Cotrimoxazole and INH preventive therapies in the era









- Slides: 21

Cotrimoxazole and INH preventive therapies in the era of ART: are they necessary? Anthony D Harries Ministry of Health, Malawi

I DO NOT KNOW!

Cotrimoxazole

COTRIMOXAZOLE (CTX) PROPHYLAXIS Useful against: • Pneumocystis jiroveci (carinii) pneumonia • Toxoplasma encephalitis • Isospora belli diarrhoea • Some bacteria and enterobacteria • Nocardiosis • Falciparum malaria

CTX prophylaxis in HIV+ve persons in industrialised countries • Patients with CD 4 counts < 200/mm 3 to prevent PCP and other opportunistic infections • Patients who have had an episode of PCP or toxoplasma encephalitis • Children born to HIV-positive mothers

UNAIDS Recommendations for CTX prophylaxis in Africa • HIV positive adults with: WHO Stage II, III or IV disease CD 4 counts of 500/mm 3 or less • HIV-exposed infants from six weeks of age (particularly any child born to an HIVinfected woman)

Advantages of CTX use • • • Widely available, even in rural Africa Easy to take Relatively safe Does not require laboratory monitoring Effective during 2 year follow-up periods Cheap [costs 6 – 12 USD per year]

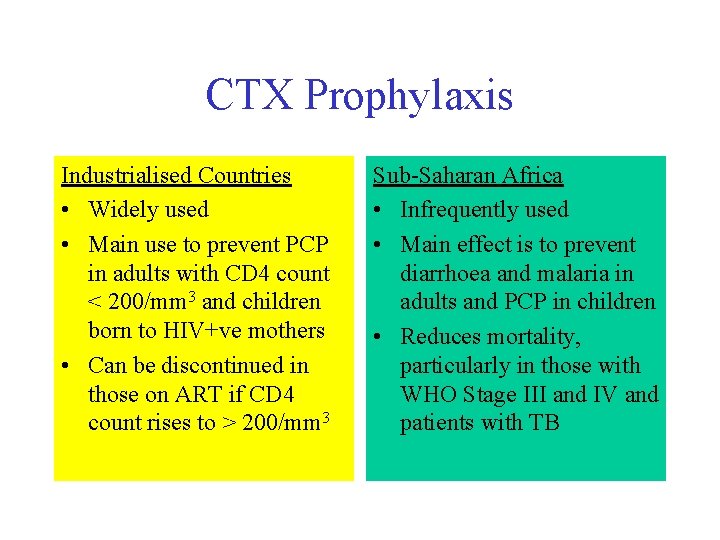
CTX Prophylaxis Industrialised Countries • Widely used • Main use to prevent PCP in adults with CD 4 count < 200/mm 3 and children born to HIV+ve mothers • Can be discontinued in those on ART if CD 4 count rises to > 200/mm 3 Sub-Saharan Africa • Infrequently used • Main effect is to prevent diarrhoea and malaria in adults and PCP in children • Reduces mortality, particularly in those with WHO Stage III and IV and patients with TB

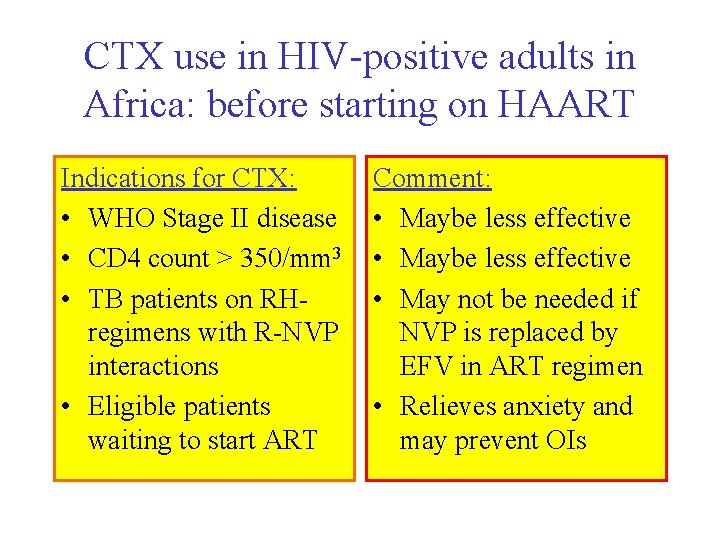
CTX use in HIV-positive adults in Africa: before starting on HAART Indications for CTX: • WHO Stage II disease • CD 4 count > 350/mm 3 • TB patients on RHregimens with R-NVP interactions • Eligible patients waiting to start ART Comment: • Maybe less effective • May not be needed if NVP is replaced by EFV in ART regimen • Relieves anxiety and may prevent OIs

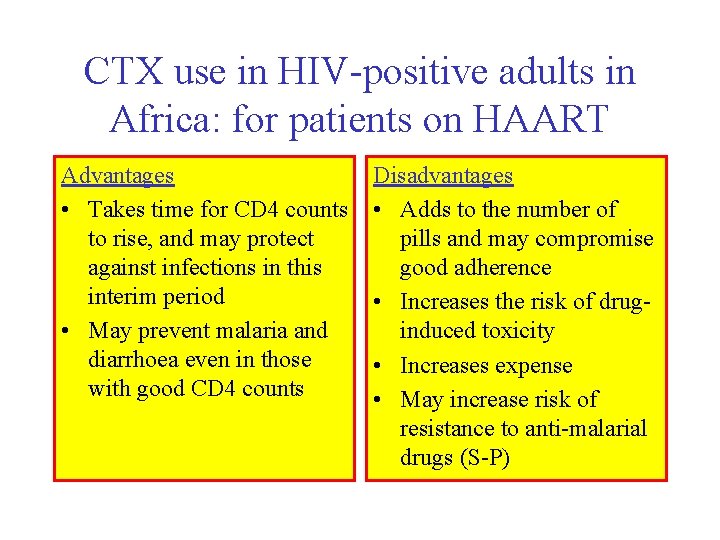
CTX use in HIV-positive adults in Africa: for patients on HAART Advantages • Takes time for CD 4 counts to rise, and may protect against infections in this interim period • May prevent malaria and diarrhoea even in those with good CD 4 counts Disadvantages • Adds to the number of pills and may compromise good adherence • Increases the risk of druginduced toxicity • Increases expense • May increase risk of resistance to anti-malarial drugs (S-P)

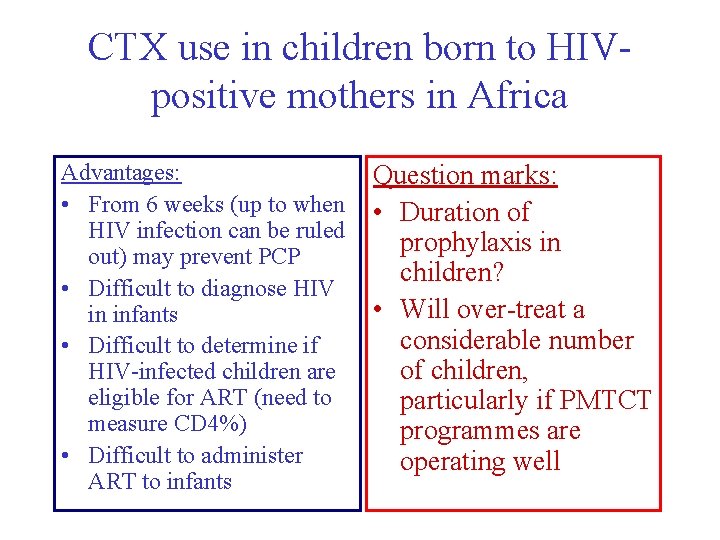
CTX use in children born to HIVpositive mothers in Africa Advantages: • From 6 weeks (up to when HIV infection can be ruled out) may prevent PCP • Difficult to diagnose HIV in infants • Difficult to determine if HIV-infected children are eligible for ART (need to measure CD 4%) • Difficult to administer ART to infants Question marks: • Duration of prophylaxis in children? • Will over-treat a considerable number of children, particularly if PMTCT programmes are operating well

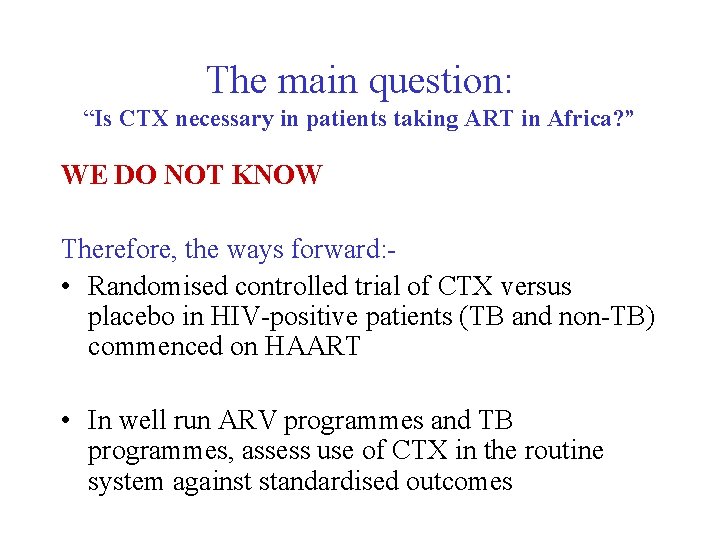
The main question: “Is CTX necessary in patients taking ART in Africa? ” WE DO NOT KNOW Therefore, the ways forward: • Randomised controlled trial of CTX versus placebo in HIV-positive patients (TB and non-TB) commenced on HAART • In well run ARV programmes and TB programmes, assess use of CTX in the routine system against standardised outcomes

Isoniazid Preventive Therapy

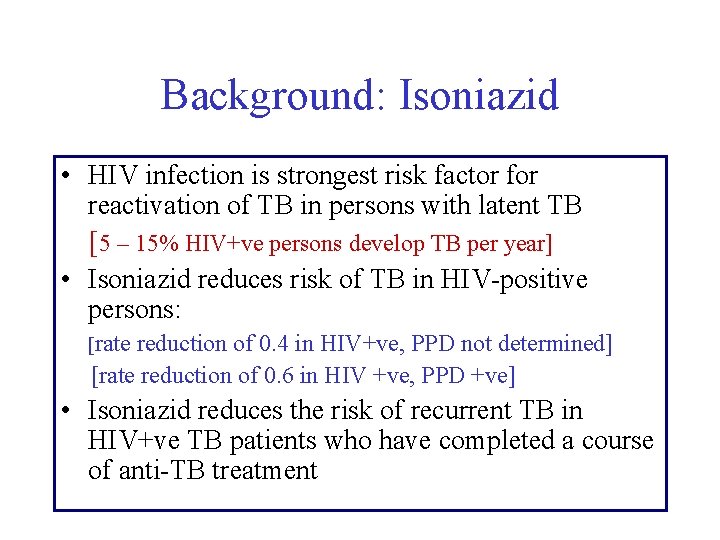
Background: Isoniazid • HIV infection is strongest risk factor for reactivation of TB in persons with latent TB [5 – 15% HIV+ve persons develop TB per year] • Isoniazid reduces risk of TB in HIV-positive persons: [rate reduction of 0. 4 in HIV+ve, PPD not determined] [rate reduction of 0. 6 in HIV +ve, PPD +ve] • Isoniazid reduces the risk of recurrent TB in HIV+ve TB patients who have completed a course of anti-TB treatment

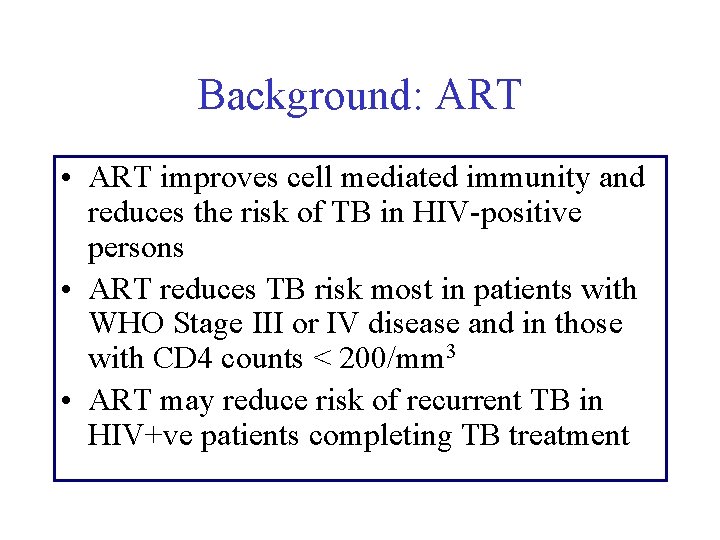
Background: ART • ART improves cell mediated immunity and reduces the risk of TB in HIV-positive persons • ART reduces TB risk most in patients with WHO Stage III or IV disease and in those with CD 4 counts < 200/mm 3 • ART may reduce risk of recurrent TB in HIV+ve patients completing TB treatment

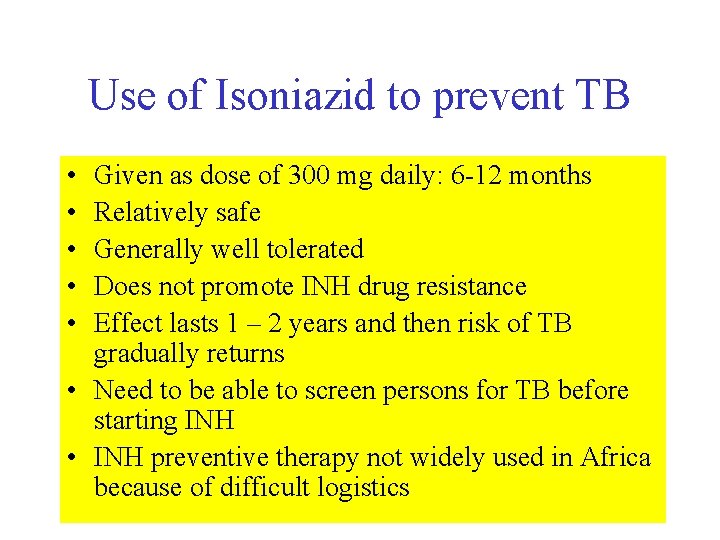
Use of Isoniazid to prevent TB • • • Given as dose of 300 mg daily: 6 -12 months Relatively safe Generally well tolerated Does not promote INH drug resistance Effect lasts 1 – 2 years and then risk of TB gradually returns • Need to be able to screen persons for TB before starting INH • INH preventive therapy not widely used in Africa because of difficult logistics

Value of Isoniazid • Useful as a measure to prevent TB for HIVpositive individuals, either first time TB or recurrent TB • Not useful as a measure to reduce the burden of TB in high HIV burden communities because: - need to have large numbers of people HIV tested - small % of HIV+ve persons complete the course - lack of structures to manage INH preventive therapy

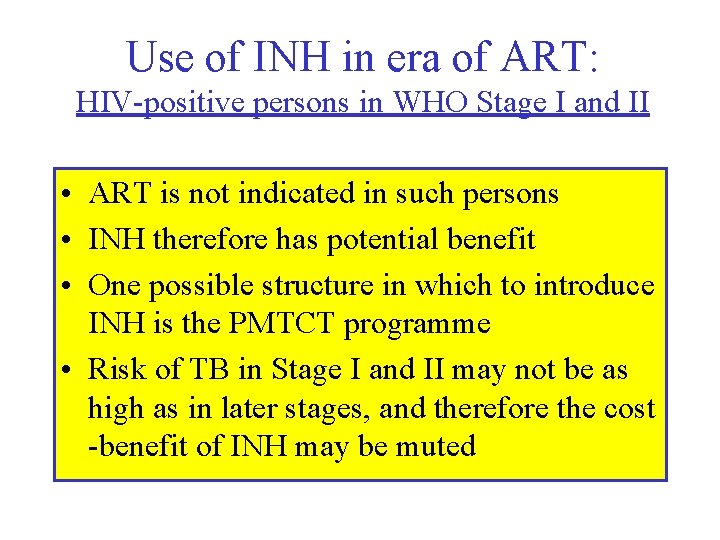
Use of INH in era of ART: HIV-positive persons in WHO Stage I and II • ART is not indicated in such persons • INH therefore has potential benefit • One possible structure in which to introduce INH is the PMTCT programme • Risk of TB in Stage I and II may not be as high as in later stages, and therefore the cost -benefit of INH may be muted

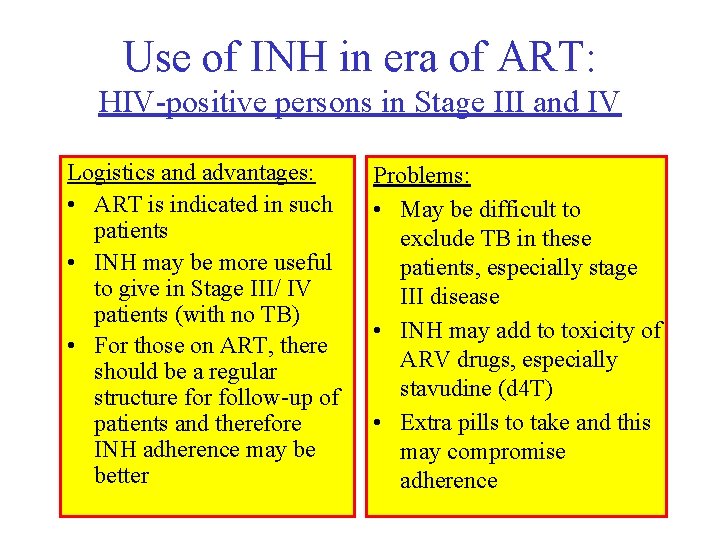
Use of INH in era of ART: HIV-positive persons in Stage III and IV Logistics and advantages: • ART is indicated in such patients • INH may be more useful to give in Stage III/ IV patients (with no TB) • For those on ART, there should be a regular structure for follow-up of patients and therefore INH adherence may be better Problems: • May be difficult to exclude TB in these patients, especially stage III disease • INH may add to toxicity of ARV drugs, especially stavudine (d 4 T) • Extra pills to take and this may compromise adherence

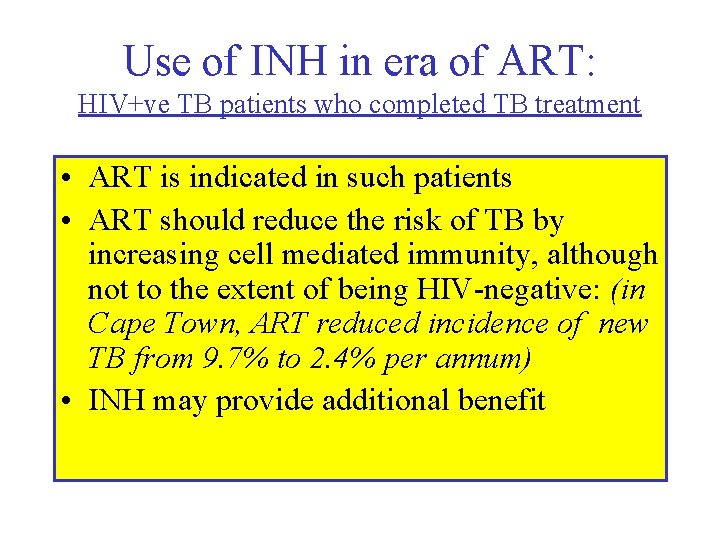
Use of INH in era of ART: HIV+ve TB patients who completed TB treatment • ART is indicated in such patients • ART should reduce the risk of TB by increasing cell mediated immunity, although not to the extent of being HIV-negative: (in Cape Town, ART reduced incidence of new TB from 9. 7% to 2. 4% per annum) • INH may provide additional benefit

Research questions on INH • Does PMTCT provide a suitable structure for INH preventive therapy in HIV-positive persons in Stage I or II? • Does the addition of INH to ART further reduce the incidence of new TB and recurrent TB in HIV-positive patients in Stage III and IV?
 Systematic desensitization therapy
Systematic desensitization therapy Mode of action of sulfonamides
Mode of action of sulfonamides Chapter 32 complementary and alternative therapies
Chapter 32 complementary and alternative therapies Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Bodywork and movement therapies
Bodywork and movement therapies Bodywork and movement therapies
Bodywork and movement therapies Pharmacological and parenteral therapies
Pharmacological and parenteral therapies Complementary therapy ppt
Complementary therapy ppt Quiz 2 the baroque era
Quiz 2 the baroque era Victorian and elizabethan era
Victorian and elizabethan era Creí que era una aventura y en realidad era la vida
Creí que era una aventura y en realidad era la vida Vi uma estrela tão alta
Vi uma estrela tão alta Preventive and predictive maintenance of hydro power plant
Preventive and predictive maintenance of hydro power plant Bachelor of public health monash
Bachelor of public health monash Changing trends in hospital care
Changing trends in hospital care Examples of corrective discipline in the classroom
Examples of corrective discipline in the classroom Preventive maintenance and troubleshooting
Preventive maintenance and troubleshooting Preventive maintenance and troubleshooting
Preventive maintenance and troubleshooting Preventive health and safety in the child care setting
Preventive health and safety in the child care setting Hardware preventive maintenance
Hardware preventive maintenance Capa meaning
Capa meaning Module 73: the biomedical therapies
Module 73: the biomedical therapies