Guidance on Cotrimoxazole Prophylactic Therapy for HIV ExposedInfected



















- Slides: 28

Guidance on Cotrimoxazole Prophylactic Therapy for HIV Exposed/Infected Children HAIVN Harvard Medical School AIDS Initiative in Vietnam 1

Learning Objectives By the end of this session, participants should be able to: n Explain indications of Cotrimoxazole Prophylactic Therapy (CPT) for HIVexposed and HIV-infected children n Describe clinical and testing follow up while taking CTX Prophylactic Therapy n Can explain when stop taking CTX Prophylactic Therapy 2

Introduction 3

OI Prophylactic Therapies n n n Anti-retrovirus treatment could prevent OIs Some medications (mainly CTX) have been proved that they could prevent OIs Counseling people who provide care for infected children: • Food hygiene • Good nutrition • Regular check up 4

Two Types of OI Prophylaxis Primary prophylaxis: n Giving medication to prevent an OI from occurring in the first place Secondary prophylaxis: n Giving medication after an OI is treated to prevent it from recurring n Also known as maintenance therapy 5

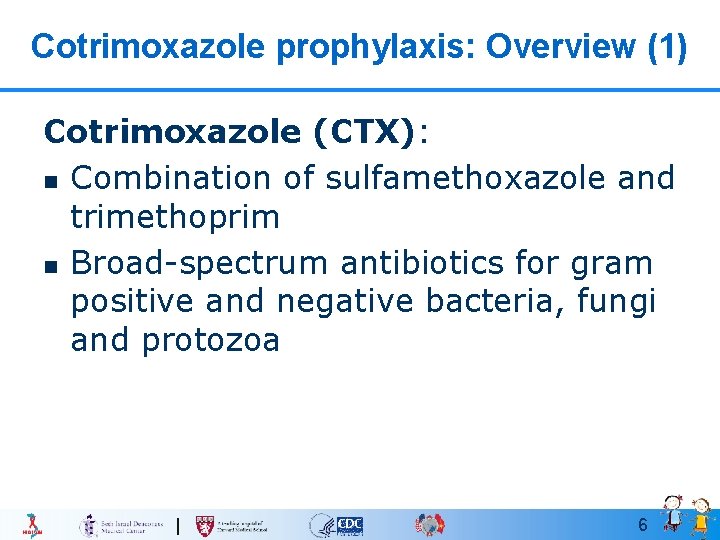
Cotrimoxazole prophylaxis: Overview (1) Cotrimoxazole (CTX): n Combination of sulfamethoxazole and trimethoprim n Broad-spectrum antibiotics for gram positive and negative bacteria, fungi and protozoa 6

Cotrimoxazole prophylaxis: Overview (2) CTX prophylaxis: n A part of a standardized Care and Treatment Package for HIV/AIDS patients n High effective, simple, and economical intervention n Need to be maintained until having evidence of immunological recovery. 7

Cotrimoxazole prophylaxis: Overview (3) n Prevents: • PCP (Pneumocystis jiroveci pneumonia) • Cerebral toxoplasmosis n Reduces the occurrence of: • Pneumonia due to streptococcus pneumoniae, Nocardia, Haemophilus Influenzae, S. aureus, gram-negative bacilli • Diarrheas due to Salmonella, Isospoiaris and protozoa • Malaria 8

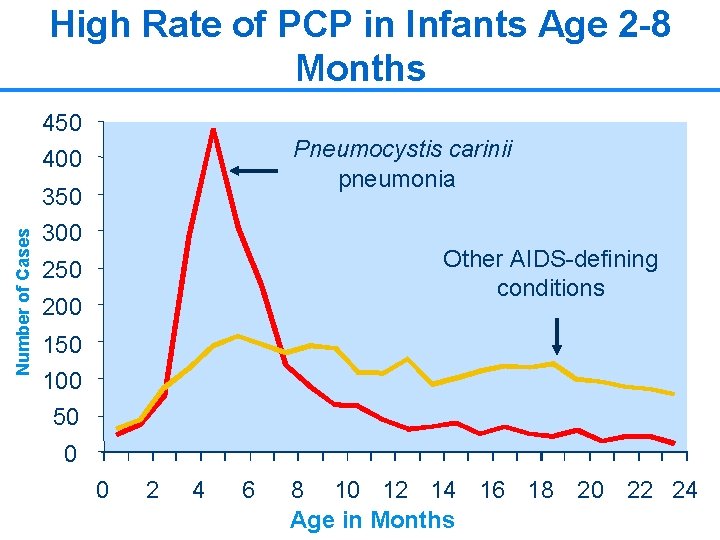
CTX prophylaxis for PCP n PCP is the most common OI in infants and young children • Mortality associated with PCP in infants is as high as 40% despite treatment n CPT is highly effective at preventing PCP • CPT has been shown to reduce mortality in infants and children 9

High Rate of PCP in Infants Age 2 -8 Months 450 400 Pneumocystis carinii pneumonia Number of Cases 350 300 Other AIDS-defining conditions 250 200 150 100 50 0 0 2 4 6 8 10 12 14 16 18 20 22 24 Age in Months 10

Indications and Dose for Cotrimoxazole Prophylaxis 11

Objective of CTX prophylaxis Prevent OIs: n PCP n n n Cerebral toxoplasmosis Some diarrheas Pneumonia due to bacterium 12

Indications for Primary Cotrimoxazole Prophylaxis All HIV-exposed infants/children: n n Starting at 4– 6 weeks of age and Continued until HIV infection is excluded Hướng dẫn Quốc gia về Chẩn đoán và Điều trị HIV/AIDS. Bộ Y tế, Việt Nam, 2011. 13

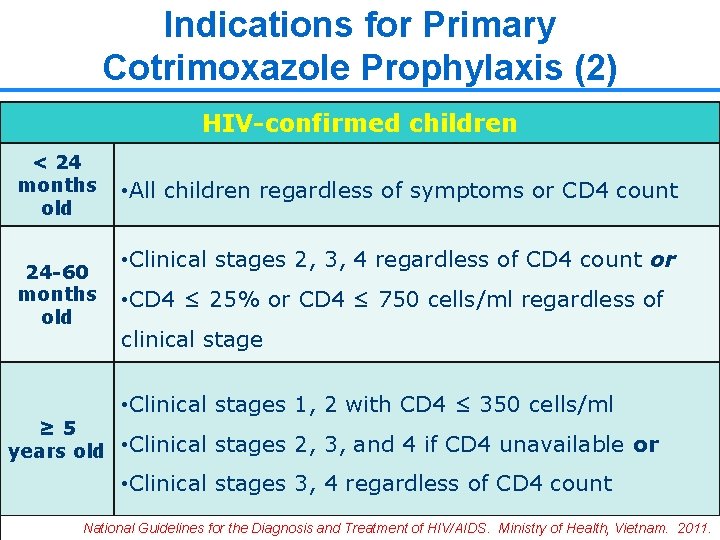
Indications for Primary Cotrimoxazole Prophylaxis (2) HIV-confirmed children < 24 months old 24 -60 months old • All children regardless of symptoms or CD 4 count • Clinical stages 2, 3, 4 regardless of CD 4 count or • CD 4 ≤ 25% or CD 4 ≤ 750 cells/ml regardless of clinical stage • Clinical stages 1, 2 with CD 4 ≤ 350 cells/ml ≥ 5 years old • Clinical stages 2, 3, and 4 if CD 4 unavailable or • Clinical stages 3, 4 regardless of CD 4 count 14 National Guidelines for the Diagnosis and Treatment of HIV/AIDS. Ministry of Health, Vietnam. 2011.

Indications for Secondary Cotrimoxazole Prophylaxis Exposed/infected children who acquired: n PCP n Cerebral toxoplasmosis Hướng dẫn Quốc gia về Chẩn đoán và Điều trị HIV/AIDS. Bộ Y tế, Việt Nam, 2011. 15

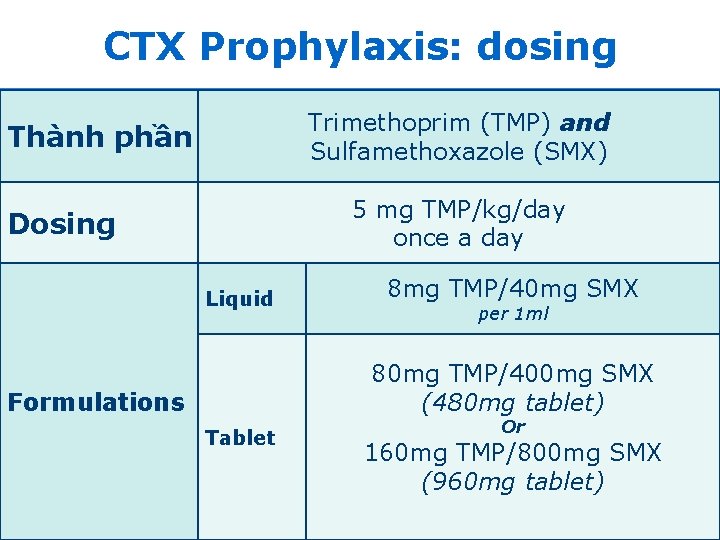
CTX Prophylaxis: dosing Trimethoprim (TMP) and Sulfamethoxazole (SMX) Thành phần 5 mg TMP/kg/day once a day Dosing Liquid 8 mg TMP/40 mg SMX per 1 ml 80 mg TMP/400 mg SMX (480 mg tablet) Formulations Tablet Or 160 mg TMP/800 mg SMX (960 mg tablet) 16

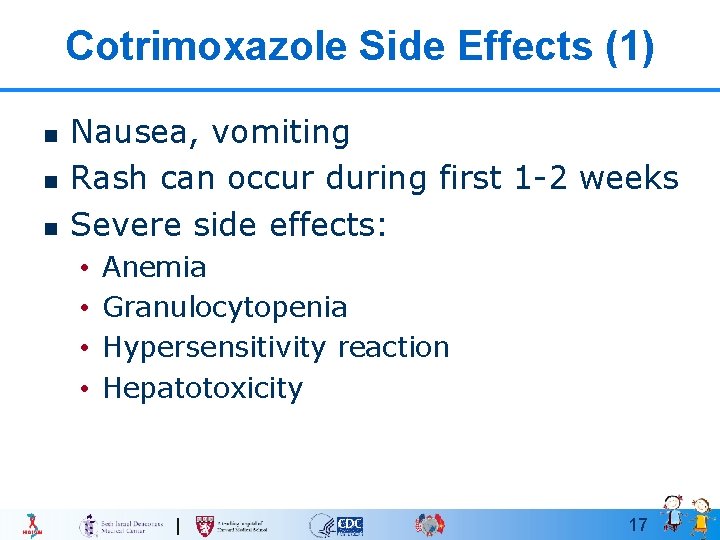
Cotrimoxazole Side Effects (1) n n n Nausea, vomiting Rash can occur during first 1 -2 weeks Severe side effects: • • Anemia Granulocytopenia Hypersensitivity reaction Hepatotoxicity 17

Cotrimoxazole Side Effects (2) n Need to counsel for care givers and children: • Side effects • How to manage • Check up immediately when suspected signs of severe side effect occur n Do complete blood count, liver enzymes measuring when anemia or hepatotoxicity is suspected 18

Group Discussion n Divided into 4 groups Topics of discussion: • Group 1: manifestations/symptoms grade 1 and how to manage • Group 2: manifestations/symptoms grade 2 and how to manage • Group 3: manifestations/symptoms grade 3 and how to manage • Group 4: manifestations/symptoms grade 4 and how to manage Time: 10 minutes of rash at 19

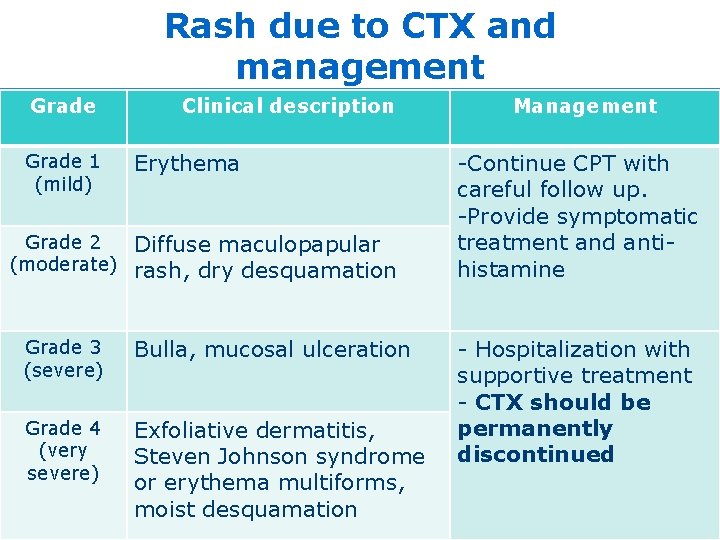
Rash due to CTX and management Grade 1 (mild) Clinical description Erythema Grade 2 Diffuse maculopapular (moderate) rash, dry desquamation Grade 3 (severe) Bulla, mucosal ulceration Grade 4 (very severe) Exfoliative dermatitis, Steven Johnson syndrome or erythema multiforms, moist desquamation Management -Continue CPT with careful follow up. -Provide symptomatic treatment and antihistamine - Hospitalization with supportive treatment - CTX should be permanently discontinued 20

Rash due to CTX and management n n There are no adequate data on CTX desensitization in children Other medications need to be noticed and may be overlapping drug toxicity: • EFV • NVP • Isoniazid… 21

Alternative Therapy: Dapson n n Indication: allergy to CTX Dose: • 2 mg/kg/day, once a day or • 4 mg/kg/time, once a week n Note: • Less effect than CTX in terms of PCP prevention • Can not prevent Toxoplasma 22

Indication of discontinuing CPT 23

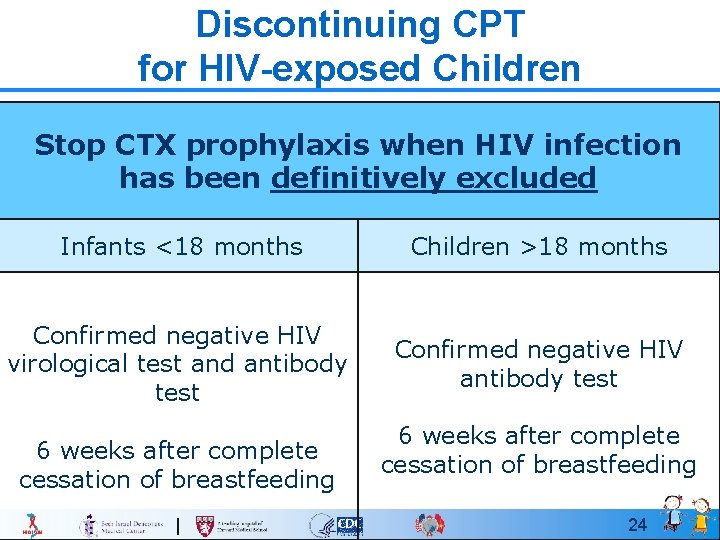
Discontinuing CPT for HIV-exposed Children Stop CTX prophylaxis when HIV infection has been definitively excluded Infants <18 months Children >18 months Confirmed negative HIV virological test and antibody test Confirmed negative HIV antibody test 6 weeks after complete cessation of breastfeeding 24

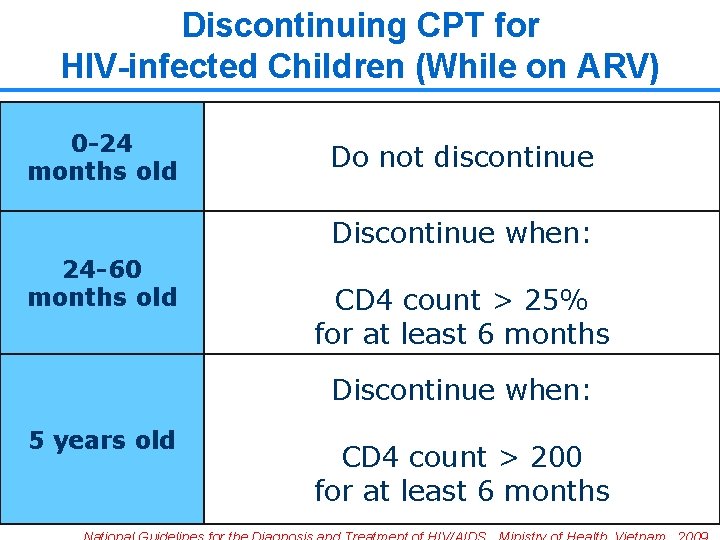
Discontinuing CPT for HIV-infected Children (While on ARV) 0 -24 months old Do not discontinue Discontinue when: 24 -60 months old CD 4 count > 25% for at least 6 months Discontinue when: 5 years old CD 4 count > 200 for at least 6 months 25

Case Study 26

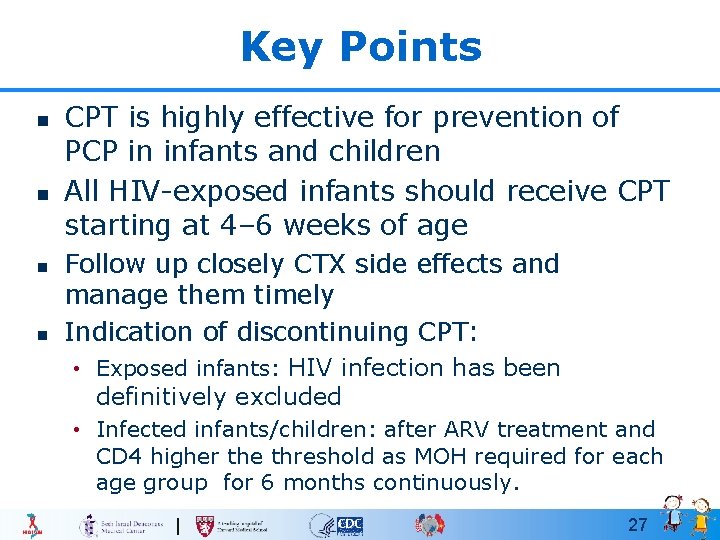
Key Points n n CPT is highly effective for prevention of PCP in infants and children All HIV-exposed infants should receive CPT starting at 4– 6 weeks of age Follow up closely CTX side effects and manage them timely Indication of discontinuing CPT: • Exposed infants: HIV infection has been definitively excluded • Infected infants/children: after ARV treatment and CD 4 higher the threshold as MOH required for each age group for 6 months continuously. 27

Thank you! Questions? 28
 Cotrimoxazole mechanism of action
Cotrimoxazole mechanism of action Direct and indirect guidance examples
Direct and indirect guidance examples Incisal guidance
Incisal guidance Prophylactic definition
Prophylactic definition Prophylactic taping
Prophylactic taping Sports taping for plantar fasciitis
Sports taping for plantar fasciitis Prophylactic lovenox
Prophylactic lovenox Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness integrated therapy system occupational therapy
Bioness integrated therapy system occupational therapy Rita perspektiv
Rita perspektiv Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Gumman cirkel sång
Gumman cirkel sång Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Claes martinsson
Claes martinsson Tillitsbaserad ledning
Tillitsbaserad ledning Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Form dikt
Form dikt Personlig tidbok för yrkesförare
Personlig tidbok för yrkesförare Mästare lärling modell
Mästare lärling modell Orubbliga rättigheter
Orubbliga rättigheter Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Big brother rösta
Big brother rösta Bat mitza
Bat mitza Exspektans eller expektans
Exspektans eller expektans Kyssande vind
Kyssande vind Sju för caesar
Sju för caesar Stig kerman
Stig kerman Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning