Chapter 10 Conjugation in Alkadienes and Allylic Systems








- Slides: 40

Chapter 10 Conjugation in Alkadienes and Allylic Systems conjugare is a Latin verb meaning "to link or yoke together"

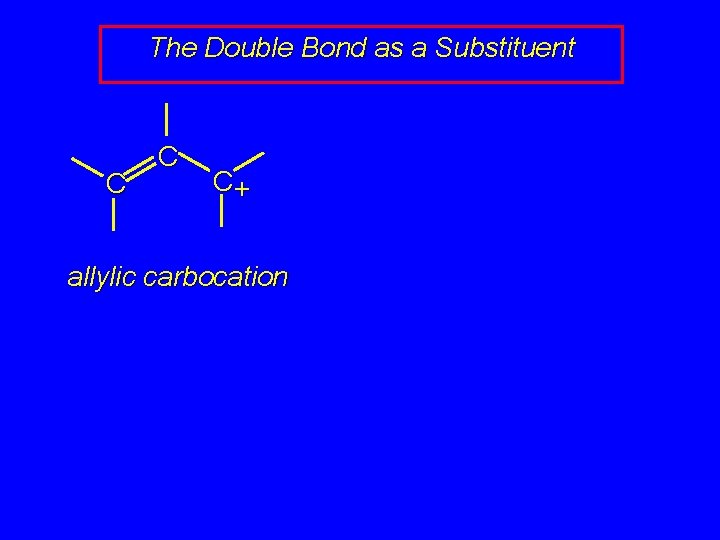
The Double Bond as a Substituent C C C+ allylic carbocation

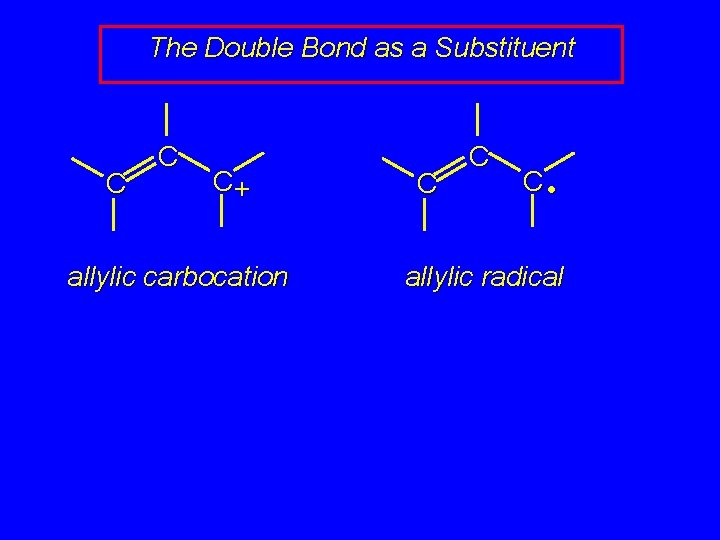
The Double Bond as a Substituent C C C+ allylic carbocation C C C • allylic radical

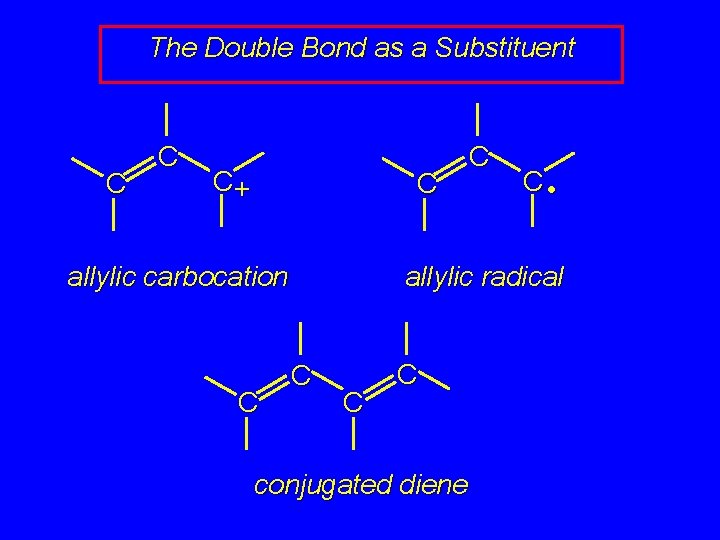
The Double Bond as a Substituent C C C+ C allylic carbocation C C C • allylic radical C C C conjugated diene

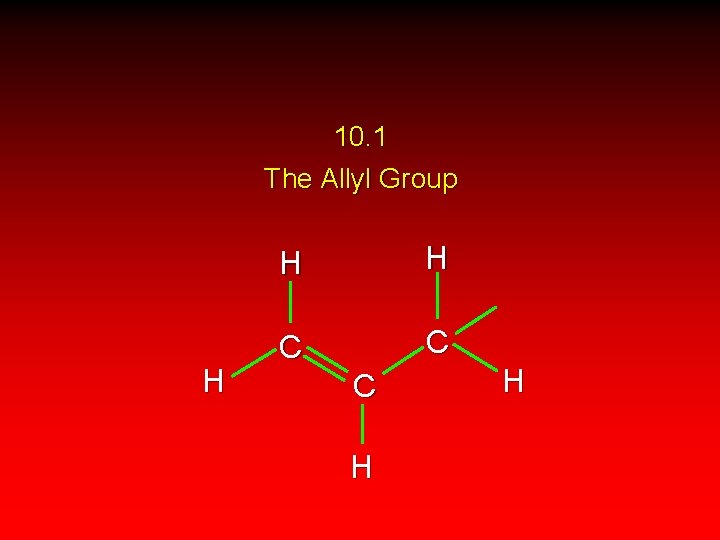
10. 1 The Allyl Group H H H C C C H H

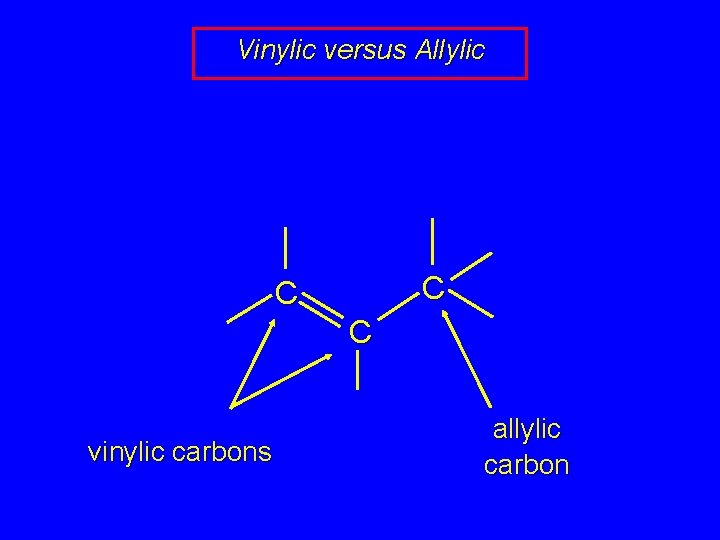
Vinylic versus Allylic C C C vinylic carbons allylic carbon

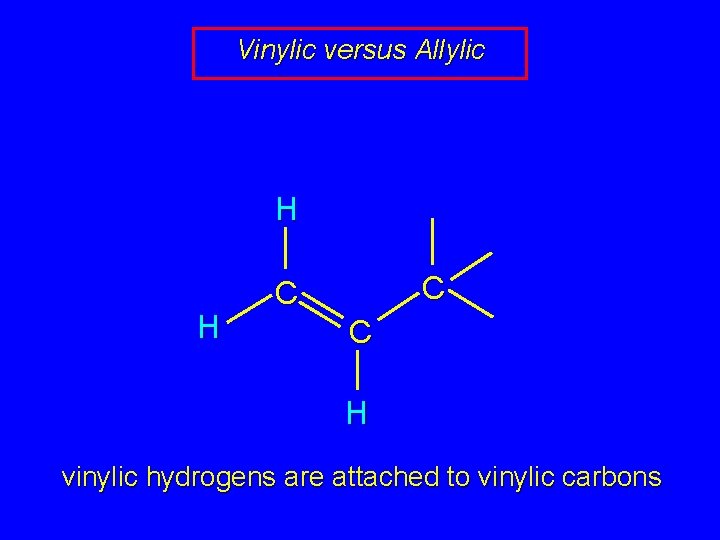
Vinylic versus Allylic H H C C C H vinylic hydrogens are attached to vinylic carbons

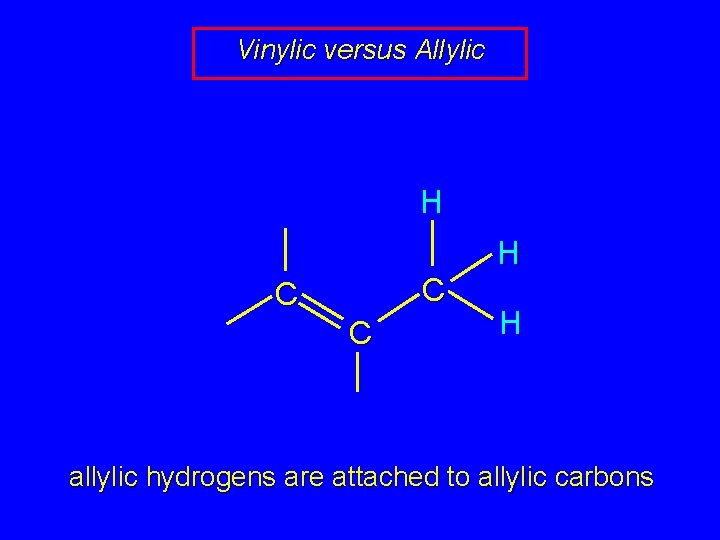
Vinylic versus Allylic H C C C H H allylic hydrogens are attached to allylic carbons

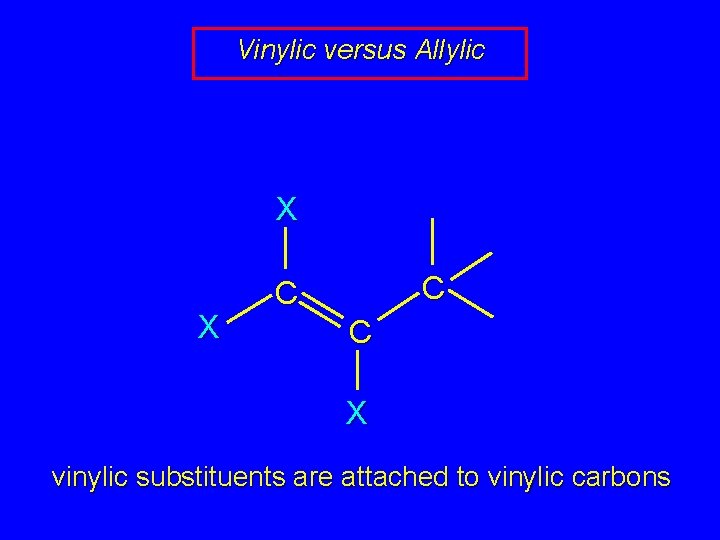
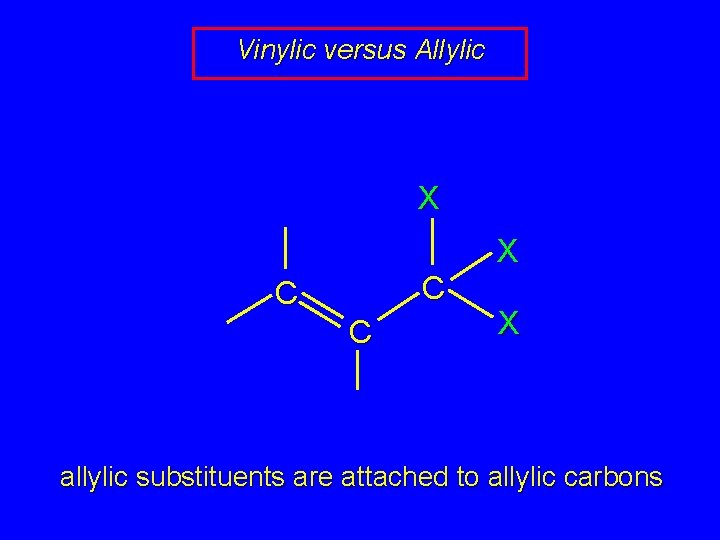
Vinylic versus Allylic X X C C C X vinylic substituents are attached to vinylic carbons

Vinylic versus Allylic X X C C C X allylic substituents are attached to allylic carbons

10. 2 Allylic Carbocations C C C +

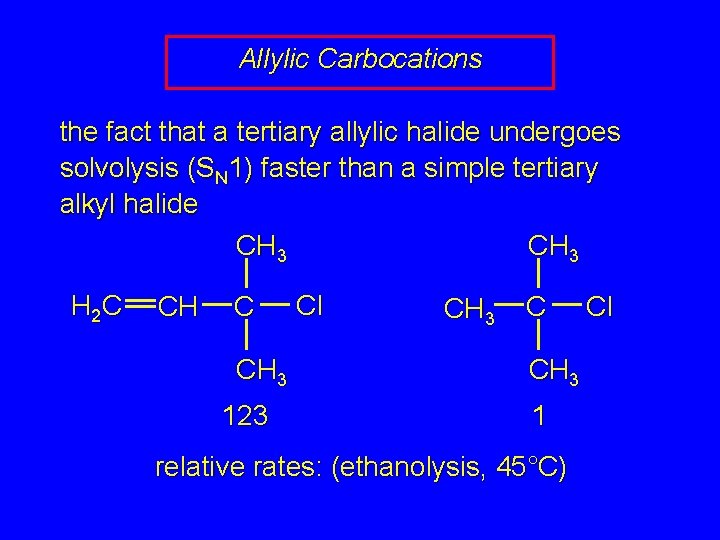
Allylic Carbocations the fact that a tertiary allylic halide undergoes solvolysis (SN 1) faster than a simple tertiary alkyl halide CH 3 H 2 C CH 3 123 Cl CH 3 C CH 3 1 relative rates: (ethanolysis, 45°C) Cl

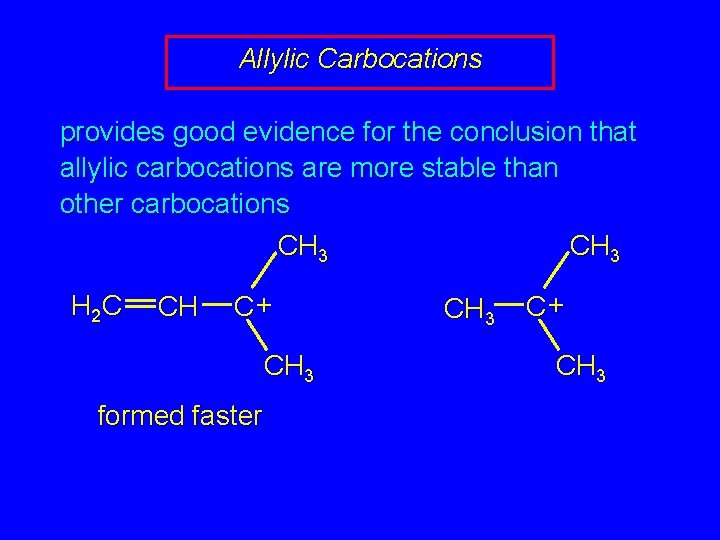
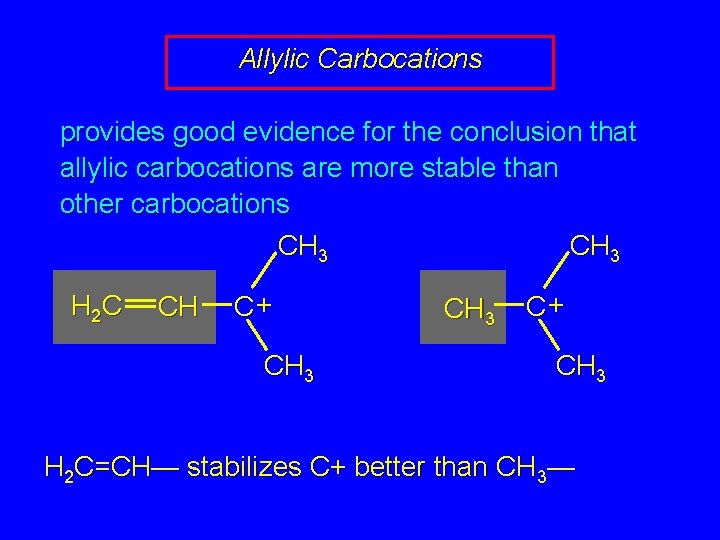
Allylic Carbocations provides good evidence for the conclusion that allylic carbocations are more stable than other carbocations CH 3 H 2 C CH C+ CH 3 formed faster CH 3 C+ CH 3

Allylic Carbocations provides good evidence for the conclusion that allylic carbocations are more stable than other carbocations CH 3 H 2 C CH C+ CH 3 H 2 C=CH— stabilizes C+ better than CH 3—

Stabilization of Allylic Carbocations Delocalization of electrons in the double bond stabilizes the carbocation resonance model orbital overlap model

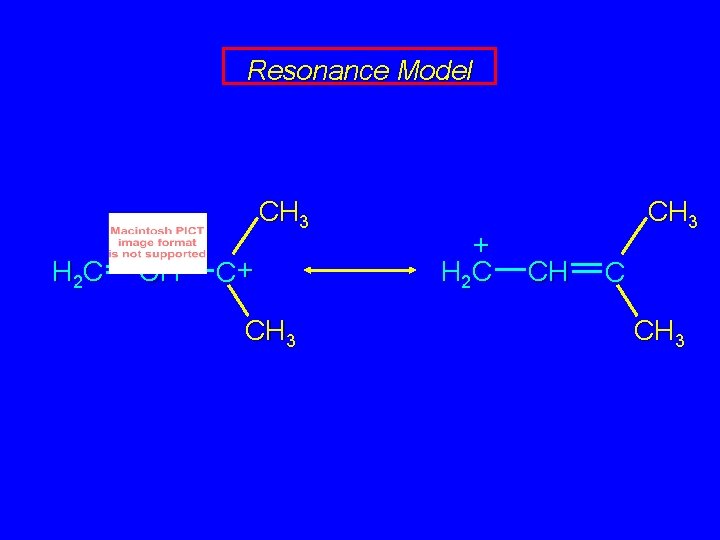
Resonance Model CH 3 H 2 C CH C+ CH 3 + H 2 C CH 3 CH C CH 3

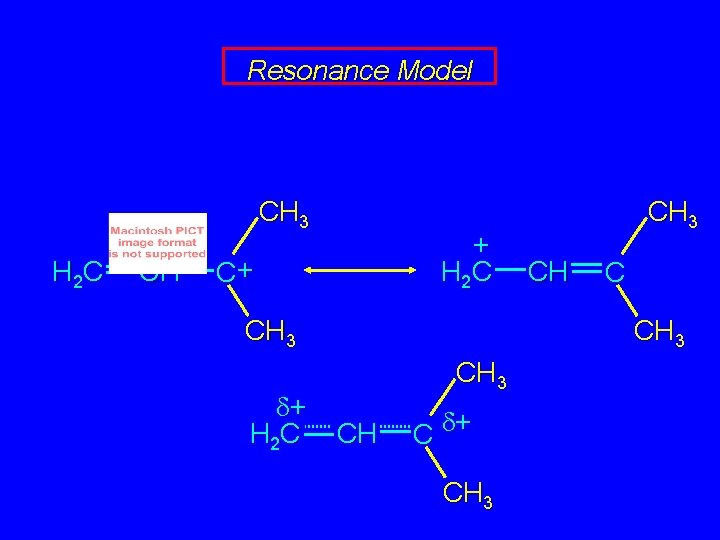
Resonance Model CH 3 H 2 C CH + H 2 C C+ CH 3 d+ H 2 C CH 3 CH C d+ CH 3

Orbital Overlap Model d+ d+

Orbital Overlap Model

Orbital Overlap Model

Orbital Overlap Model

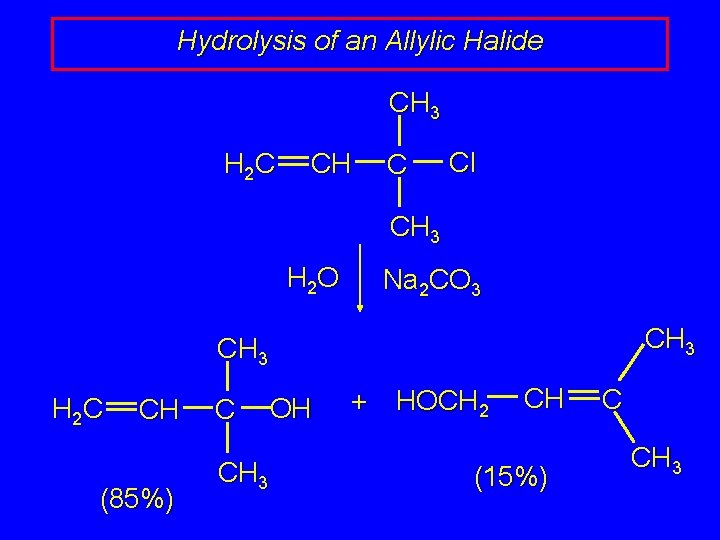
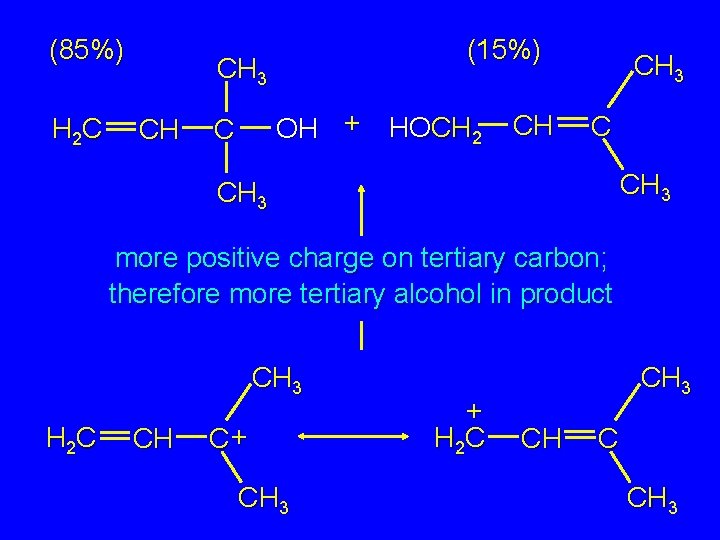
Hydrolysis of an Allylic Halide CH 3 H 2 C CH C Cl CH 3 H 2 O Na 2 CO 3 CH 3 H 2 C CH (85%) C CH 3 OH + HOCH 2 CH (15%) C CH 3

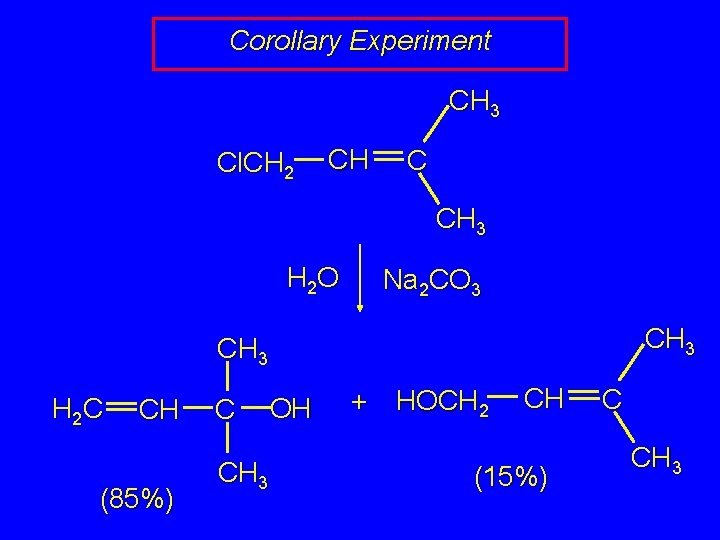
Corollary Experiment CH 3 Cl. CH 2 CH C CH 3 H 2 O Na 2 CO 3 CH 3 H 2 C CH (85%) C CH 3 OH + HOCH 2 CH (15%) C CH 3

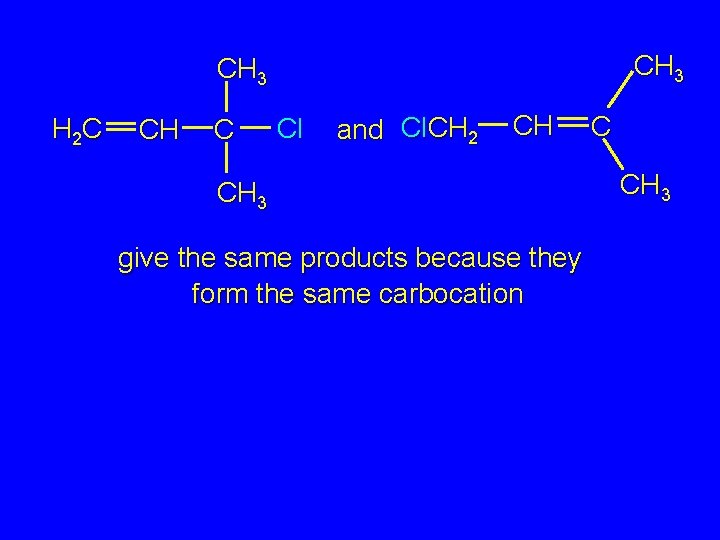
CH 3 H 2 C CH C Cl and Cl. CH 2 CH CH 3 give the same products because they form the same carbocation C CH 3

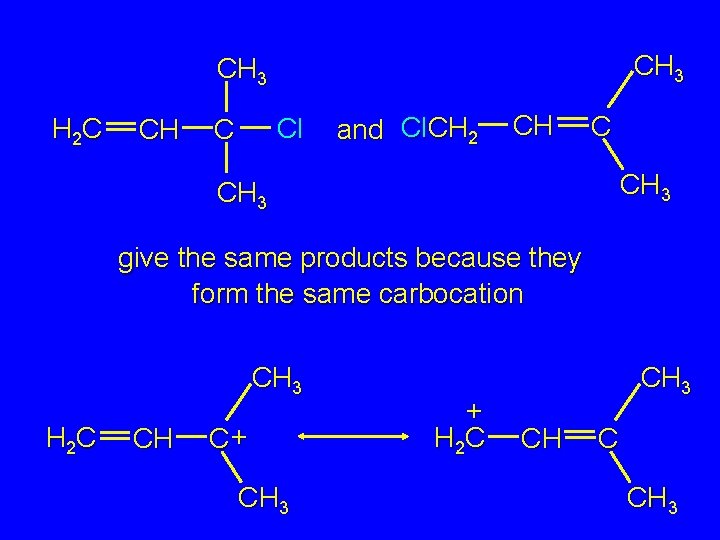
CH 3 H 2 C CH Cl C and Cl. CH 2 CH C CH 3 give the same products because they form the same carbocation CH 3 H 2 C CH C+ CH 3 + H 2 C CH 3 CH C CH 3

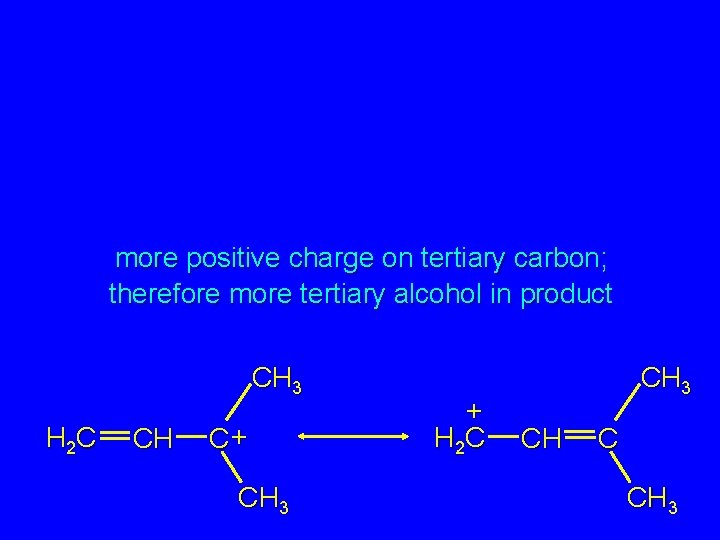
more positive charge on tertiary carbon; therefore more tertiary alcohol in product CH 3 H 2 C CH C+ CH 3 + H 2 C CH 3 CH C CH 3

(85%) H 2 C (15%) CH 3 CH OH + HOCH 2 C CH CH 3 more positive charge on tertiary carbon; therefore more tertiary alcohol in product CH 3 H 2 C CH C+ CH 3 + H 2 C CH 3 CH C CH 3

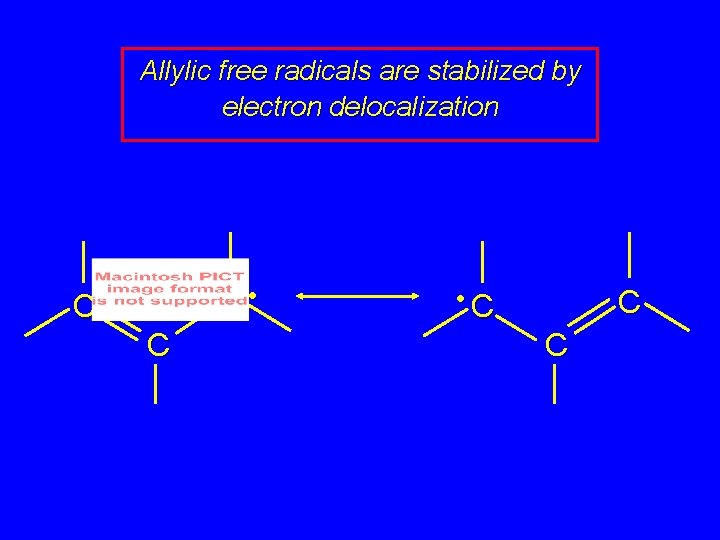
10. 3 Allylic Free Radicals C • C C

Allylic free radicals are stabilized by electron delocalization C • C C • C C C

Free-radical stabilities are related to bond-dissociation energies CH 3 CH 2—H H 2 C CHCH 2—H 410 k. J/mol • CH 3 CH 2 + H • 368 k. J/mol • CHCH 2 + H • H 2 C C—H bond is weaker in propene because resulting radical (allyl) is more stable than radical (propyl) from propane

10. 4 Allylic Halogenation

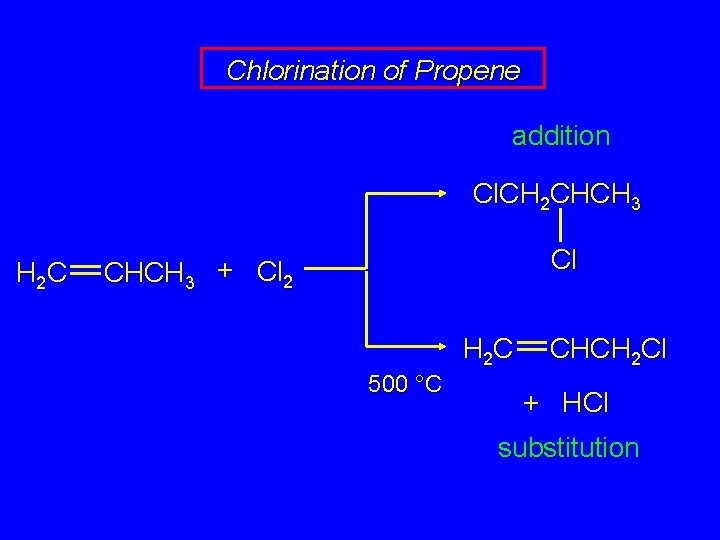
Chlorination of Propene addition Cl. CH 2 CHCH 3 H 2 C Cl CHCH 3 + Cl 2 H 2 C 500 °C CHCH 2 Cl + HCl substitution

Allylic Halogenation selective for replacement of allylic hydrogen free radical mechanism allylic radical is intermediate

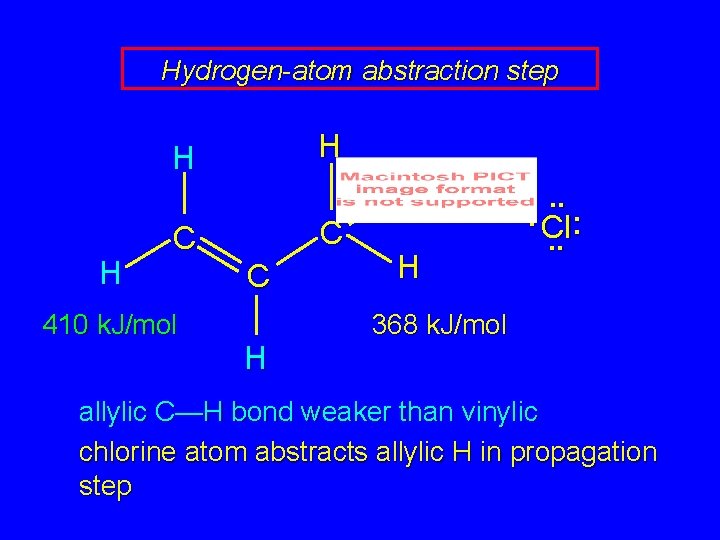
Hydrogen-atom abstraction step H H H C C 410 k. J/mol C H H H . . . Cl: . . 368 k. J/mol allylic C—H bond weaker than vinylic chlorine atom abstracts allylic H in propagation step

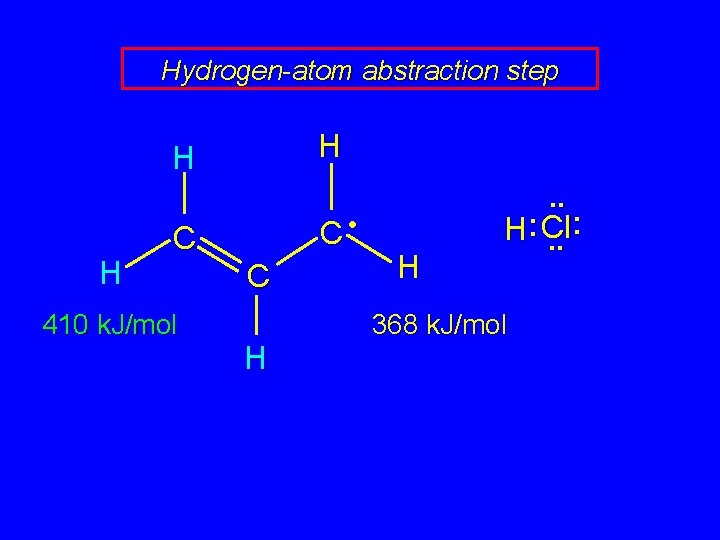
Hydrogen-atom abstraction step H H H C • C 410 k. J/mol C H H . . : H : Cl. . 368 k. J/mol

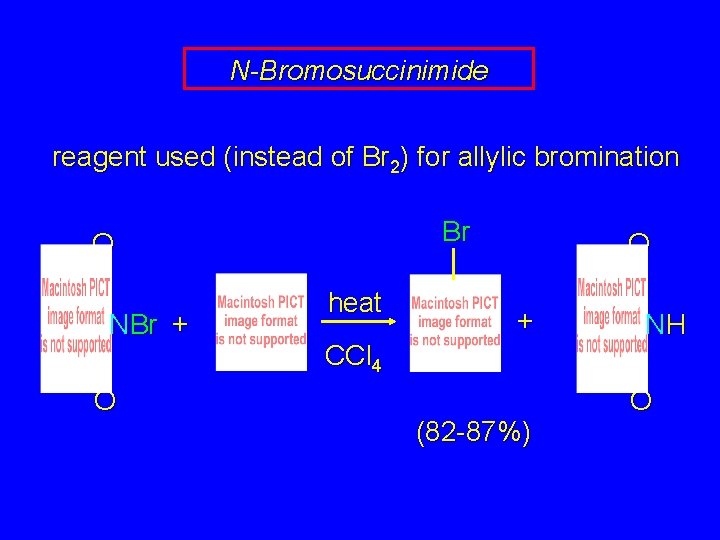
N-Bromosuccinimide reagent used (instead of Br 2) for allylic bromination Br O NBr + O heat O + CCl 4 (82 -87%) NH O

Limited Scope Allylic halogenation is only used when: all of the allylic hydrogens are equivalent and the resonance forms of allylic radical are equivalent

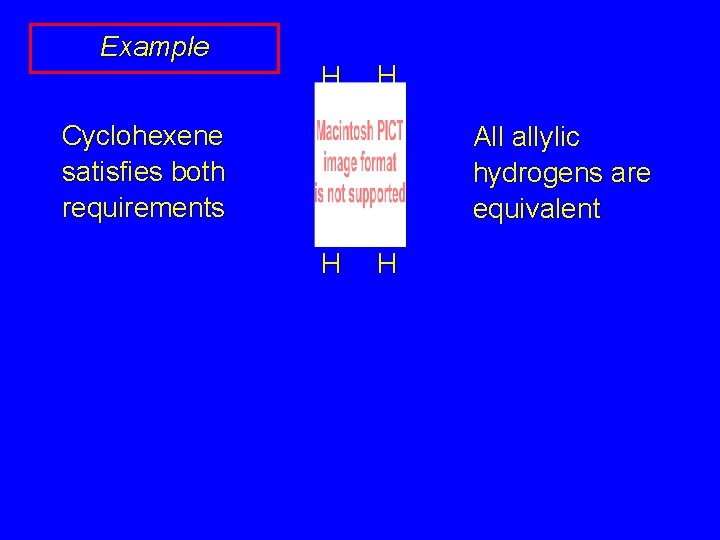
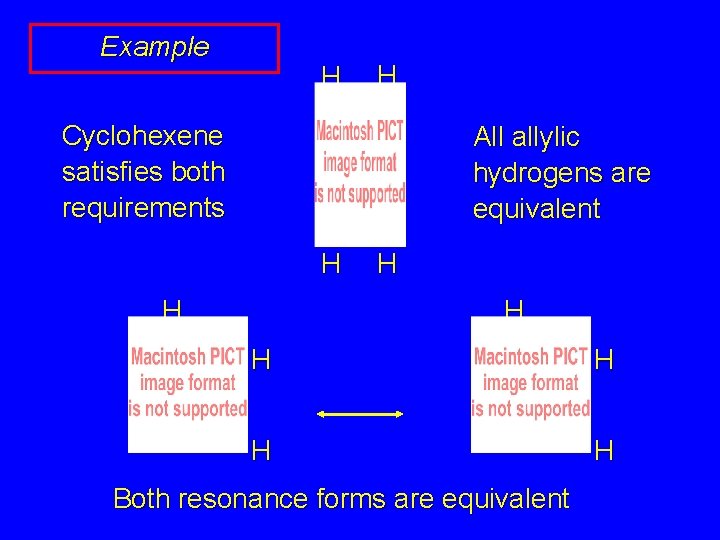
Example H H Cyclohexene satisfies both requirements All allylic hydrogens are equivalent H H

Example H H Cyclohexene satisfies both requirements All allylic hydrogens are equivalent H H • H H H • Both resonance forms are equivalent H

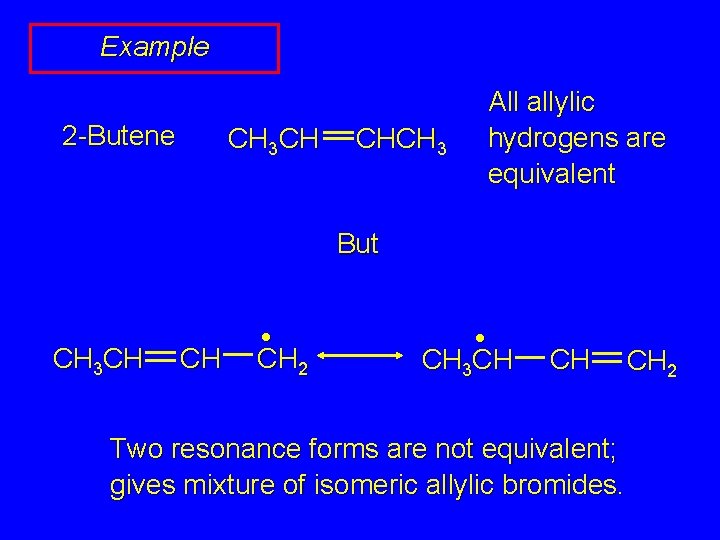
Example 2 -Butene CH 3 CH CHCH 3 All allylic hydrogens are equivalent But CH 3 CH CH • CH 2 • CH 3 CH CH Two resonance forms are not equivalent; gives mixture of isomeric allylic bromides. CH 2