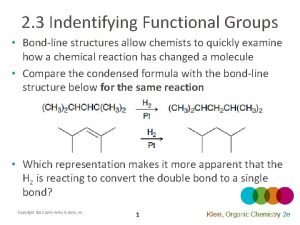
Conjugation in Alkadienes and Allylic Systems Conjugation in















- Slides: 57

Conjugation in Alkadienes and Allylic Systems

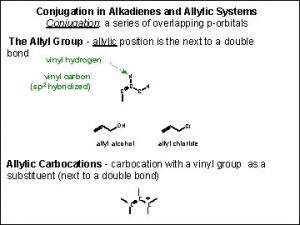
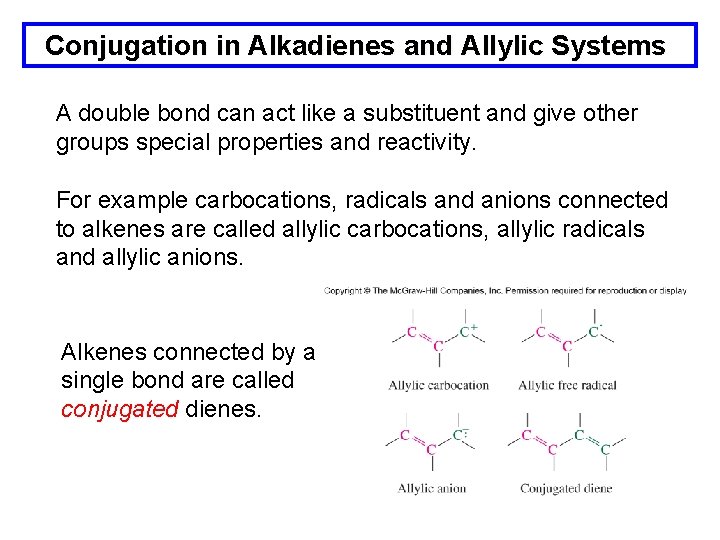
Conjugation in Alkadienes and Allylic Systems A double bond can act like a substituent and give other groups special properties and reactivity. For example carbocations, radicals and anions connected to alkenes are called allylic carbocations, allylic radicals and allylic anions. Alkenes connected by a single bond are called conjugated dienes.

The Allylic Group Allyl is both a common name and a permissible IUPAC name for the H 2 C=CHCH 2 group. The sp 3 hybridized carbon of an allyl carbon is the allylic carbon.

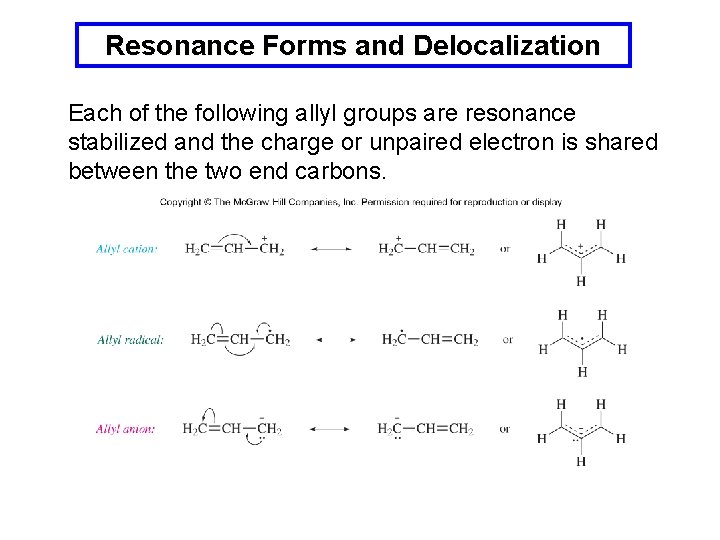
Resonance Forms and Delocalization Each of the following allyl groups are resonance stabilized and the charge or unpaired electron is shared between the two end carbons.

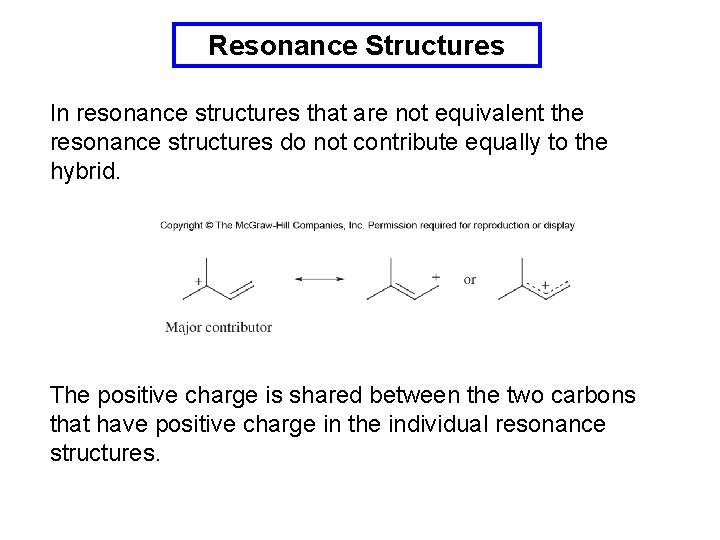
Resonance Structures In resonance structures that are not equivalent the resonance structures do not contribute equally to the hybrid. The positive charge is shared between the two carbons that have positive charge in the individual resonance structures.

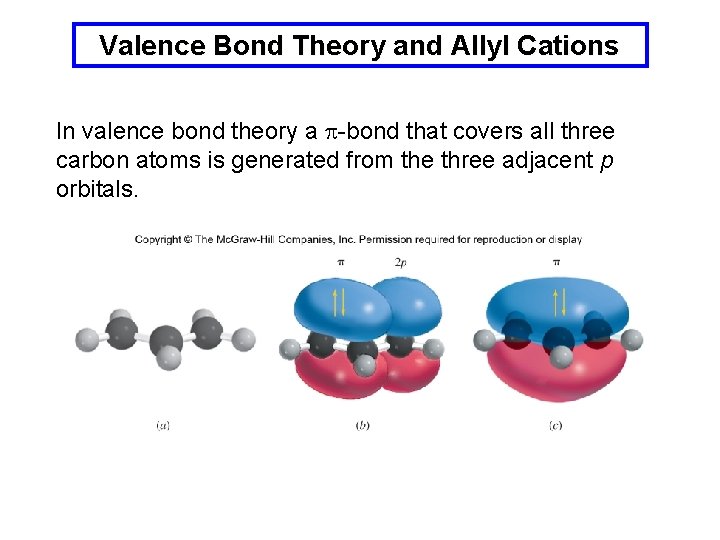
Valence Bond Theory and Allyl Cations In valence bond theory a p-bond that covers all three carbon atoms is generated from the three adjacent p orbitals.

Allylic Halides and SN 1 Reactions Allylic halides react much faster than tertiary alkyl halides. For example: The resonance stabilized carbocation is much more stable so the rate determining ionization step is much faster.

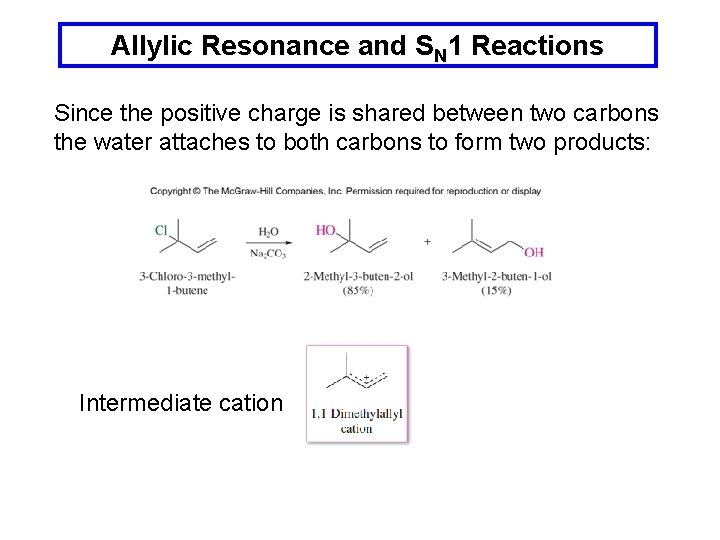
Allylic Resonance and SN 1 Reactions Since the positive charge is shared between two carbons the water attaches to both carbons to form two products: Intermediate cation

Mechanism of Hydrolysis of Allylic Chlorides Reaction Equation. Step 1. Ionization. Step 2 a. Addition of water to one end. Step 2 b. Addition of water to the other end.

Mechanism of Hydrolysis of Allylic Chlorides Step 3 a. Deprotonation. Step 3 b. Deprotonation. The major product corresponds to the more stable resonance structure.

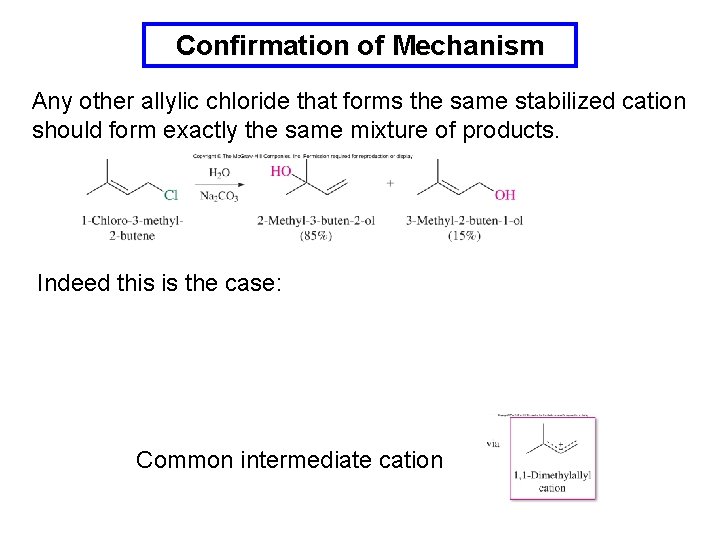
Confirmation of Mechanism Any other allylic chloride that forms the same stabilized cation should form exactly the same mixture of products. Indeed this is the case: Common intermediate cation

Allylic Halides and SN 2 Reactions For SN 2 reactions we compare reactions of a strong nucleophile with a series of primary alkyl halides including allylic halides.

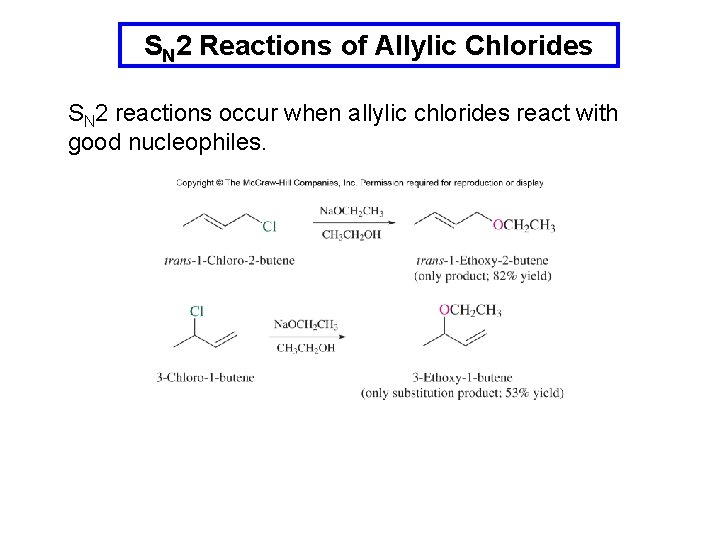
SN 2 Reactions of Allylic Chlorides SN 2 reactions occur when allylic chlorides react with good nucleophiles.

Allylic Free-Radical Halogenation

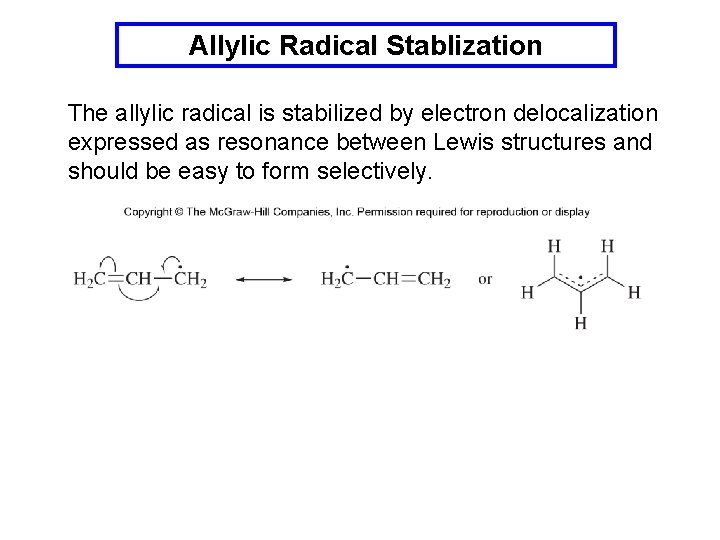
Allylic Radical Stablization The allylic radical is stabilized by electron delocalization expressed as resonance between Lewis structures and should be easy to form selectively.

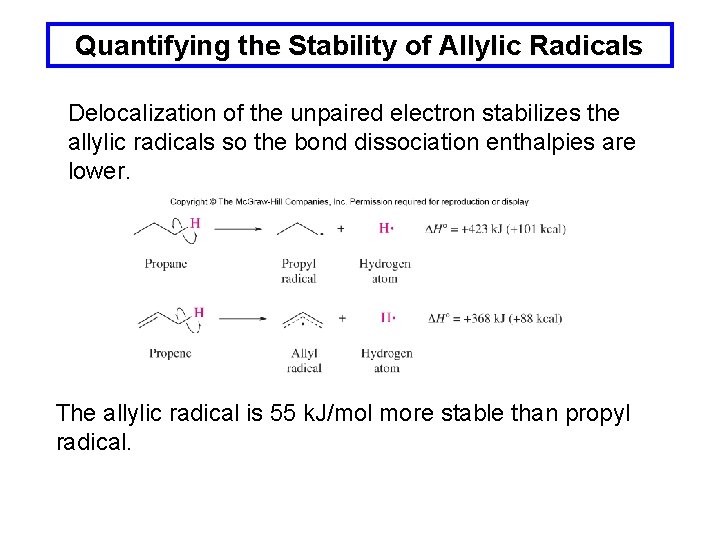
Quantifying the Stability of Allylic Radicals Delocalization of the unpaired electron stabilizes the allylic radicals so the bond dissociation enthalpies are lower. The allylic radical is 55 k. J/mol more stable than propyl radical.

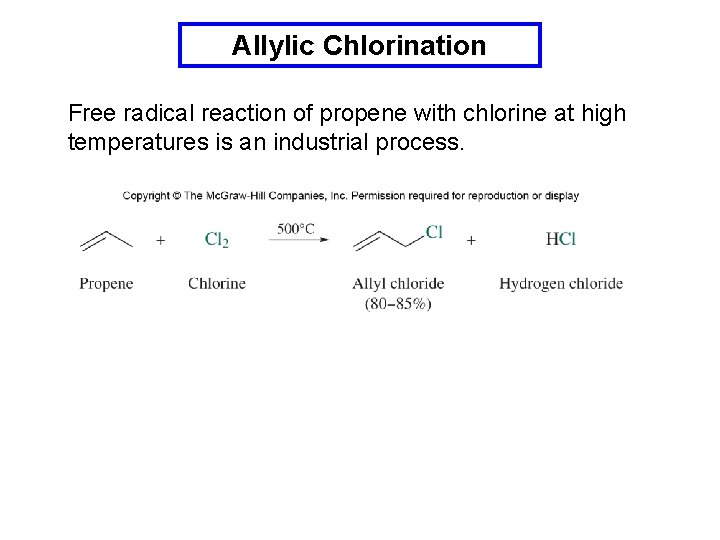
Allylic Chlorination Free radical reaction of propene with chlorine at high temperatures is an industrial process.

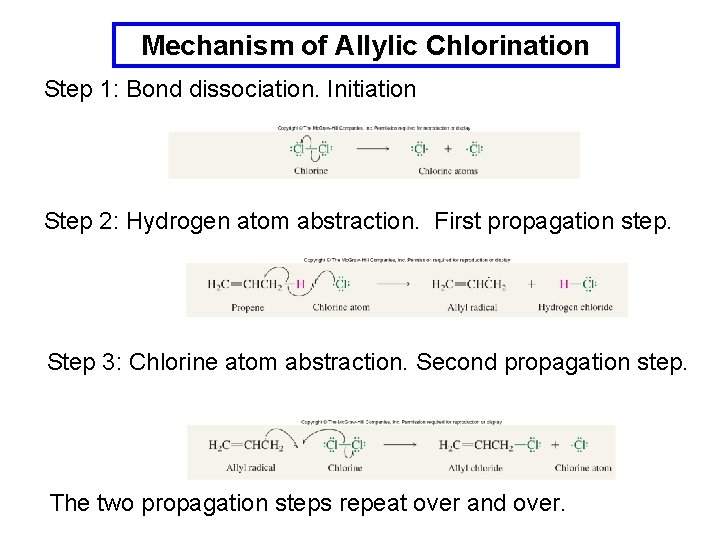
Mechanism of Allylic Chlorination Step 1: Bond dissociation. Initiation Step 2: Hydrogen atom abstraction. First propagation step. Step 3: Chlorine atom abstraction. Second propagation step. The two propagation steps repeat over and over.

Allylic Halogenation Allylic brominations are usually carried out with N-bromosuccinimide.

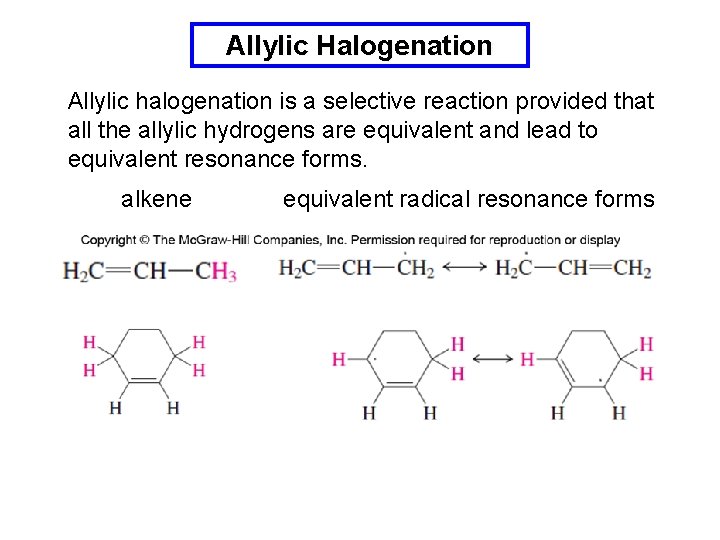
Allylic Halogenation Allylic halogenation is a selective reaction provided that all the allylic hydrogens are equivalent and lead to equivalent resonance forms. alkene equivalent radical resonance forms

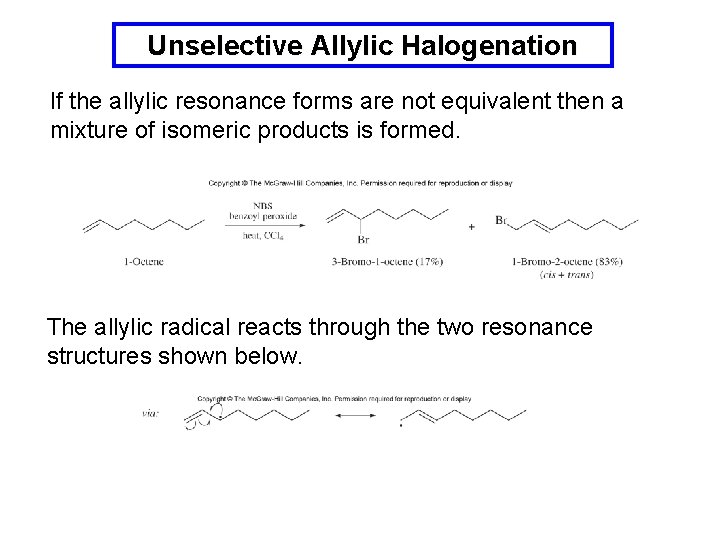
Unselective Allylic Halogenation If the allylic resonance forms are not equivalent then a mixture of isomeric products is formed. The allylic radical reacts through the two resonance structures shown below.

Allylic Anions

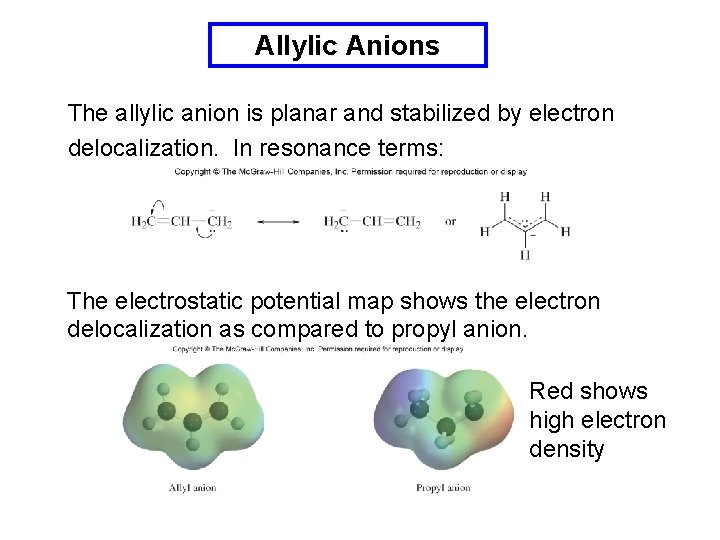
Allylic Anions The allylic anion is planar and stabilized by electron delocalization. In resonance terms: The electrostatic potential map shows the electron delocalization as compared to propyl anion. Red shows high electron density

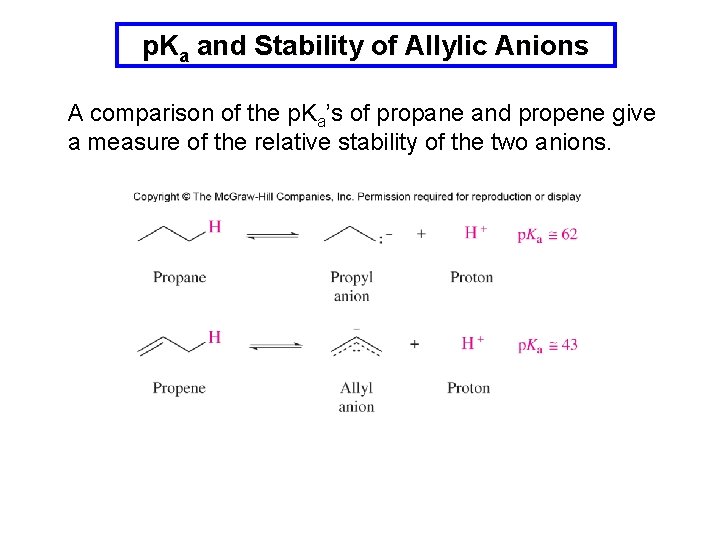
p. Ka and Stability of Allylic Anions A comparison of the p. Ka’s of propane and propene give a measure of the relative stability of the two anions.

Classes of Dienes: Conjugated and Otherwise

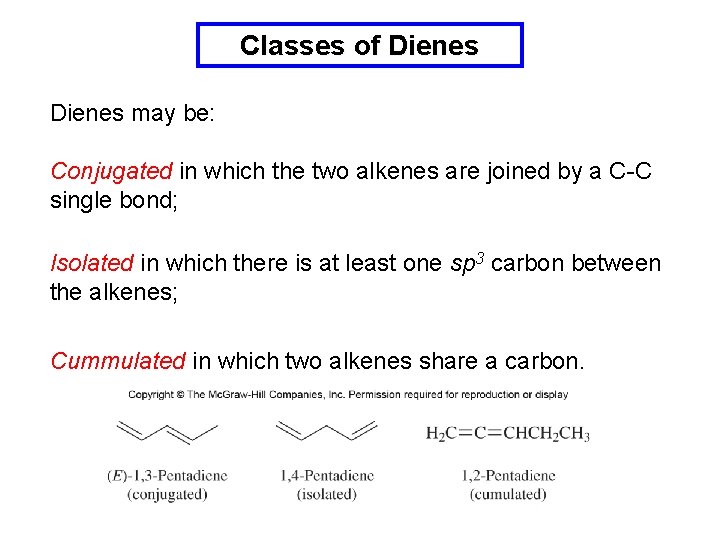
Classes of Dienes may be: Conjugated in which the two alkenes are joined by a C-C single bond; Isolated in which there is at least one sp 3 carbon between the alkenes; Cummulated in which two alkenes share a carbon.

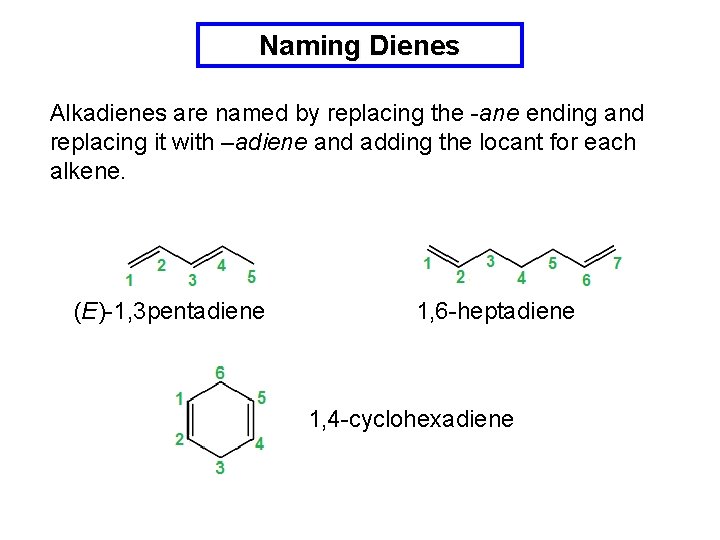
Naming Dienes Alkadienes are named by replacing the -ane ending and replacing it with –adiene and adding the locant for each alkene. (E)-1, 3 pentadiene 1, 6 -heptadiene 1, 4 -cyclohexadiene

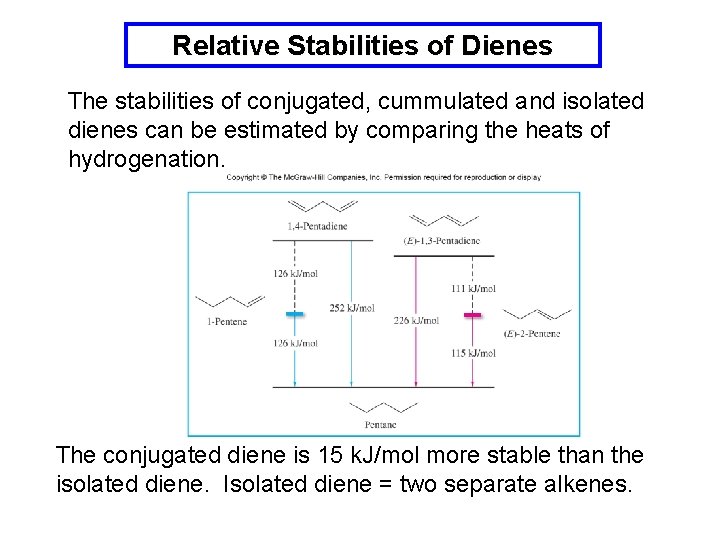
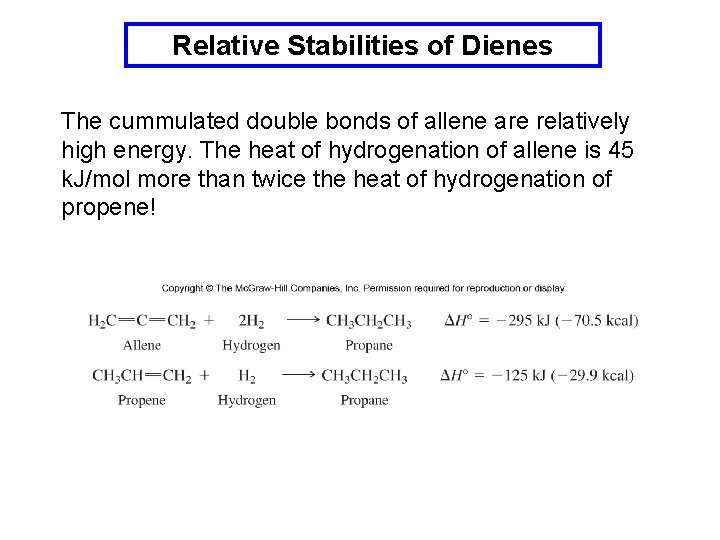
Relative Stabilities of Dienes The stabilities of conjugated, cummulated and isolated dienes can be estimated by comparing the heats of hydrogenation. The conjugated diene is 15 k. J/mol more stable than the isolated diene. Isolated diene = two separate alkenes.

Relative Stabilities of Dienes The cummulated double bonds of allene are relatively high energy. The heat of hydrogenation of allene is 45 k. J/mol more than twice the heat of hydrogenation of propene!

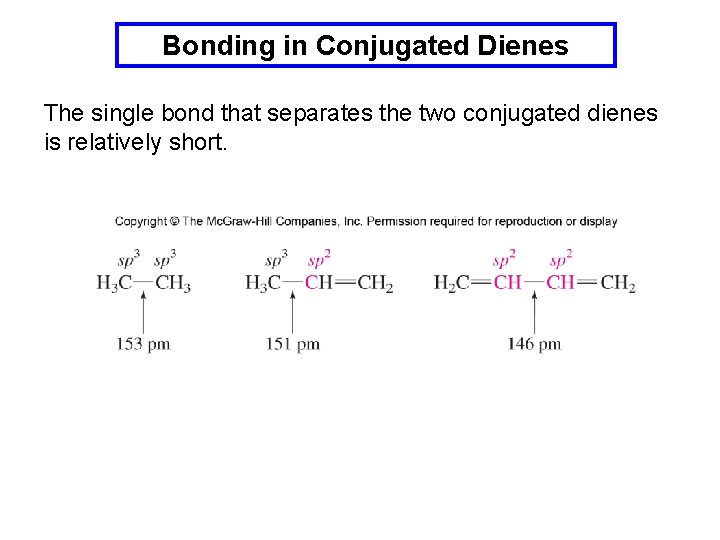
Bonding in Conjugated Dienes The single bond that separates the two conjugated dienes is relatively short.

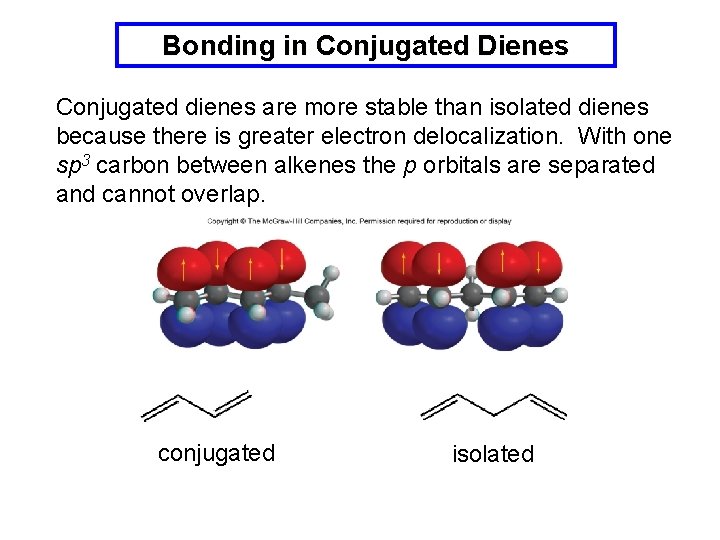
Bonding in Conjugated Dienes Conjugated dienes are more stable than isolated dienes because there is greater electron delocalization. With one sp 3 carbon between alkenes the p orbitals are separated and cannot overlap. conjugated isolated

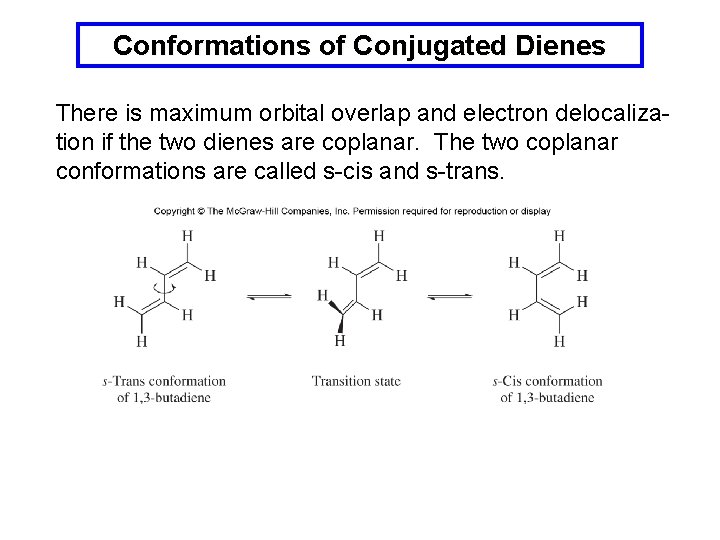
Conformations of Conjugated Dienes There is maximum orbital overlap and electron delocalization if the two dienes are coplanar. The two coplanar conformations are called s-cis and s-trans.

Bonding in Allenes

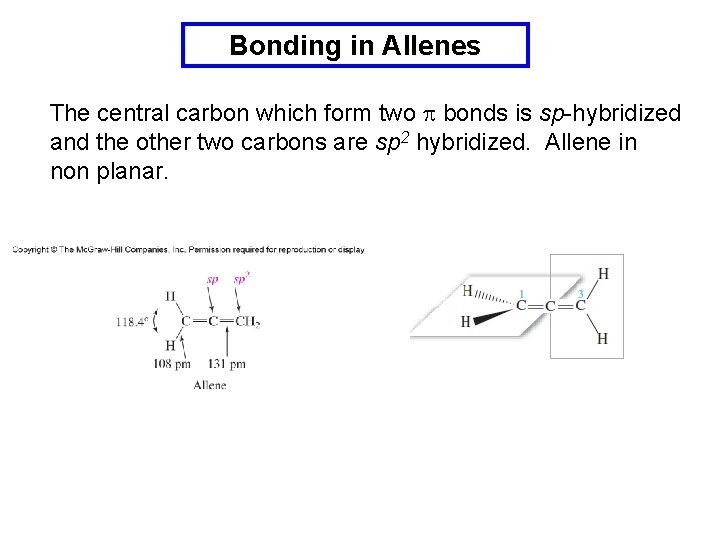
Bonding in Allenes The central carbon which form two p bonds is sp-hybridized and the other two carbons are sp 2 hybridized. Allene in non planar.

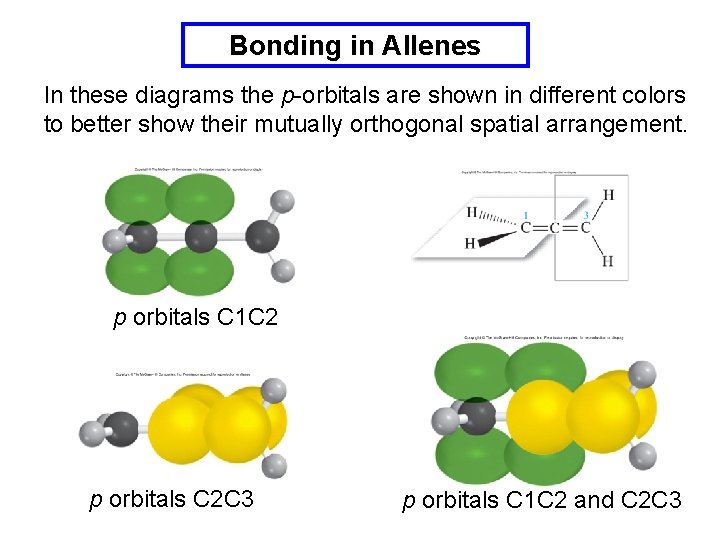
Bonding in Allenes In these diagrams the p-orbitals are shown in different colors to better show their mutually orthogonal spatial arrangement. p orbitals C 1 C 2 p orbitals C 2 C 3 p orbitals C 1 C 2 and C 2 C 3

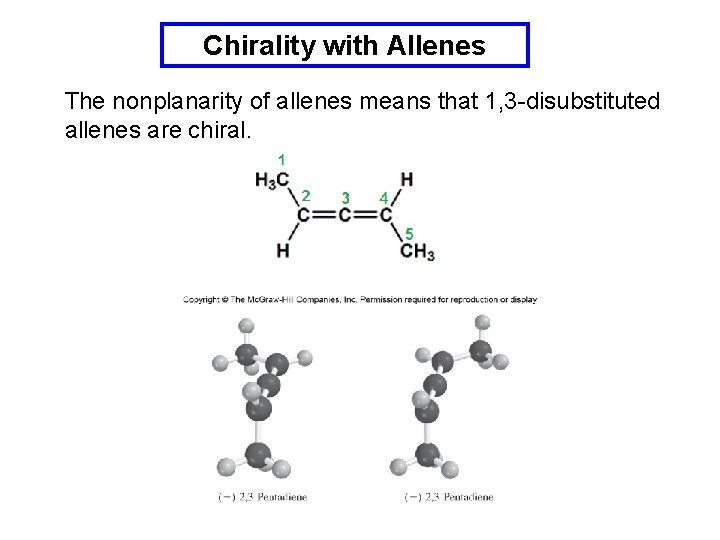
Chirality with Allenes The nonplanarity of allenes means that 1, 3 -disubstituted allenes are chiral.

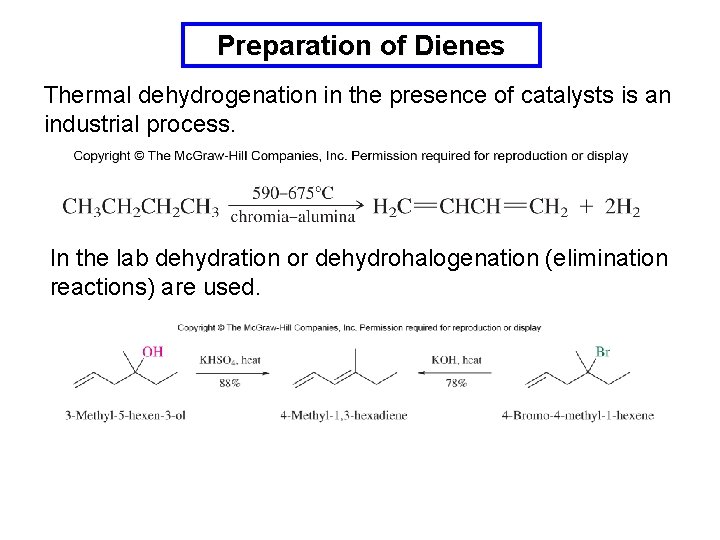
Preparation of Dienes Thermal dehydrogenation in the presence of catalysts is an industrial process. In the lab dehydration or dehydrohalogenation (elimination reactions) are used.

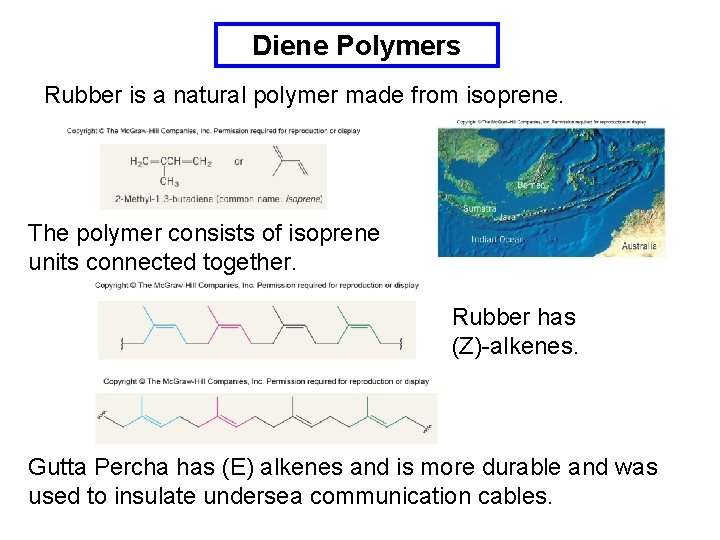
Diene Polymers Rubber is a natural polymer made from isoprene. The polymer consists of isoprene units connected together. Rubber has (Z)-alkenes. Gutta Percha has (E) alkenes and is more durable and was used to insulate undersea communication cables.

Addition of Hydrogen Halides to Conjugated Dienes

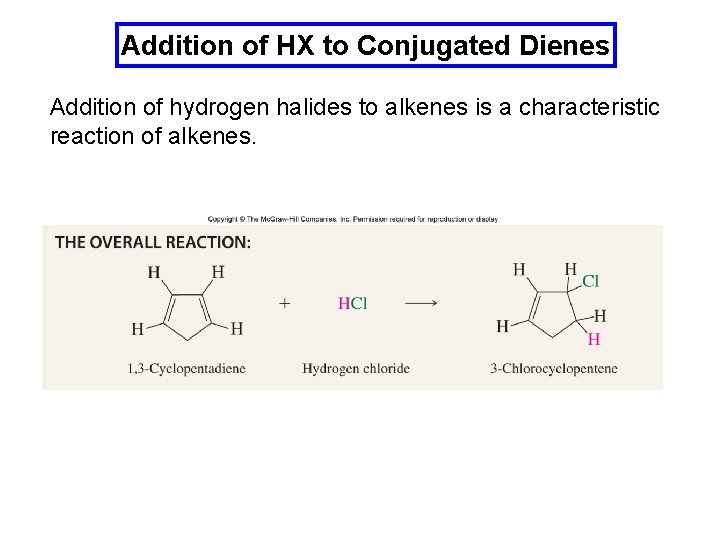
Addition of HX to Conjugated Dienes Addition of hydrogen halides to alkenes is a characteristic reaction of alkenes.

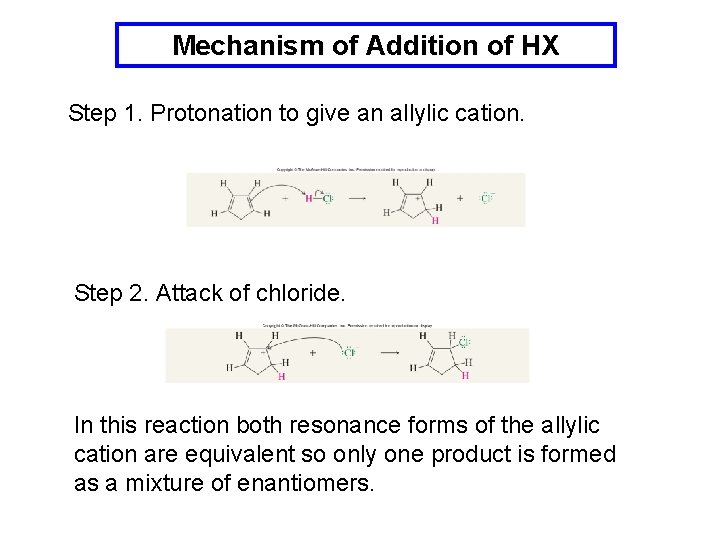
Mechanism of Addition of HX Step 1. Protonation to give an allylic cation. Step 2. Attack of chloride. In this reaction both resonance forms of the allylic cation are equivalent so only one product is formed as a mixture of enantiomers.

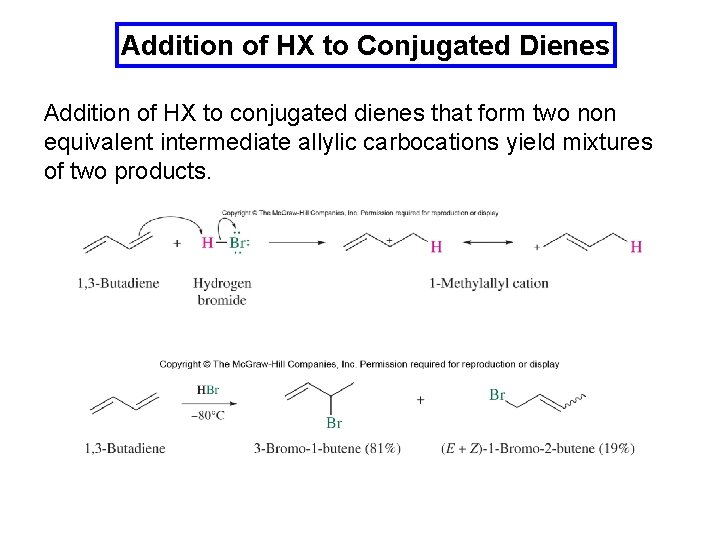
Addition of HX to Conjugated Dienes Addition of HX to conjugated dienes that form two non equivalent intermediate allylic carbocations yield mixtures of two products.

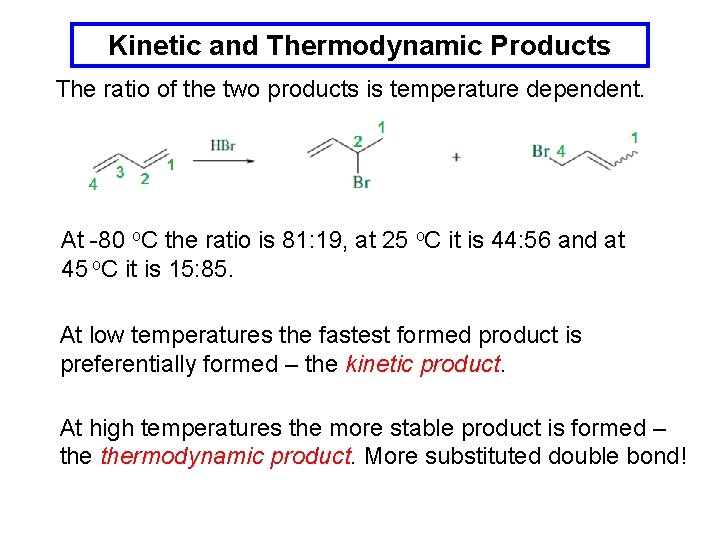
Kinetic and Thermodynamic Products The ratio of the two products is temperature dependent. At -80 o. C the ratio is 81: 19, at 25 o. C it is 44: 56 and at 45 o. C it is 15: 85. At low temperatures the fastest formed product is preferentially formed – the kinetic product. At high temperatures the more stable product is formed – thermodynamic product. More substituted double bond!

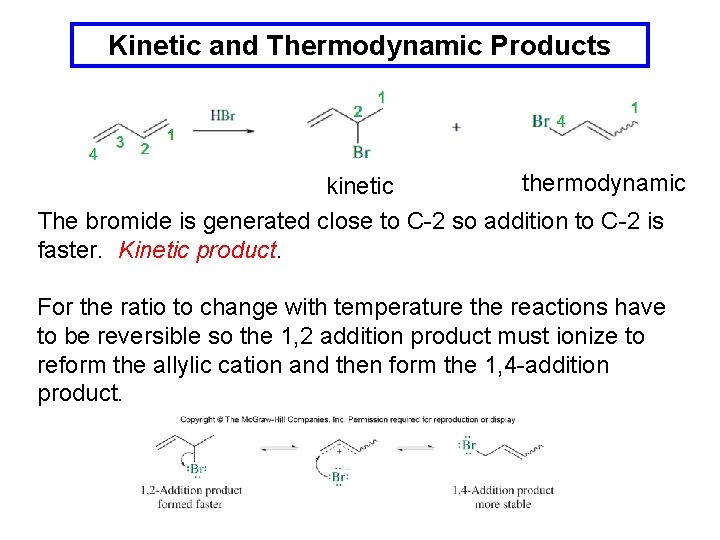
Kinetic and Thermodynamic Products thermodynamic kinetic The bromide is generated close to C-2 so addition to C-2 is faster. Kinetic product. For the ratio to change with temperature the reactions have to be reversible so the 1, 2 addition product must ionize to reform the allylic cation and then form the 1, 4 -addition product.

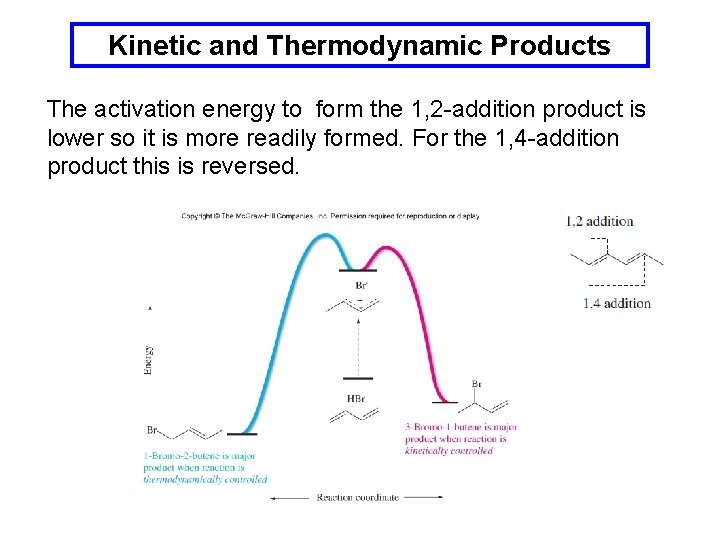
Kinetic and Thermodynamic Products The activation energy to form the 1, 2 -addition product is lower so it is more readily formed. For the 1, 4 -addition product this is reversed.

Halogen Addition to Dienes

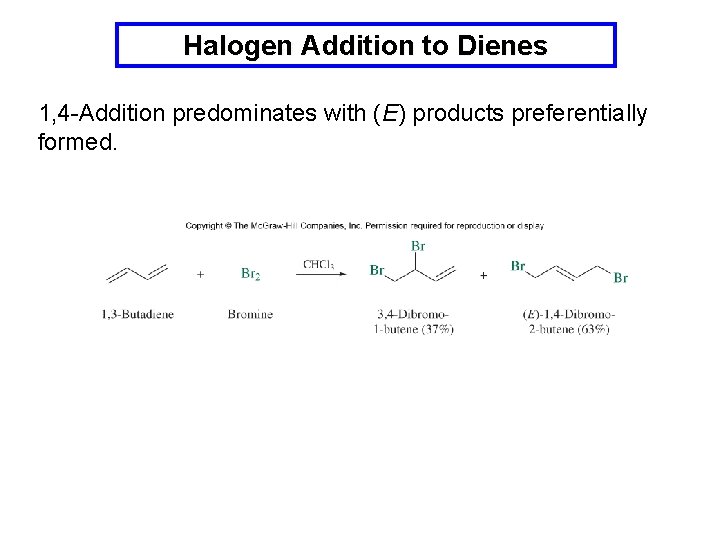
Halogen Addition to Dienes 1, 4 -Addition predominates with (E) products preferentially formed.

The Diels Alder Reaction

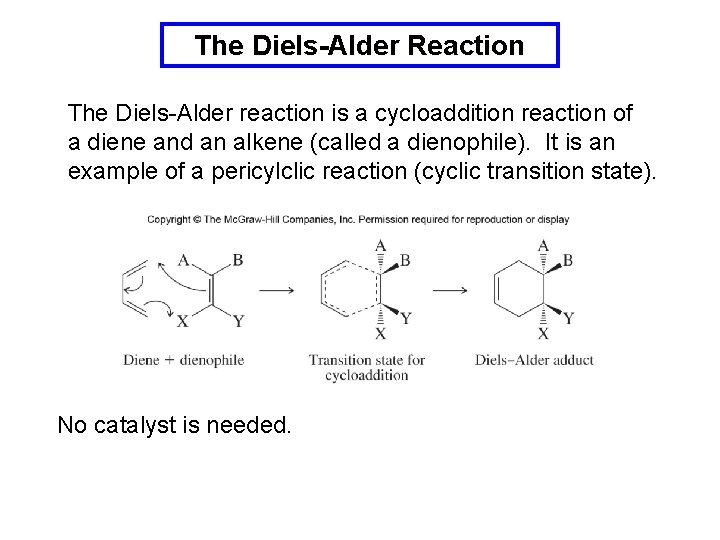
The Diels-Alder Reaction The Diels-Alder reaction is a cycloaddition reaction of a diene and an alkene (called a dienophile). It is an example of a pericylclic reaction (cyclic transition state). No catalyst is needed.

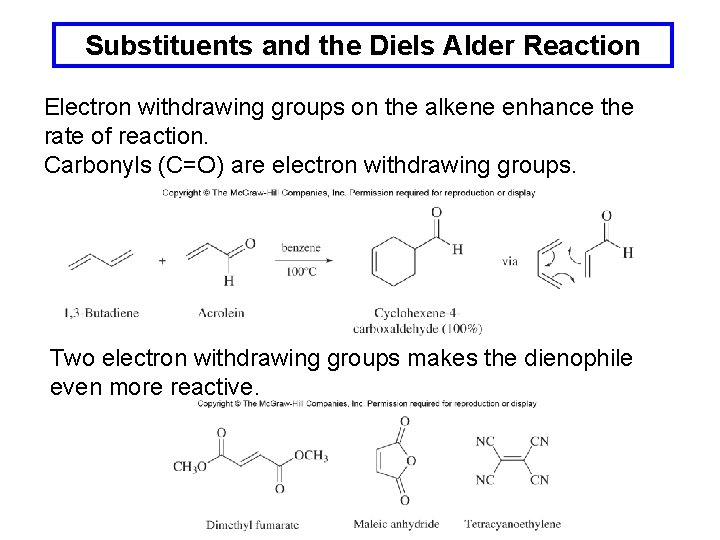
Substituents and the Diels Alder Reaction Electron withdrawing groups on the alkene enhance the rate of reaction. Carbonyls (C=O) are electron withdrawing groups. Two electron withdrawing groups makes the dienophile even more reactive.

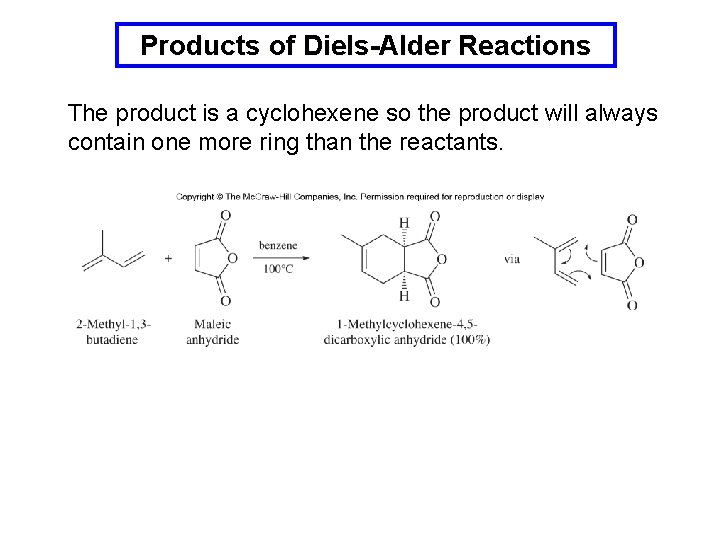
Products of Diels-Alder Reactions The product is a cyclohexene so the product will always contain one more ring than the reactants.

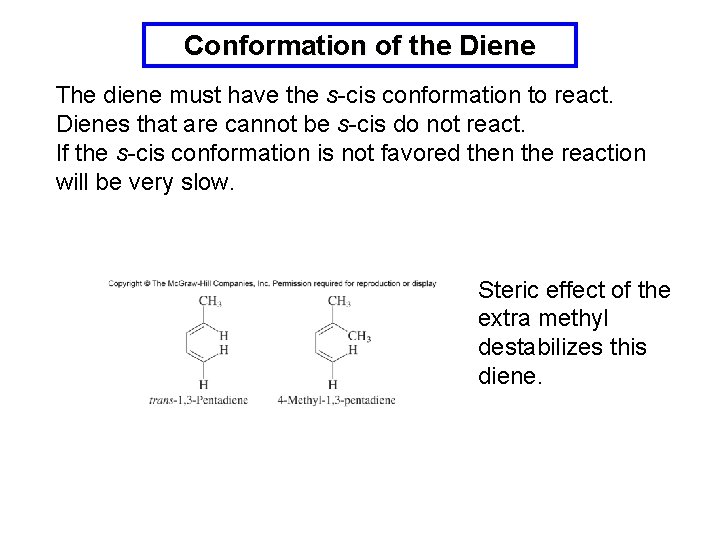
Conformation of the Diene The diene must have the s-cis conformation to react. Dienes that are cannot be s-cis do not react. If the s-cis conformation is not favored then the reaction will be very slow. Steric effect of the extra methyl destabilizes this diene.

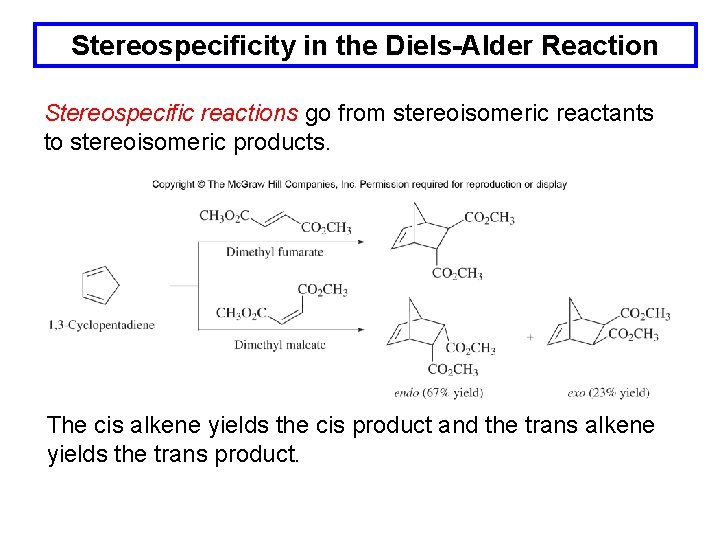
Stereospecificity in the Diels-Alder Reaction Stereospecific reactions go from stereoisomeric reactants to stereoisomeric products. The cis alkene yields the cis product and the trans alkene yields the trans product.

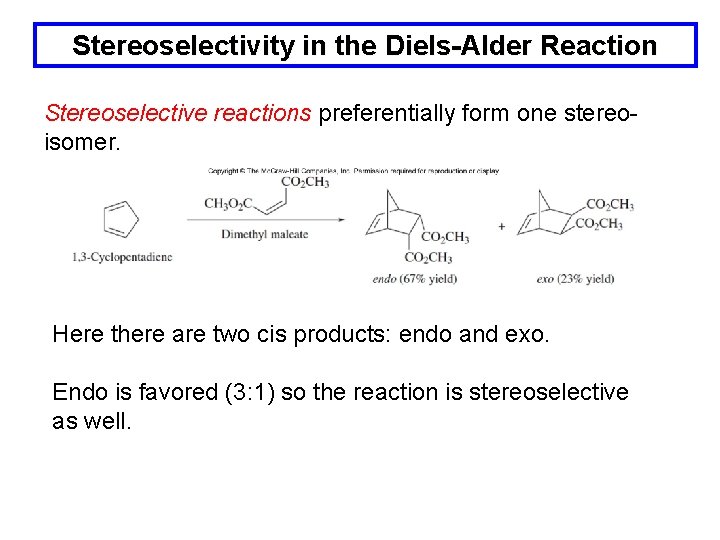
Stereoselectivity in the Diels-Alder Reaction Stereoselective reactions preferentially form one stereoisomer. Here there are two cis products: endo and exo. Endo is favored (3: 1) so the reaction is stereoselective as well.

Retrosynthetic Analysis and the Diels-Alder Reaction

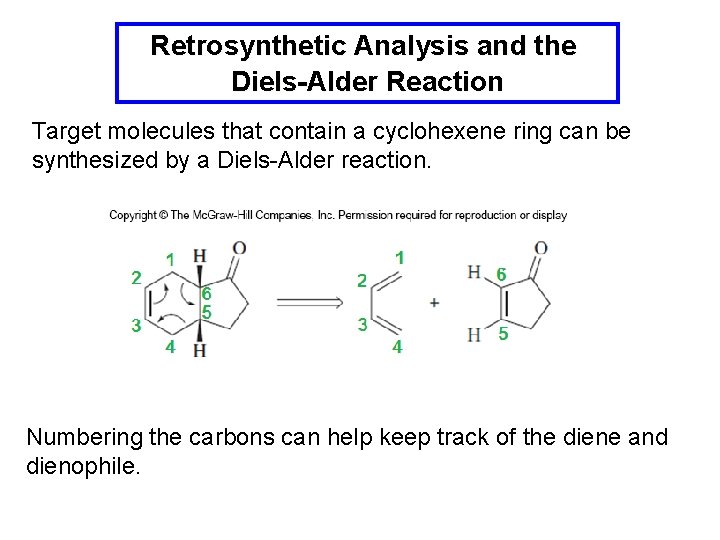
Retrosynthetic Analysis and the Diels-Alder Reaction Target molecules that contain a cyclohexene ring can be synthesized by a Diels-Alder reaction. Numbering the carbons can help keep track of the diene and dienophile.

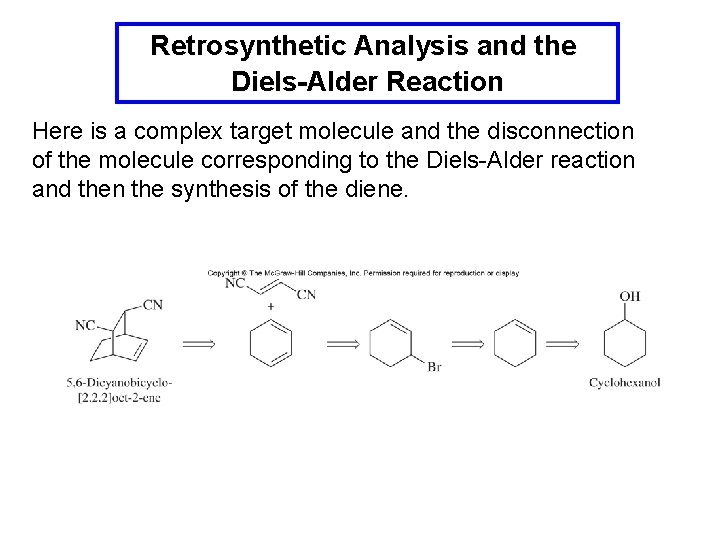
Retrosynthetic Analysis and the Diels-Alder Reaction Here is a complex target molecule and the disconnection of the molecule corresponding to the Diels-Alder reaction and then the synthesis of the diene.
 Allylic lone pair resonance
Allylic lone pair resonance Allylic carbon
Allylic carbon Nbs allylic bromination
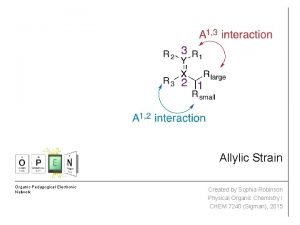
Nbs allylic bromination Allylic strain
Allylic strain Allylic lone pair
Allylic lone pair Decision support systems and intelligent systems
Decision support systems and intelligent systems Principles of complex systems for systems engineering
Principles of complex systems for systems engineering Embedded systems vs cyber physical systems
Embedded systems vs cyber physical systems Elegant systems
Elegant systems Linking vowel to vowel
Linking vowel to vowel Conoscere vs sapere
Conoscere vs sapere Reflexive levantarse
Reflexive levantarse Spanish future tense endings
Spanish future tense endings Aprender conjugation chart
Aprender conjugation chart Caerse preterite
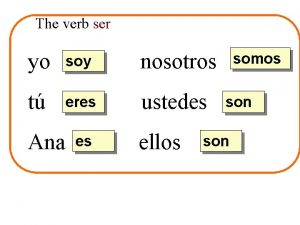
Caerse preterite Eres verbo ser
Eres verbo ser Latin conjugations 1-4
Latin conjugations 1-4 Ser yo soy tu eres
Ser yo soy tu eres Viajar ar verbs
Viajar ar verbs En un sándwich, prefiero
En un sándwich, prefiero Reciclar future tense
Reciclar future tense Conditional simple spanish
Conditional simple spanish Surd from
Surd from Ar conjugation chart
Ar conjugation chart Take conjugation
Take conjugation Audio conjugation
Audio conjugation Vivir present tense
Vivir present tense Vocare conjugation
Vocare conjugation Pintarse reflexive conjugation
Pintarse reflexive conjugation Linking verb chart
Linking verb chart Aggiungi le forme mancanti dei verbi riflessivi indicati.
Aggiungi le forme mancanti dei verbi riflessivi indicati. Sonreir stem change
Sonreir stem change Sacar present subjunctive
Sacar present subjunctive Glucuronide conjugation
Glucuronide conjugation Past tense of practicar
Past tense of practicar Cepillarse reflexive verb conjugation
Cepillarse reflexive verb conjugation Ser mandatos informales
Ser mandatos informales Mandatos informales ejemplos
Mandatos informales ejemplos Mandato meaning
Mandato meaning Repetir verb
Repetir verb Essere and avere
Essere and avere Futurus esse
Futurus esse Regular preterite endings
Regular preterite endings Ir conjugation
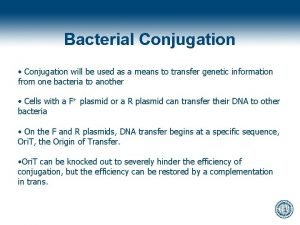
Ir conjugation Binary fission vs conjugation
Binary fission vs conjugation Se lever présent
Se lever présent Presact
Presact Sienten preterite
Sienten preterite Be estar
Be estar Poser conjugation
Poser conjugation Double bond extending conjugation
Double bond extending conjugation Tener que conjugation
Tener que conjugation Miran conjugation
Miran conjugation Vestirse conjugation preterite
Vestirse conjugation preterite Re verb french
Re verb french Ir verb conjugation french
Ir verb conjugation french Conjugating er verbs
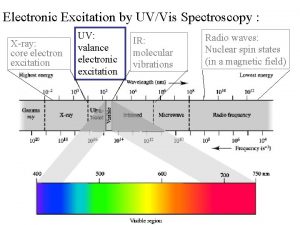
Conjugating er verbs Uv spectra of dienes
Uv spectra of dienes