Focus Activity What is the conservation of matter
























- Slides: 35

Focus Activity: What is the conservation of matter? What does it show you? Homework: Read and Answer 6. 2 questions 1, 2, 3 and Section 6. 3 all questions due Wed

• Place your model on the appropriate table around the room. • Make sure your name is on it. • Put your name(s) on your rubric and put the rubric on TOP of your model. • There will be a sign up sheet coming aroundfill in who you handed your project into and if it was an email attachment/poster/shared on google docs.

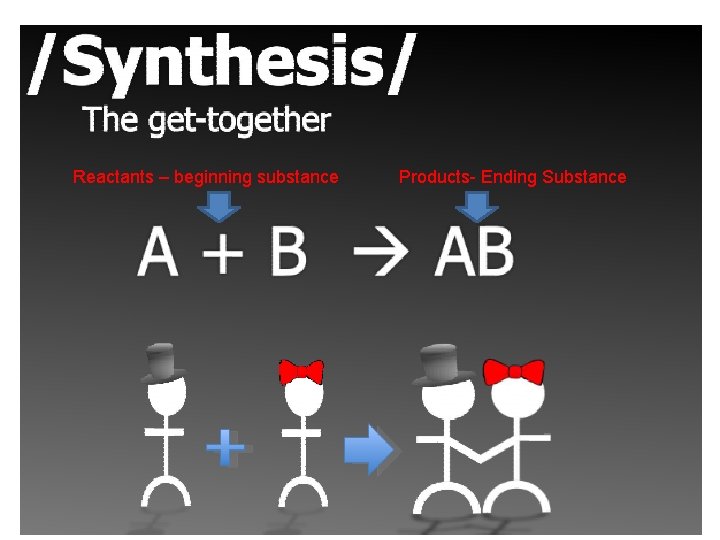
Types of Chemical Reactions *Synthesis – The get together *Decomposition- The break up *Single Replacement- The Cheater *Double Replacement- The Swap *Combustion **The Red names are “helpful hints”



Reactants – beginning substance Products- Ending Substance

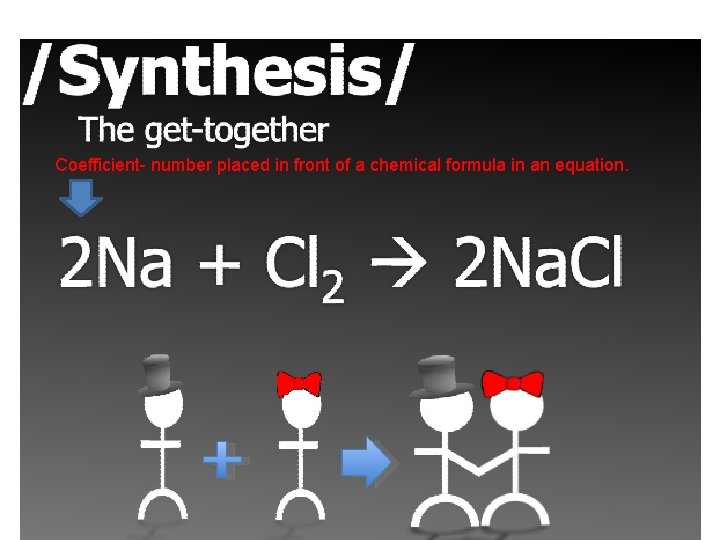
Coefficient- number placed in front of a chemical formula in an equation.














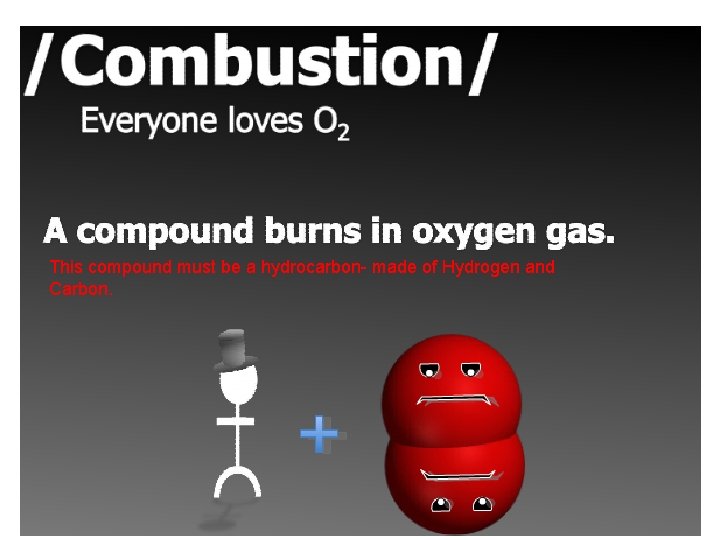
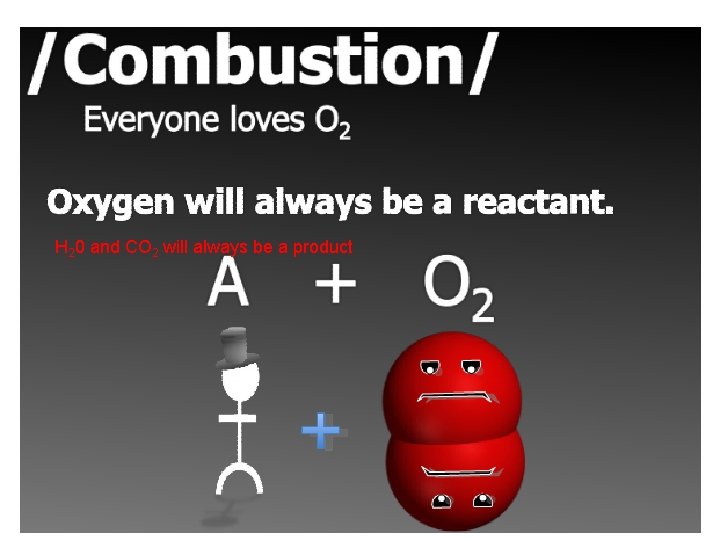
This compound must be a hydrocarbon- made of Hydrogen and Carbon.

H 20 and CO 2 will always be a product

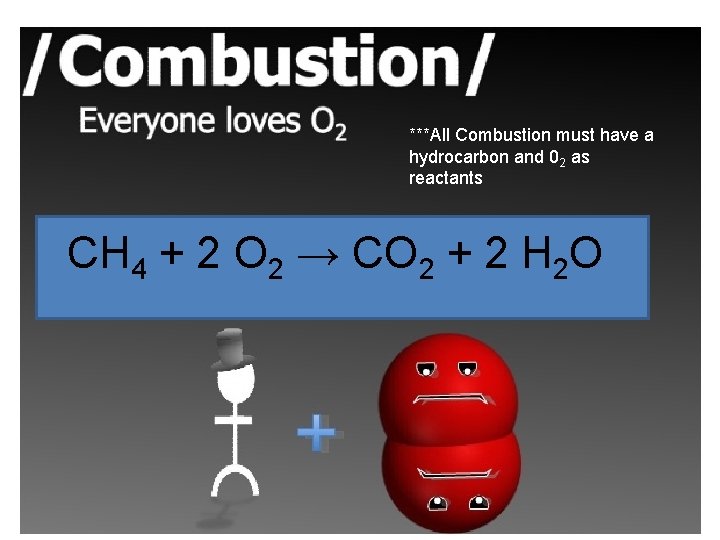
***All Combustion must have a hydrocarbon and 02 as reactants CH 4 + 2 O 2 → CO 2 + 2 H 2 O

Some fun with Chemical Reactions!! http: //www. youtube. com/watch? v=dxl. Wts. Fin. TM

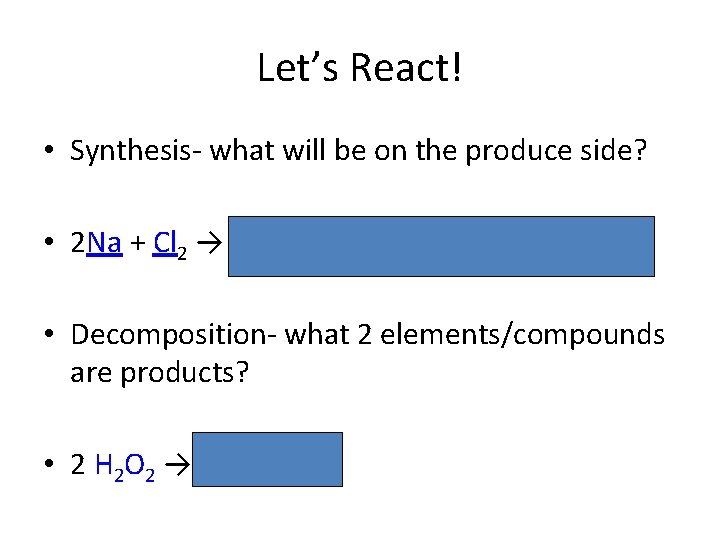
Let’s React! • Synthesis- what will be on the produce side? • 2 Na + Cl 2 → 2 Na. Cl (formation of table salt) • Decomposition- what 2 elements/compounds are products? • 2 H 2 O 2 → 2 H 2 O + O 2

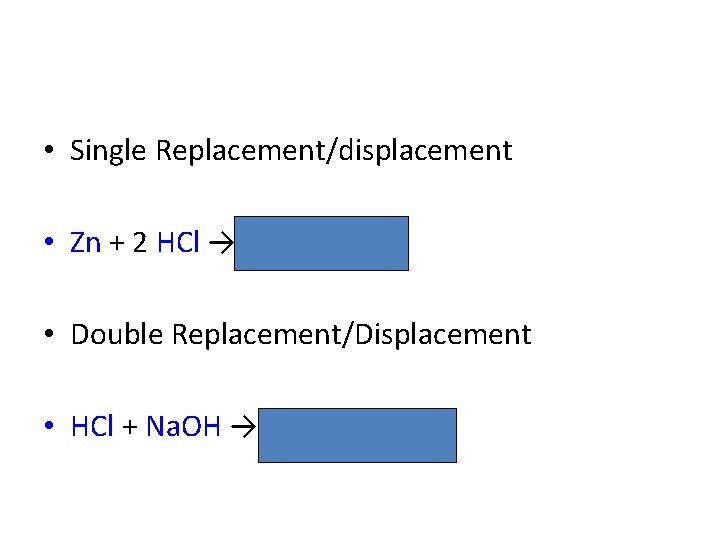
• Single Replacement/displacement • Zn + 2 HCl → Zn. Cl 2 + H 2 • Double Replacement/Displacement • HCl + Na. OH → Na. Cl + H 2 O

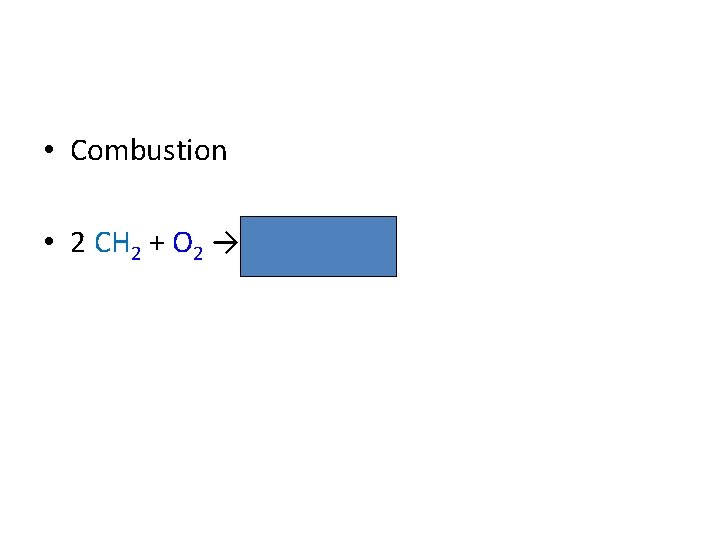
• Combustion • 2 CH 2 + O 2 → 2 C 02 2 H 2 O

Focus Activity: Describe in your own words why you think the different types of chemical reactions could be called “The Get Together”, “The Break Up”, “The Cheater”, and “The Swap”. • Study for your quiz on Thursday- Information from Friday, Monday, Tuesday, and Wednesday’s notes will be on the quiz.

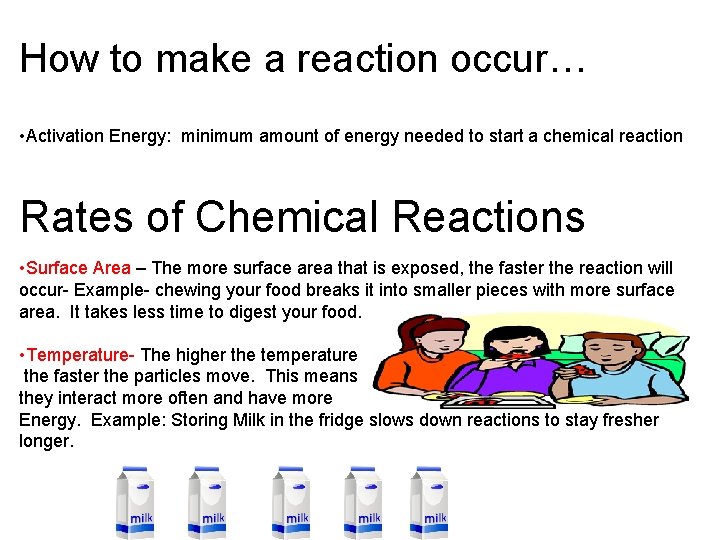
How to make a reaction occur… • Activation Energy: minimum amount of energy needed to start a chemical reaction Rates of Chemical Reactions • Surface Area – The more surface area that is exposed, the faster the reaction will occur- Example- chewing your food breaks it into smaller pieces with more surface area. It takes less time to digest your food. • Temperature- The higher the temperature the faster the particles move. This means they interact more often and have more Energy. Example: Storing Milk in the fridge slows down reactions to stay fresher longer.

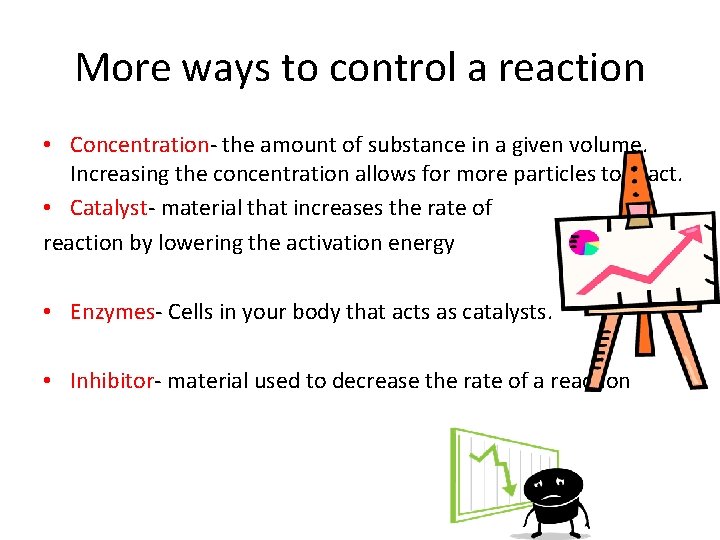
More ways to control a reaction • Concentration- the amount of substance in a given volume. Increasing the concentration allows for more particles to react. • Catalyst- material that increases the rate of reaction by lowering the activation energy • Enzymes- Cells in your body that acts as catalysts. • Inhibitor- material used to decrease the rate of a reaction

Every Chemical Reaction displays a change in Energy!!!!! http: //www. youtube. com/watch? v=t. E 4668 aarck

Time for Practice Worksheets!!

Answers to 6. 1 • • • 1 A. Physical Properties can be observed without changing the substance into another. Chemical Property: Characteristic of a substance that describes its ability to change into another substance. B. You could ask whether the black crust formed from silver and water? If it is, then the silver underwent a chemical change, because a new substance was formed. C. Chemical Bonds form between atoms that share, gain, or lose electrons, or bonds break and new bonds form. 2 A. Change in color, precipitate forms, gas bubbles, change in texture, change in energy. B. Cooked eggs are solid, raw are liquid. Color changes. C. Both types of reactions show a change in energy. Endothermic absorbs energy, exothermic releases energy.

Answers to 6. 2 • 1 A. Formulas tell you the elements and compounds involved in the reaction, arrow means “Yields” and points to the products. + indications 2 or more reactants or products. • B. Both reactants and products are written as formulas. Reactants are placed to the left of the arrow and products are on the right. 2 A Conservation of Mass means no matter is created or destroyed during a chemical reaction. B 250 g 3 A. Synthesis, decomposition, and replacement B 2 C Synthesis

Answers to 6. 3 • 1 A Minimum amount of energy needed to start a chemical reaction. • B All chemical reactions need a certain amount of activation energy to get started. • C Students might say that both endothermic and exothermic reactions need a similar level of activation energy in order to begin. • 2 A Chemists can control the rates of chemical reactions by changing factors such as surface area, temperature, and concentration, and by using substances called catalysts and inhibitors. • B Sugar crystals, because more particles of sugar are exposed than in a sugar cube.
 For adult
For adult Porter competitive strategy
Porter competitive strategy Cost leadership and differentiation
Cost leadership and differentiation Actor focus vs object focus
Actor focus vs object focus Law of conservation of matter definition
Law of conservation of matter definition Law of conservation of mass
Law of conservation of mass Focus activity
Focus activity Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is white matter made of
What is white matter made of Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Dural septa
Dural septa Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Ncl. caudatus
Ncl. caudatus Ecological succession
Ecological succession Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế