Inf Dis 2017 0 WB positive Ora. Quick Unigold Sure. Check HIV-1/2 STAT-PAK Multi-Spot Reveal G 3, DPP INSTI Determine Ag/Ab Combo Advia Centaur Ortho Vitros GS 1/2+O Window Period 5 10 15 Bio-Plex Ag/Ab Combo Architect Ag/Ab Combo Bio-Rad Ag/Ab Combo Advia Centaur Ag/Ab APTIMA Sequence of Test Positivity Relative to WB (plasma) 166 specimens, 17 Seroconverters - 50 % Positive Cumulative Frequency

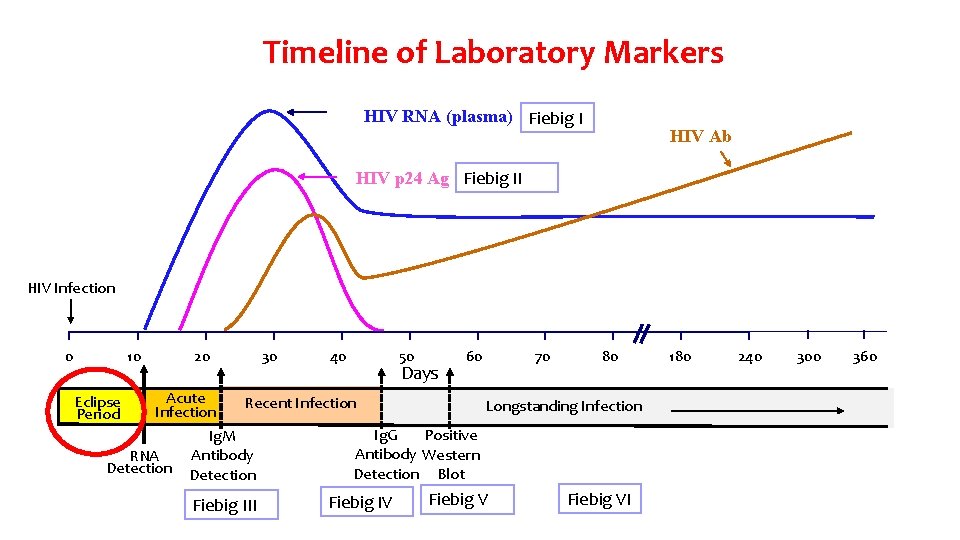
Timeline of Laboratory Markers HIV RNA (plasma) Fiebig I HIV Ab HIV p 24 Ag Fiebig II HIV Infection 0 10 20 30 40 50 Days 60 70 80 Acute Recent Infection Longstanding Infection Ig. G Positive Ig. M Antibody Western Antibody RNA Detection Blot Eclipse Period Fiebig III Fiebig IV Fiebig VI 180 240 300 360

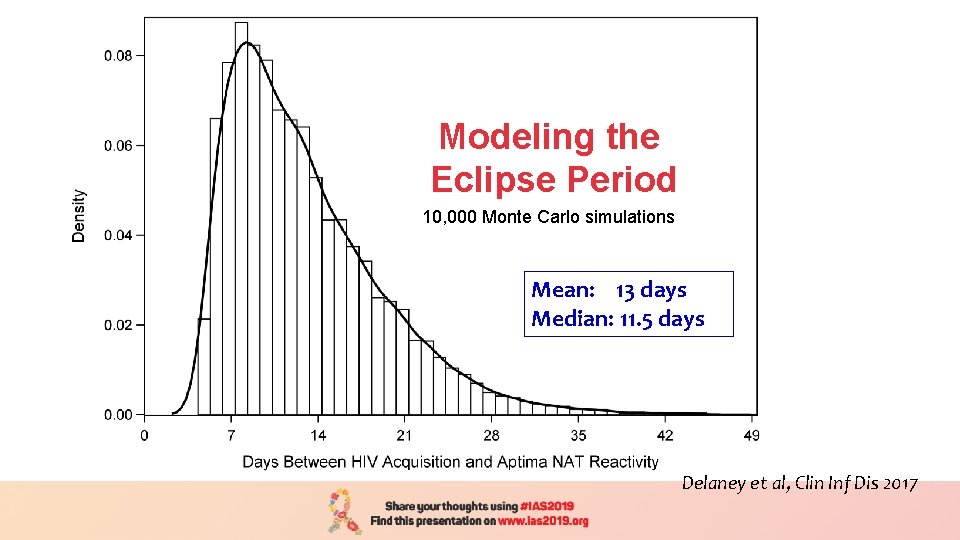
Modeling the Eclipse Period 10, 000 Monte Carlo simulations Mean: 13 days Median: 11. 5 days Delaney et al, Clin Inf Dis 2017

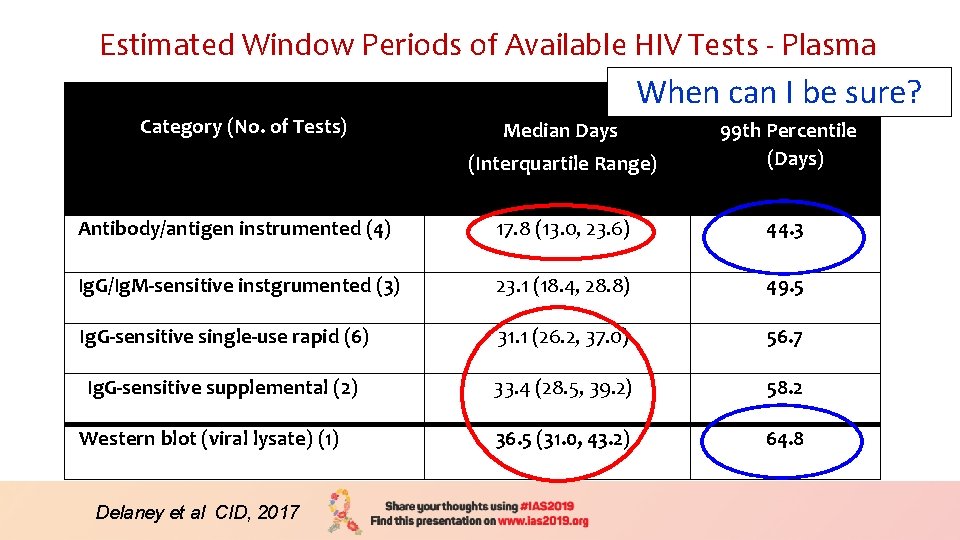
Estimated Window Periods of Available HIV Tests - Plasma When can I be sure? Category (No. of Tests) (Interquartile Range) 99 th Percentile (Days) Antibody/antigen instrumented (4) 17. 8 (13. 0, 23. 6) 44. 3 Ig. G/Ig. M-sensitive instgrumented (3) 23. 1 (18. 4, 28. 8) 49. 5 Ig. G-sensitive single-use rapid (6) 31. 1 (26. 2, 37. 0) 56. 7 Ig. G-sensitive supplemental (2) 33. 4 (28. 5, 39. 2) 58. 2 36. 5 (31. 0, 43. 2) 64. 8 Western blot (viral lysate) (1) Delaney et al CID, 2017 Median Days

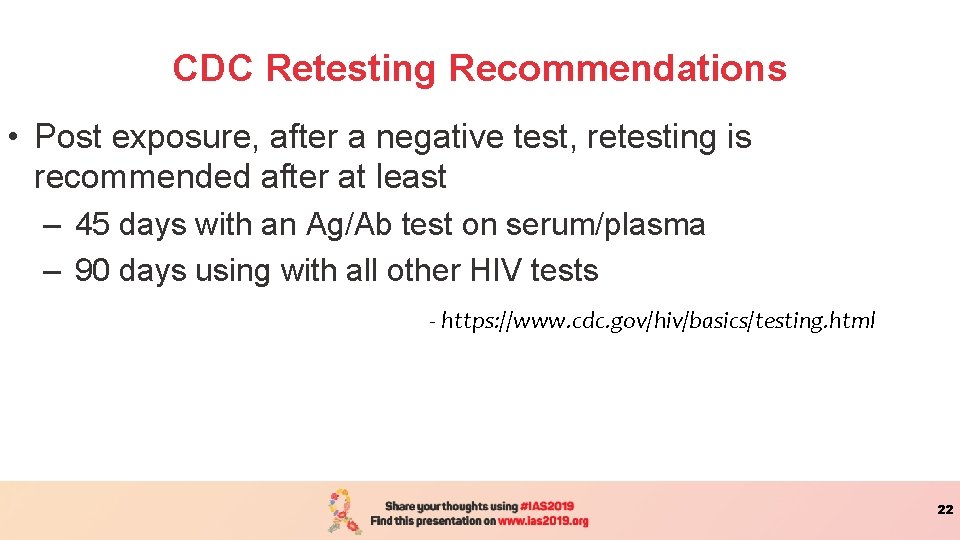
CDC Retesting Recommendations • Post exposure, after a negative test, retesting is recommended after at least – 45 days with an Ag/Ab test on serum/plasma – 90 days using with all other HIV tests - https: //www. cdc. gov/hiv/basics/testing. html 22

ART is being initiated earlier and earlier, sometimes during Acute HIV Infection

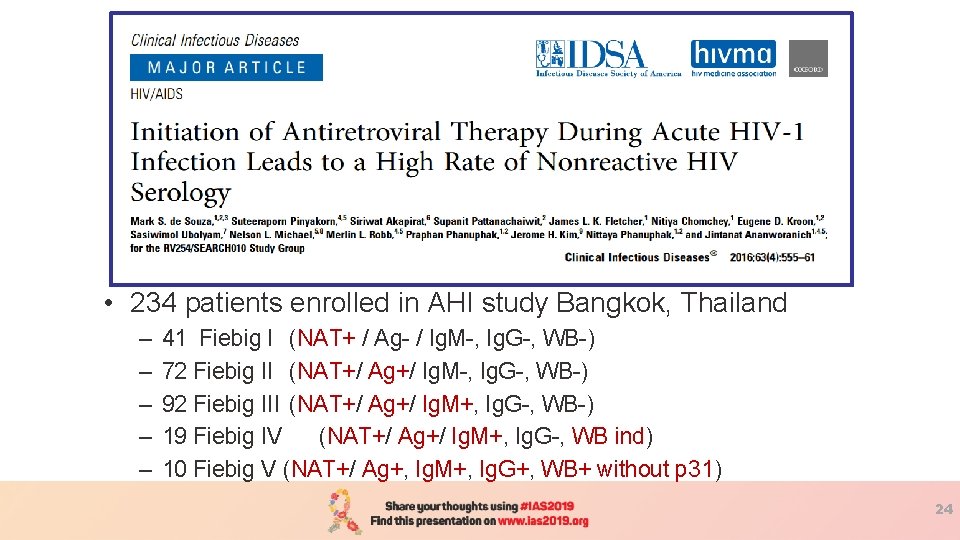
• 234 patients enrolled in AHI study Bangkok, Thailand – – – 41 Fiebig I (NAT+ / Ag- / Ig. M-, Ig. G-, WB-) 72 Fiebig II (NAT+/ Ag+/ Ig. M-, Ig. G-, WB-) 92 Fiebig III (NAT+/ Ag+/ Ig. M+, Ig. G-, WB-) 19 Fiebig IV (NAT+/ Ag+/ Ig. M+, Ig. G-, WB ind) 10 Fiebig V (NAT+/ Ag+, Ig. M+, Ig. G+, WB+ without p 31) 24

Antibody Response to HIV (without treatment) - Keating et al CID, 2016

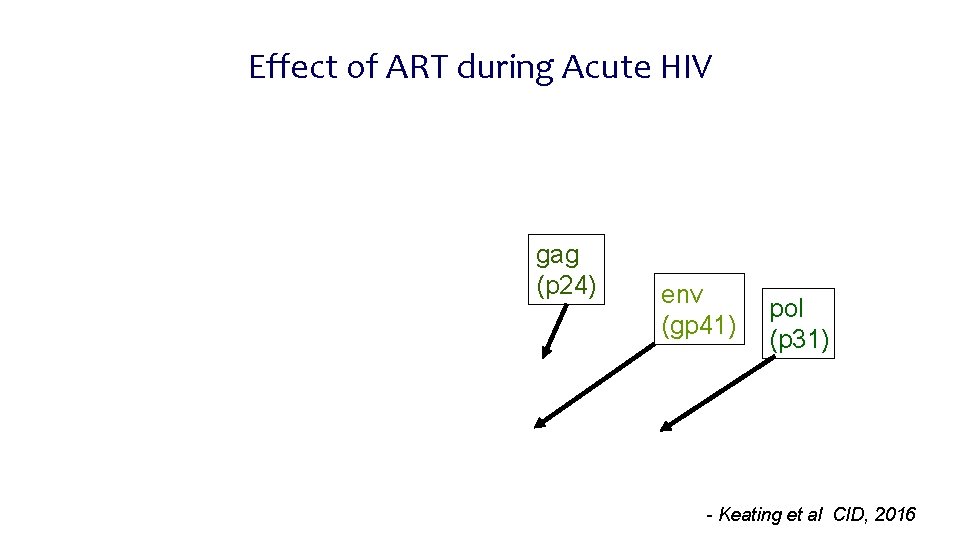
Effect of ART during Acute HIV gag (p 24) env (gp 41) pol (p 31) - Keating et al CID, 2016

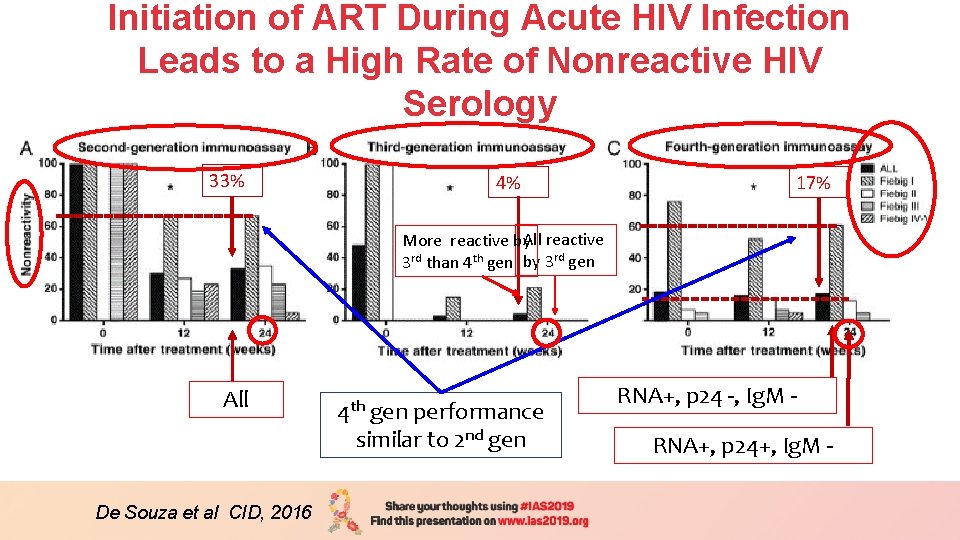
Initiation of ART During Acute HIV Infection Leads to a High Rate of Nonreactive HIV Serology 33% 4% 17% More reactive by. All reactive 3 rd than 4 th gen by 3 rd gen All De Souza et al CID, 2016 4 th gen performance similar to 2 nd gen RNA+, p 24 -, Ig. M RNA+, p 24+, Ig. M -

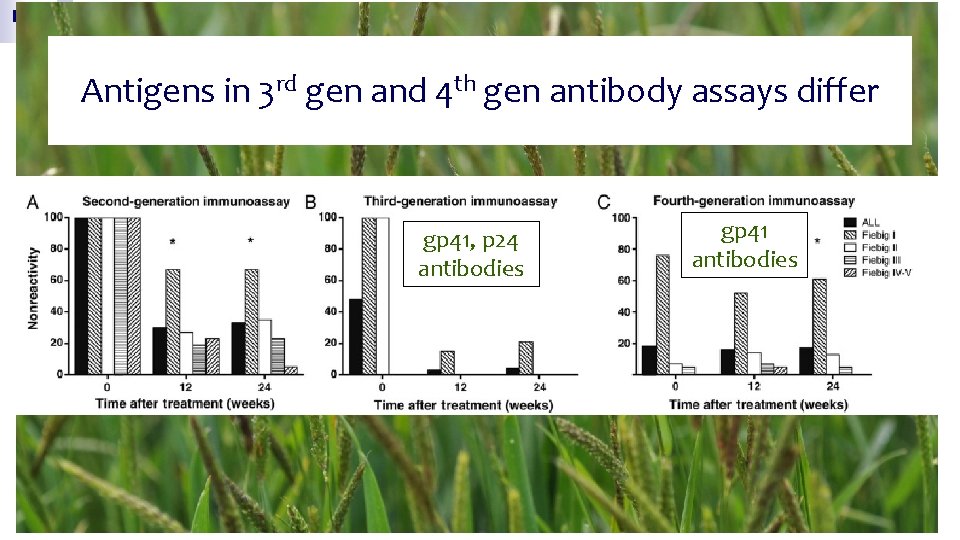
Antigens in 3 rd gen and 4 th gen antibody assays differ gp 41, p 24 antibodies gp 41 antibodies

On the Horizon…

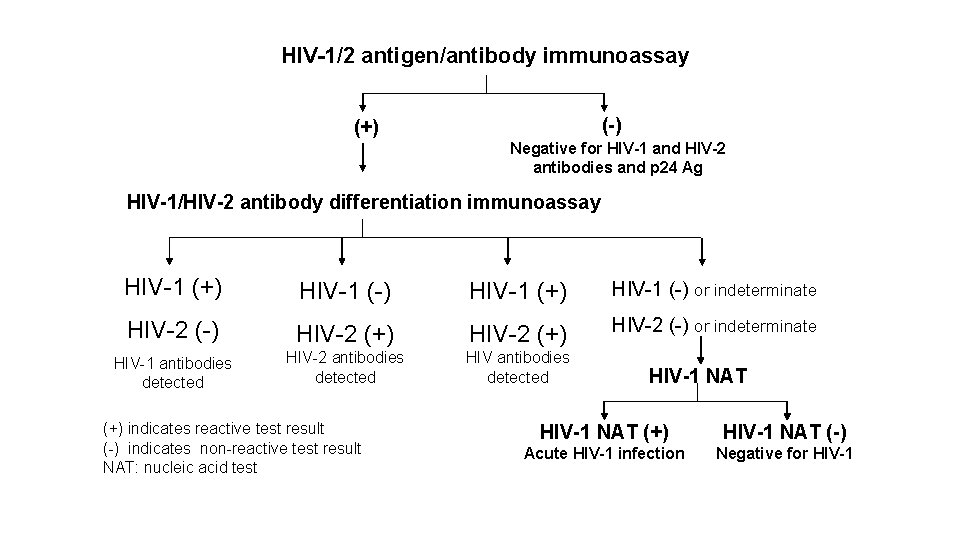
HIV-1/2 antigen/antibody immunoassay (-) (+) Negative for HIV-1 and HIV-2 antibodies and p 24 Ag HIV-1/HIV-2 antibody differentiation immunoassay HIV-1 (+) HIV-1 (-) or indeterminate HIV-2 (-) HIV-2 (+) HIV-2 (-) or indeterminate HIV-1 antibodies detected HIV-2 antibodies detected HIV antibodies detected (+) indicates reactive test result (-) indicates non-reactive test result NAT: nucleic acid test HIV-1 NAT (+) HIV-1 NAT (-) Acute HIV-1 infection Negative for HIV-1

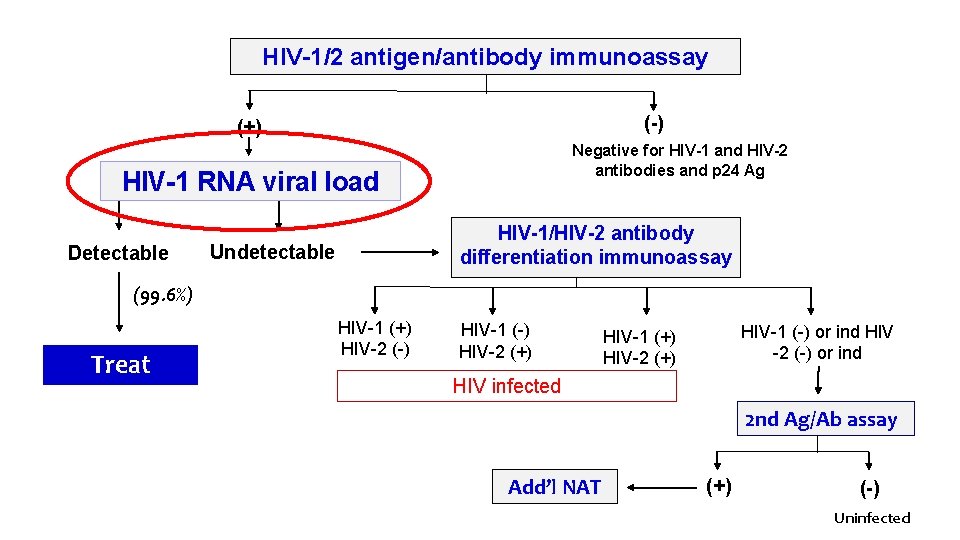
HIV-1/2 antigen/antibody immunoassay (-) (+) Negative for HIV-1 and HIV-2 antibodies and p 24 Ag HIV-1 RNA viral load Detectable HIV-1/HIV-2 antibody differentiation immunoassay Undetectable (99. 6%) Treat HIV-1 (+) HIV-2 (-) HIV-1 (-) HIV-2 (+) HIV-1 (-) or ind HIV -2 (-) or ind HIV-1 (+) HIV-2 (+) HIV infected 2 nd Ag/Ab assay Add’l NAT (+) (-) Uninfected

“Point-of-Care” Nucleic Acid Tests • Xpert HIV-1 viral load – 1 ml plasma – Results in 90 minutes – LOD 32 copies/m. L – CE-marked December 2014 Not yet available in U. S. Gene. Xpert

“Point-of-Care” Nucleic Acid Tests SAMBA II m-PIMA

3 4 Summary • HIV tests differ in subtle ways that affect different circumstances • RNA & viral load will play an increasingly important role in HIV diagnosis • Early treatment (and Pr. EP) can lead to non-reactive serology and undetectable RNA – New techniques (such as whole blood total nucleic acid) are needed.