Helsinki3 Direct Estimate of Conjugation Hyperconjugation and Aromaticity




















- Slides: 50

Helsinki-3 Direct Estimate of Conjugation, Hyperconjugation and Aromaticity With an Energy Decomposition Analysis Gernot Frenking Fachbereich Chemie, Philipps-Universität Marburg

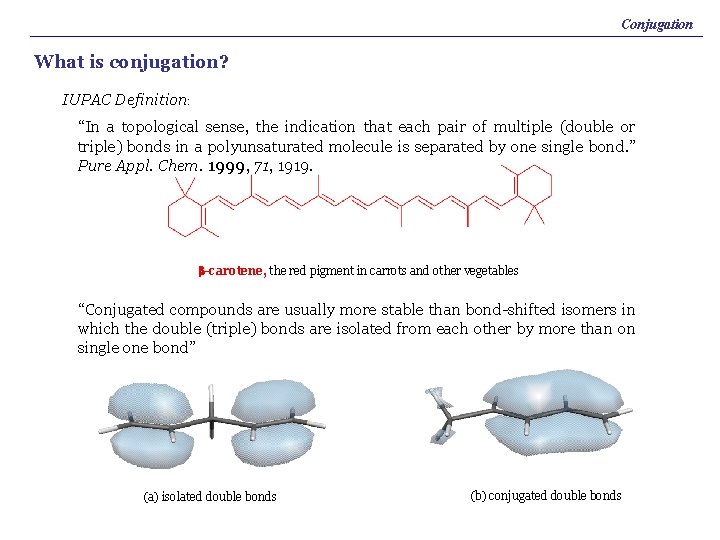
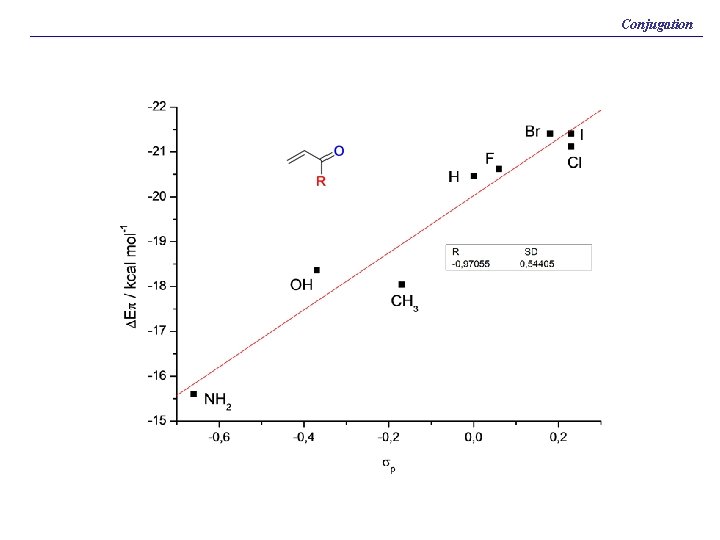
Conjugation What is conjugation? IUPAC Definition: “In a topological sense, the indication that each pair of multiple (double or triple) bonds in a polyunsaturated molecule is separated by one single bond. ” Pure Appl. Chem. 1999, 71, 1919. b-carotene, the red pigment in carrots and other vegetables “Conjugated compounds are usually more stable than bond-shifted isomers in which the double (triple) bonds are isolated from each other by more than on single one bond” (a) isolated double bonds (b) conjugated double bonds

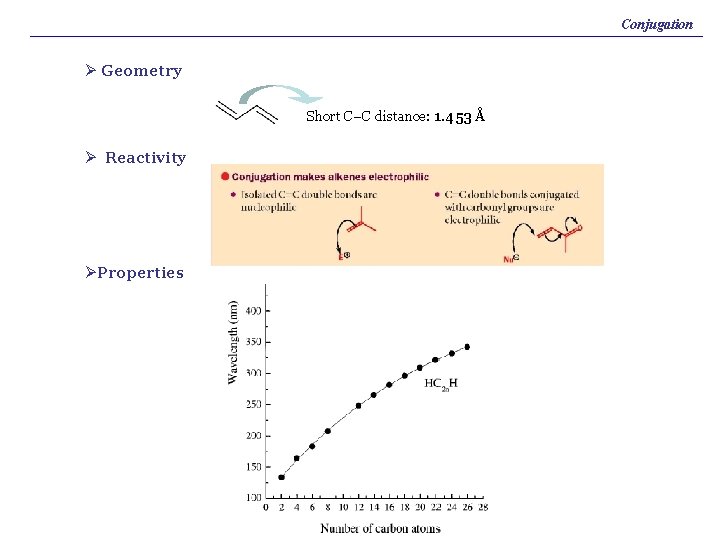
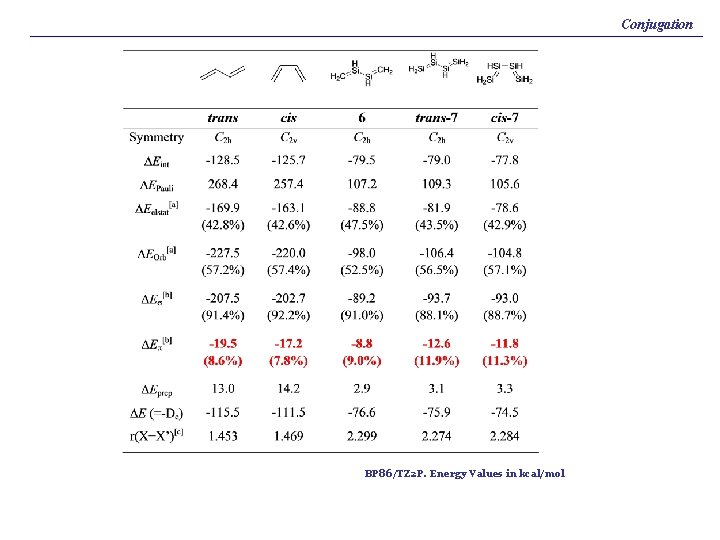
Conjugation Ø Geometry Short C–C distance: 1. 453 Å Ø Reactivity ØProperties


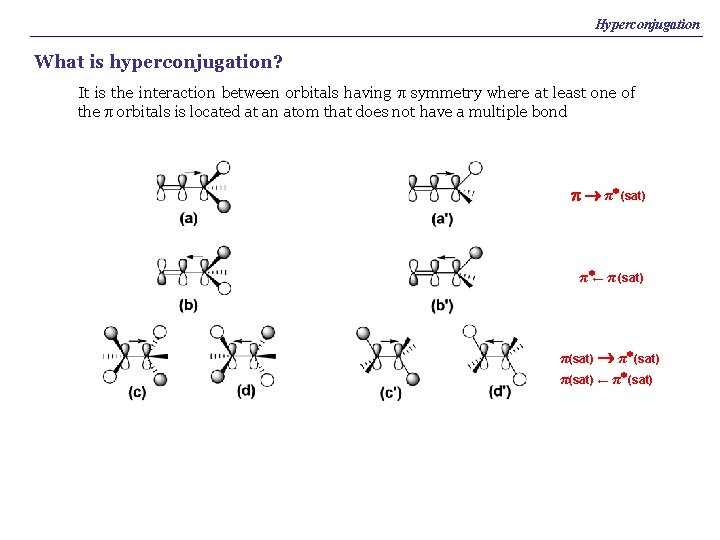
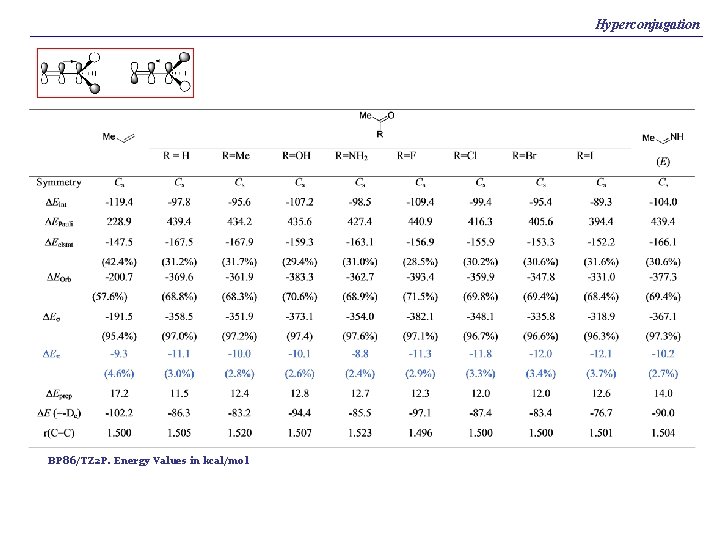
Hyperconjugation What is hyperconjugation? It is the interaction between orbitals having p symmetry where at least one of the p orbitals is located at an atom that does not have a multiple bond p π* (sat) π*← π (sat) π(sat) π* (sat) π(sat) ← π* (sat)


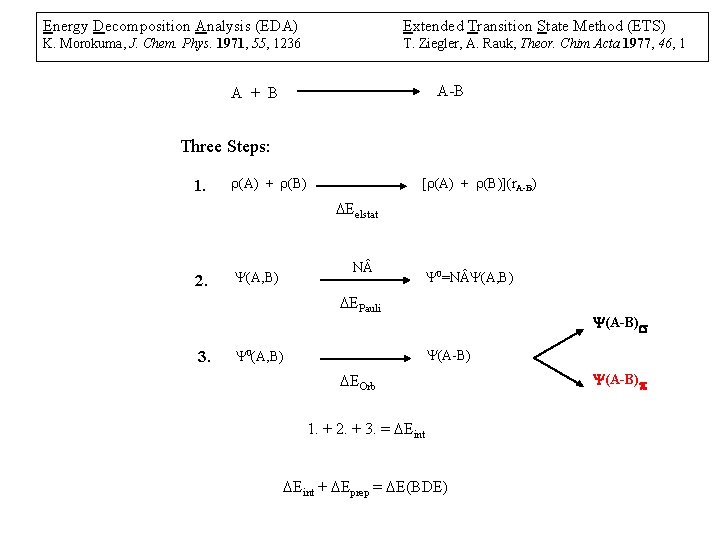
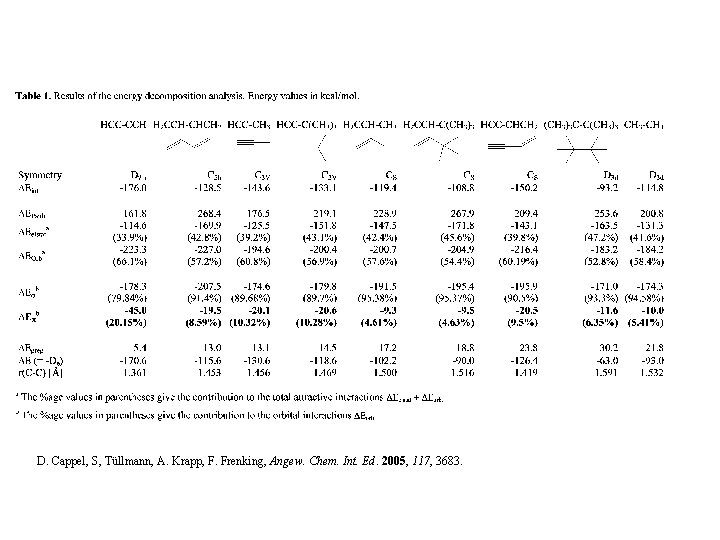
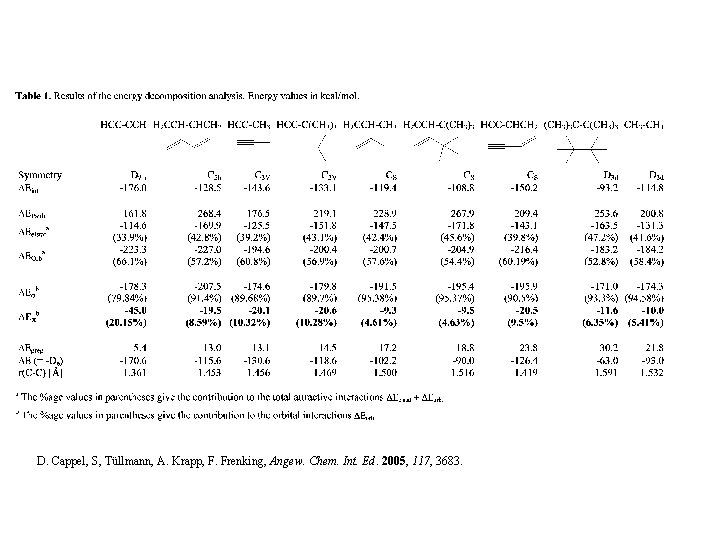
Energy Decomposition Analysis (EDA) Extended Transition State Method (ETS) K. Morokuma, J. Chem. Phys. 1971, 55, 1236 T. Ziegler, A. Rauk, Theor. Chim Acta 1977, 46, 1 A-B A + B Three Steps: 1. r(A) + r(B) [r(A) + r(B)](r. A-B) DEelstat 2. N (A, B) 0=N (A, B) DEPauli 3. (A-B)s (A-B) 0(A, B) DEOrb 1. + 2. + 3. = DEint + DEprep = DE(BDE) (A-B)p

D. Cappel, S, Tüllmann, A. Krapp, F. Frenking, Angew. Chem. Int. Ed. 2005, 117, 3683.

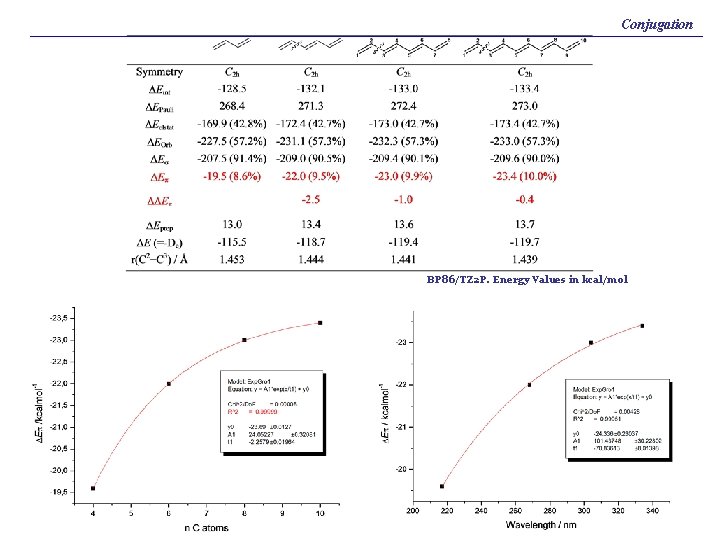
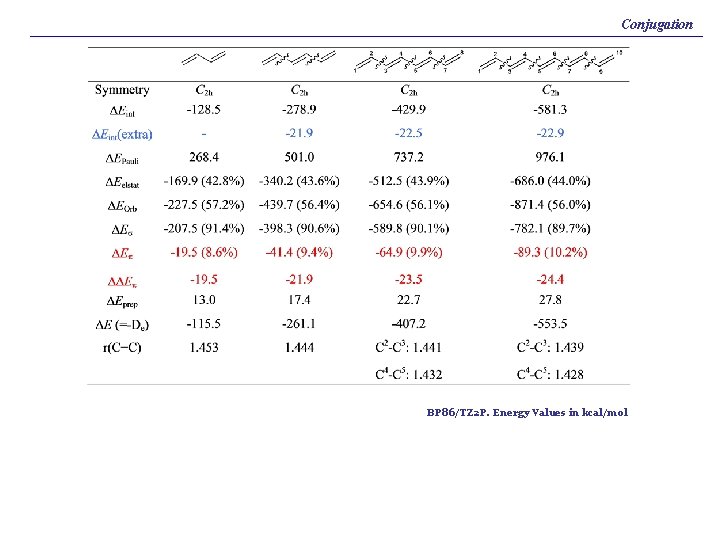
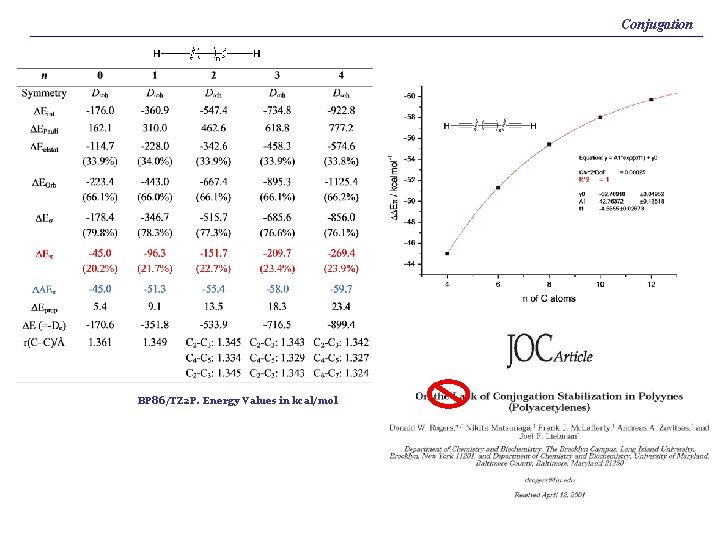
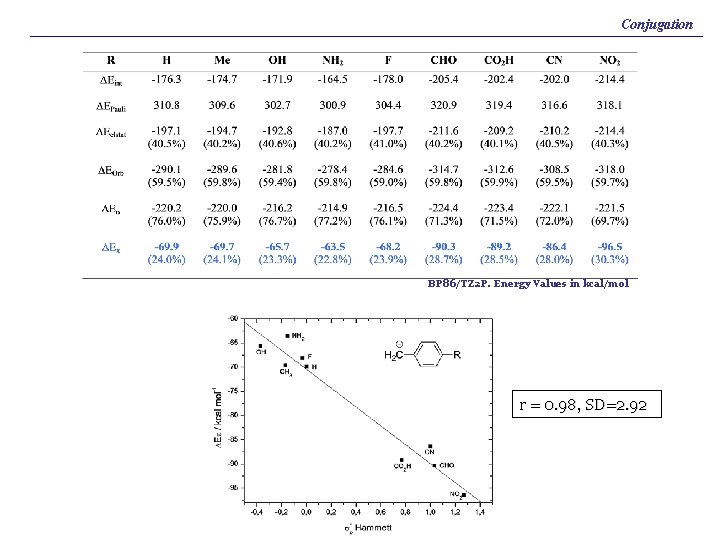
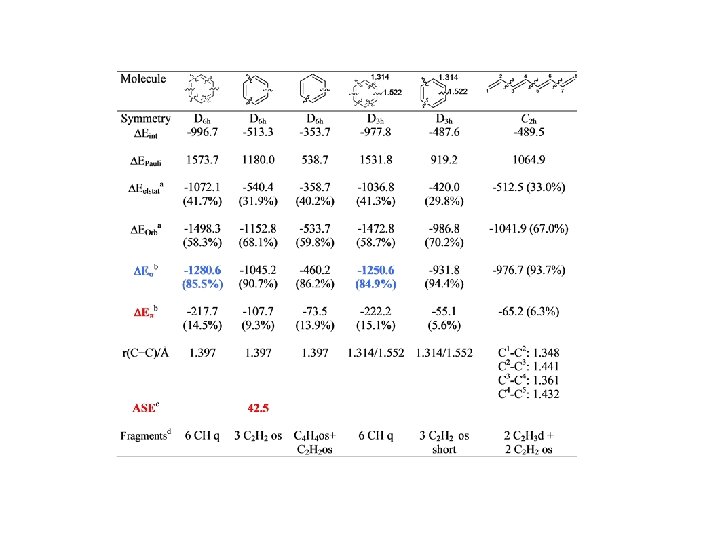
Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol

Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol

Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol

Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol

Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol

Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol

Conjugation

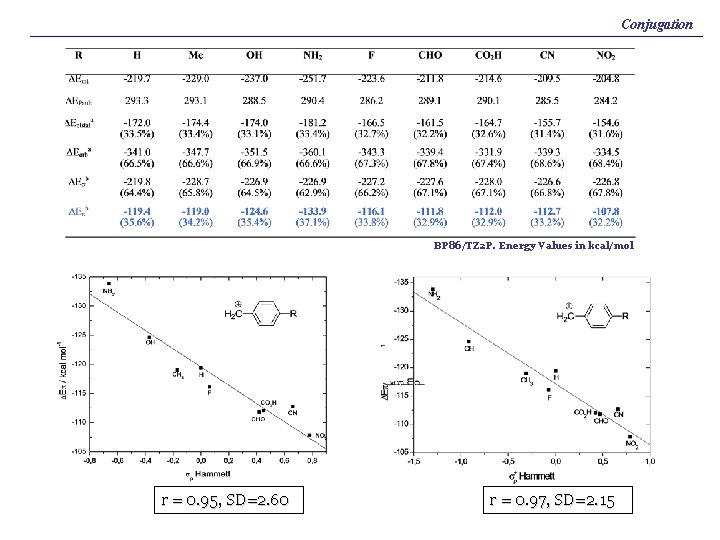
Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol r = 0. 95, SD=2. 60 r = 0. 97, SD=2. 15

Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol r = 0. 98, SD=2. 92

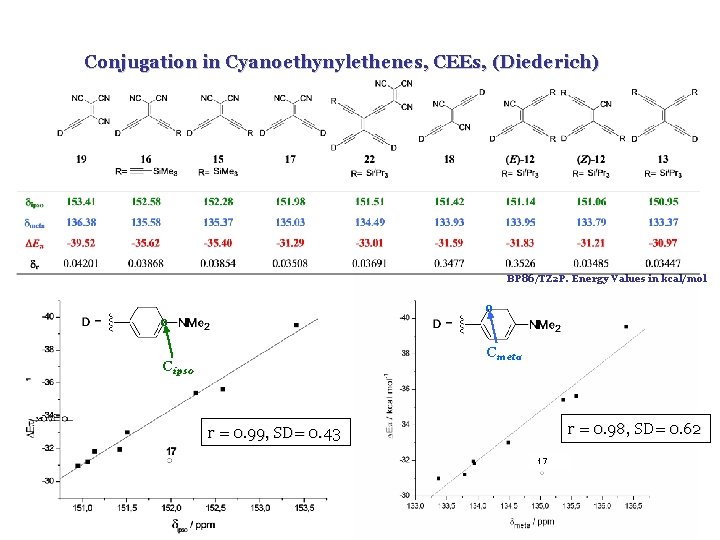
Conjugation in Cyanoethynylethenes, CEEs, (Diederich) BP 86/TZ 2 P. Energy Values in kcal/mol o o Cmeta Cipso r = 0. 98, SD= 0. 62 r = 0. 99, SD= 0. 43 17

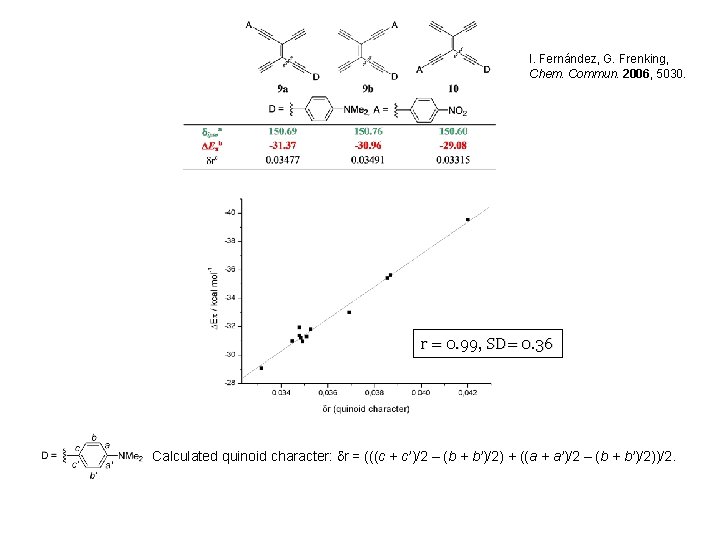
I. Fernández, G. Frenking, Chem. Commun. 2006, 5030. r = 0. 99, SD= 0. 36 Calculated quinoid character: dr = (((c + c’)/2 – (b + b’)/2) + ((a + a’)/2 – (b + b’)/2))/2.

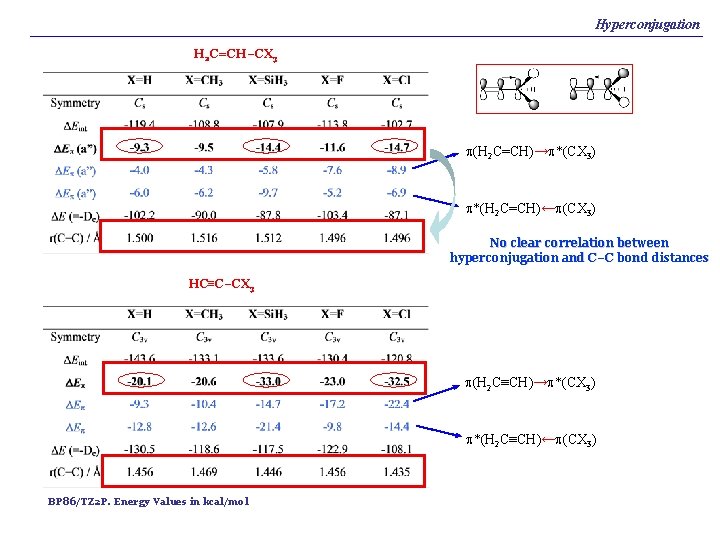
Hyperconjugation H 2 C=CH–CX 3 p(H 2 C=CH)→p*(CX 3) p*(H 2 C=CH)←p(CX 3) No clear correlation between hyperconjugation and C–C bond distances HC≡C–CX 3 p(H 2 C≡CH)→p*(CX 3) p*(H 2 C≡CH)←p(CX 3) BP 86/TZ 2 P. Energy Values in kcal/mol

Hyperconjugation BP 86/TZ 2 P. Energy Values in kcal/mol

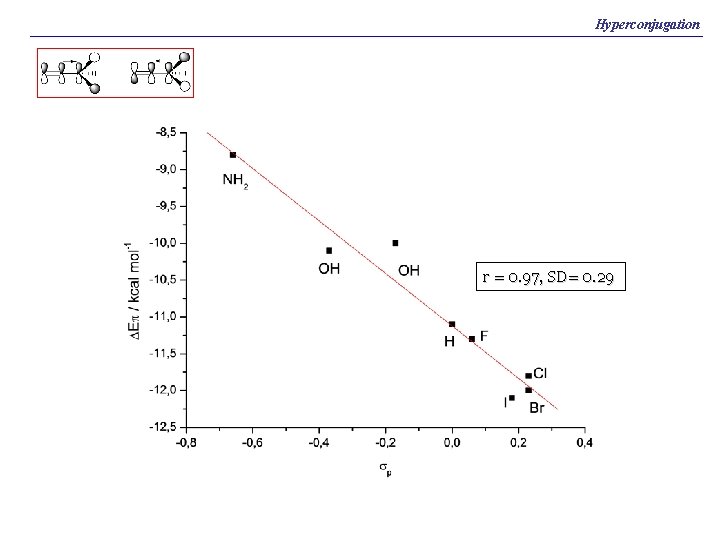
Hyperconjugation r = 0. 97, SD= 0. 29

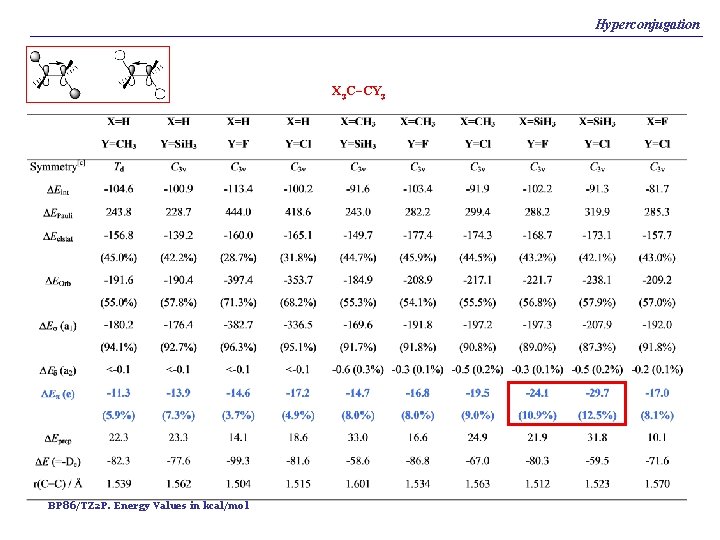
Hyperconjugation X 3 C–CY 3 BP 86/TZ 2 P. Energy Values in kcal/mol

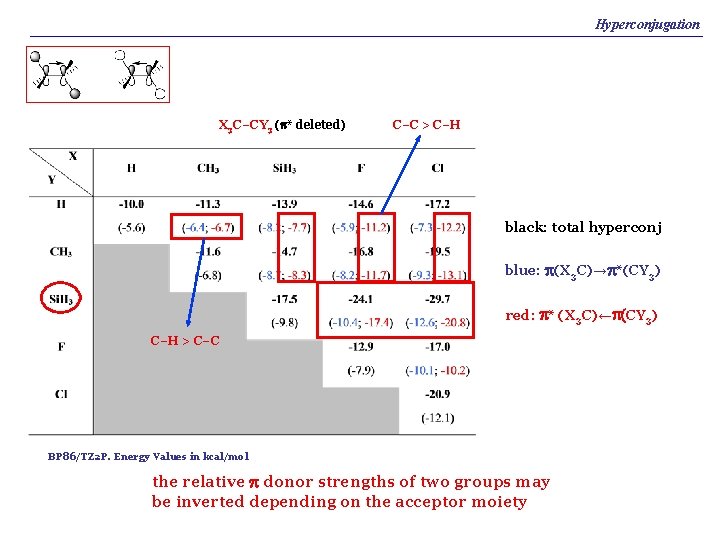
Hyperconjugation X 3 C–CY 3 (p* deleted) C–C > C–H black: total hyperconj blue: p(X 3 C)→p*(CY 3) red: p* (X 3 C)←p(CY 3) C–H > C–C BP 86/TZ 2 P. Energy Values in kcal/mol the relative p donor strengths of two groups may be inverted depending on the acceptor moiety







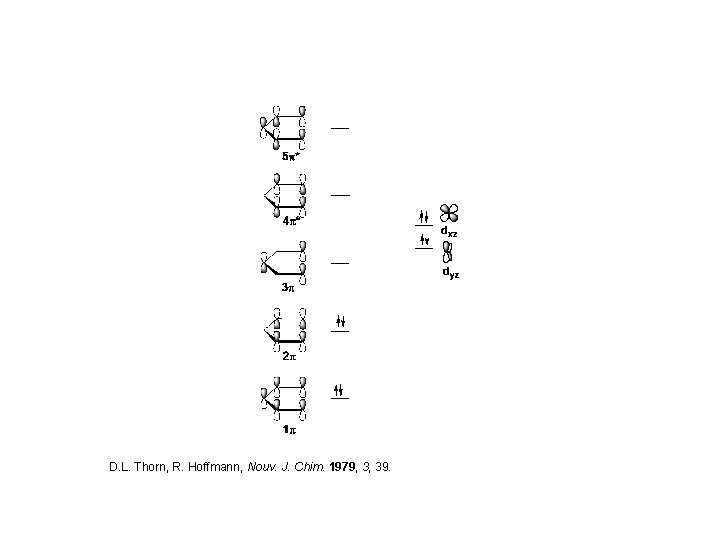
D. L. Thorn, R. Hoffmann, Nouv. J. Chim. 1979, 3, 39.


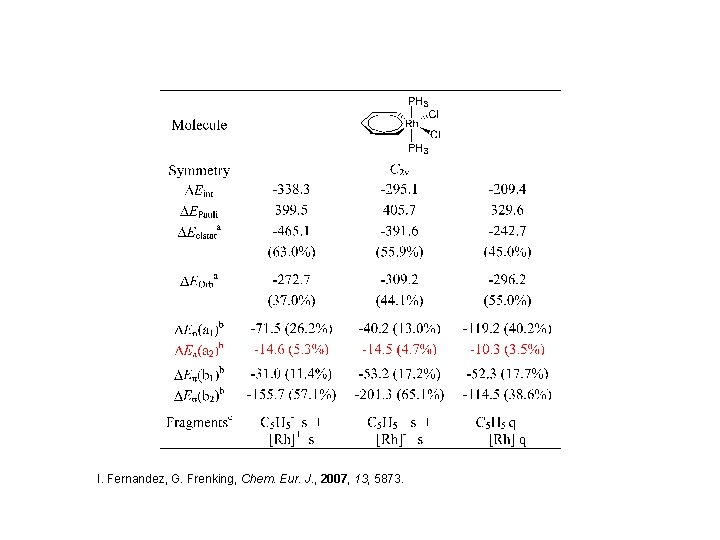
I. Fernandez, G. Frenking, Chem. Eur. J. , 2007, 13, 5873.



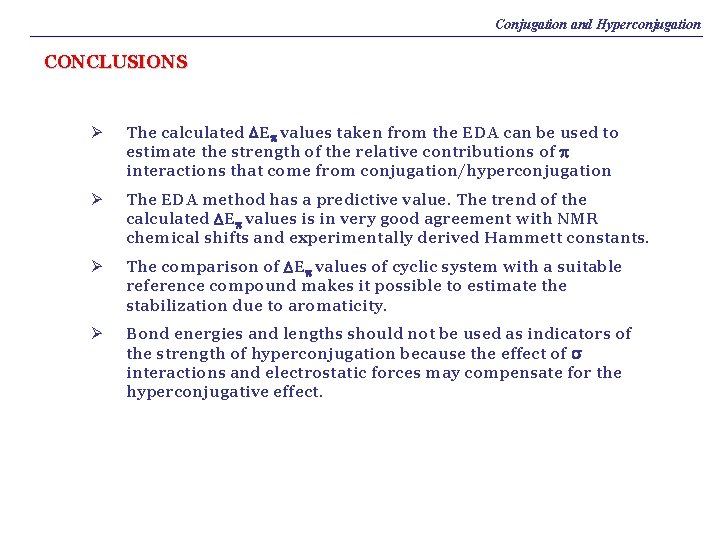
Conjugation and Hyperconjugation CONCLUSIONS Ø The calculated DEp values taken from the EDA can be used to estimate the strength of the relative contributions of p interactions that come from conjugation/hyperconjugation Ø The EDA method has a predictive value. The trend of the calculated DEp values is in very good agreement with NMR chemical shifts and experimentally derived Hammett constants. Ø The comparison of DEp values of cyclic system with a suitable reference compound makes it possible to estimate the stabilization due to aromaticity. Ø Bond energies and lengths should not be used as indicators of the strength of hyperconjugation because the effect of s interactions and electrostatic forces may compensate for the hyperconjugative effect.








D. Cappel, S, Tüllmann, A. Krapp, F. Frenking, Angew. Chem. Int. Ed. 2005, 117, 3683.

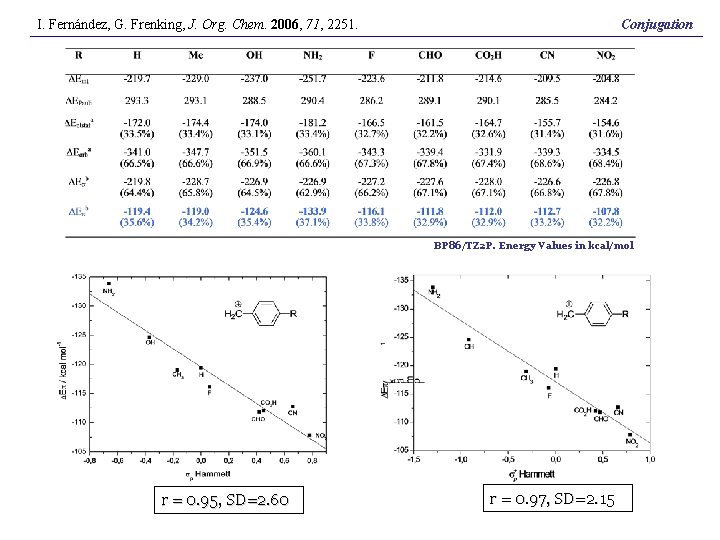
I. Fernández, G. Frenking, J. Org. Chem. 2006, 71, 2251. Conjugation BP 86/TZ 2 P. Energy Values in kcal/mol r = 0. 95, SD=2. 60 r = 0. 97, SD=2. 15

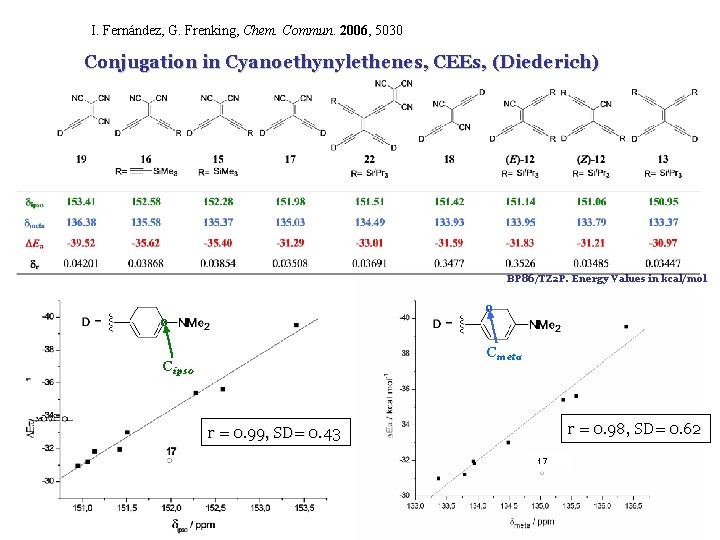
I. Fernández, G. Frenking, Chem. Commun. 2006, 5030 Conjugation in Cyanoethynylethenes, CEEs, (Diederich) BP 86/TZ 2 P. Energy Values in kcal/mol o o Cmeta Cipso r = 0. 98, SD= 0. 62 r = 0. 99, SD= 0. 43 17


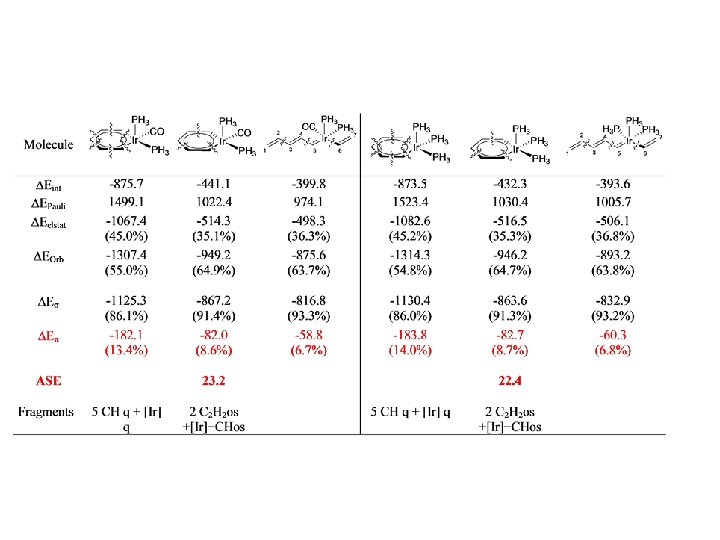
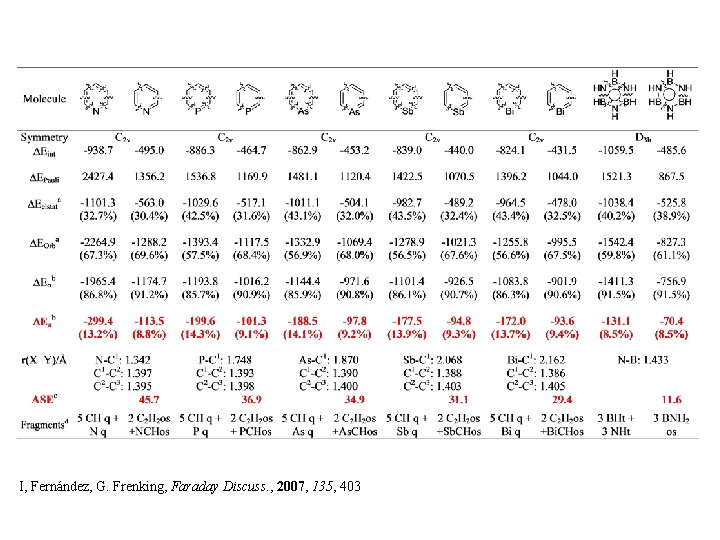
I, Fernández, G. Frenking, Faraday Discuss. , 2007, 135, 403

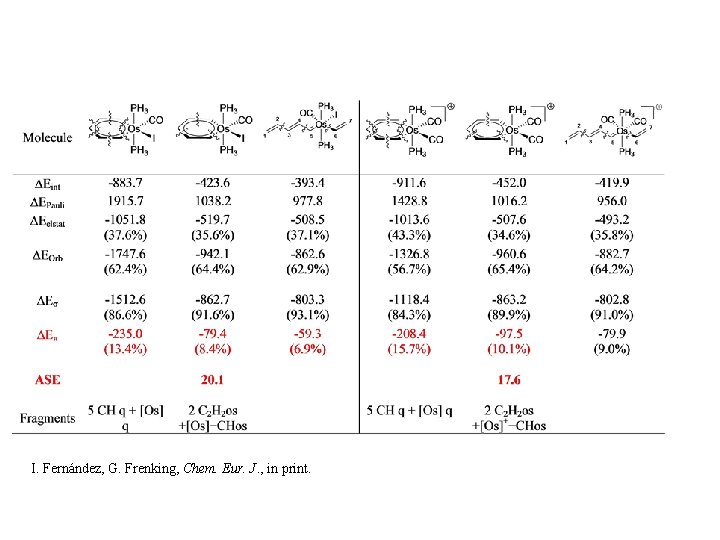
I. Fernández, G. Frenking, Chem. Eur. J. , in print.

 Benzene
Benzene Aromaticity in benzenoid and non benzenoid compounds
Aromaticity in benzenoid and non benzenoid compounds Aromaticity of benzenoid and non benzenoid compounds
Aromaticity of benzenoid and non benzenoid compounds Aromaticity of pyrrole
Aromaticity of pyrrole Benzene was discovered by
Benzene was discovered by Direct stafford loan estimate $9 500
Direct stafford loan estimate $9 500 Estimate and rounding
Estimate and rounding Estimating sums and differences of fractions
Estimating sums and differences of fractions Excavation and backfill calculation
Excavation and backfill calculation Estimate powers and roots
Estimate powers and roots Clustering estimation with decimals
Clustering estimation with decimals 1-4 practice extrema and average rates of change
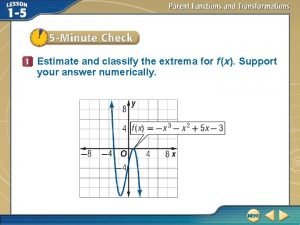
1-4 practice extrema and average rates of change Estimate and classify the extrema for the graph
Estimate and classify the extrema for the graph Lesson 2 estimate products
Lesson 2 estimate products Round the factors and estimate the products exit ticket
Round the factors and estimate the products exit ticket Concealed wiring
Concealed wiring Wecan95
Wecan95 Kruskal wallis test
Kruskal wallis test Statistical inference point estimation
Statistical inference point estimation Single time estimate
Single time estimate Section
Section Political wish
Political wish What is bac project management
What is bac project management Contoh manajemen biaya proyek
Contoh manajemen biaya proyek How to find point estimate
How to find point estimate Contoh cost baseline
Contoh cost baseline Contoh cost estimate
Contoh cost estimate Carolyn has 20 biscuits in a tin
Carolyn has 20 biscuits in a tin Upholstery estimate chart
Upholstery estimate chart Cal
Cal How to estimate nails for formworks
How to estimate nails for formworks Estimate the average drawdown over an area where 25 million
Estimate the average drawdown over an area where 25 million 47 rounded to the nearest ten
47 rounded to the nearest ten Fermi estimations
Fermi estimations Estimate at completion
Estimate at completion College board
College board Work breakdown structure for birthday party
Work breakdown structure for birthday party Estimate each one-sided or two-sided limit if it exists
Estimate each one-sided or two-sided limit if it exists Definitive estimate range
Definitive estimate range How to find correlation coefficient
How to find correlation coefficient Construx estimate
Construx estimate What is the point estimate of μ?
What is the point estimate of μ? Pooled variance estimate formula
Pooled variance estimate formula Standard error of the estimate
Standard error of the estimate Example of population mean
Example of population mean Definitive estimate range
Definitive estimate range Project cost breakdown
Project cost breakdown Surveyor pro project cost estimate
Surveyor pro project cost estimate Estimating parameters and determining sample sizes
Estimating parameters and determining sample sizes Coefficient estimate interpretation
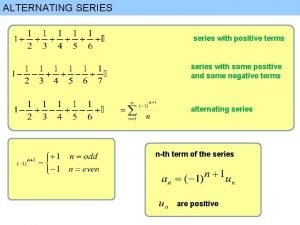
Coefficient estimate interpretation Alternating series estimation theorem
Alternating series estimation theorem