Fluorescence Yield detection XPS Ground state analysis Fluorescence







![CTM 4 DOC 48 [Delgado et al. J. Synchrot. Rad 23, 1264 (2016)] 48 CTM 4 DOC 48 [Delgado et al. J. Synchrot. Rad 23, 1264 (2016)] 48](https://slidetodoc.com/presentation_image_h2/7a76a7e78bb3153d31ed4811a94ad9fe/image-48.jpg)
- Slides: 51

• Fluorescence Yield detection • XPS • Ground state analysis

Fluorescence Yield detection • • FY (1. dilute limit) FY (2. state dependent decay) FY (3. inverse PFY) FY (4. dips and peaks)

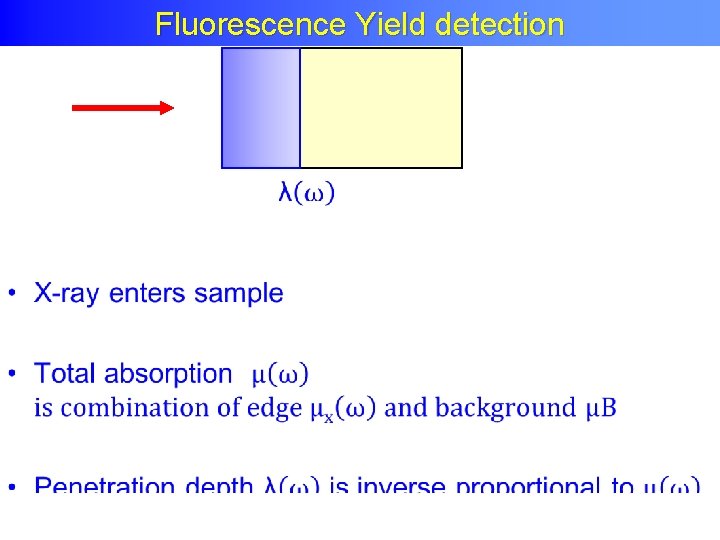
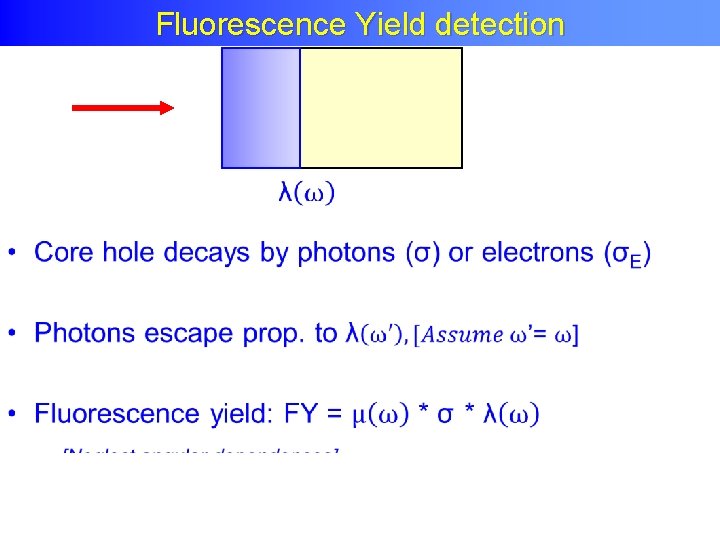
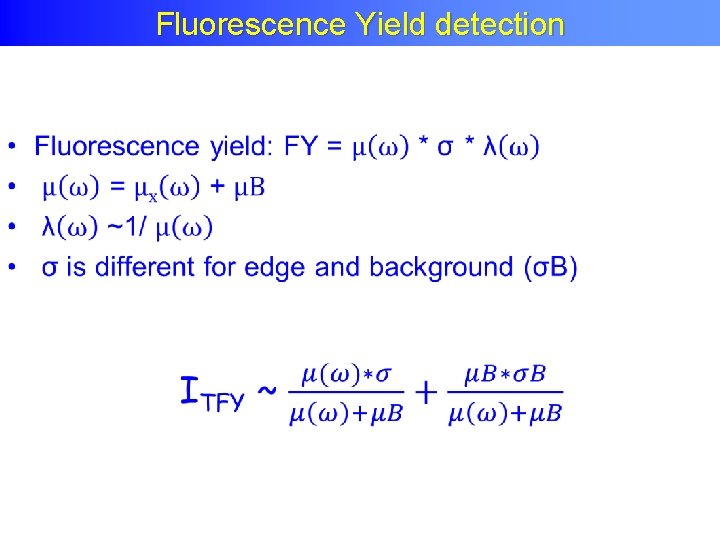
Fluorescence Yield detection •

Fluorescence Yield detection •

Fluorescence Yield detection •

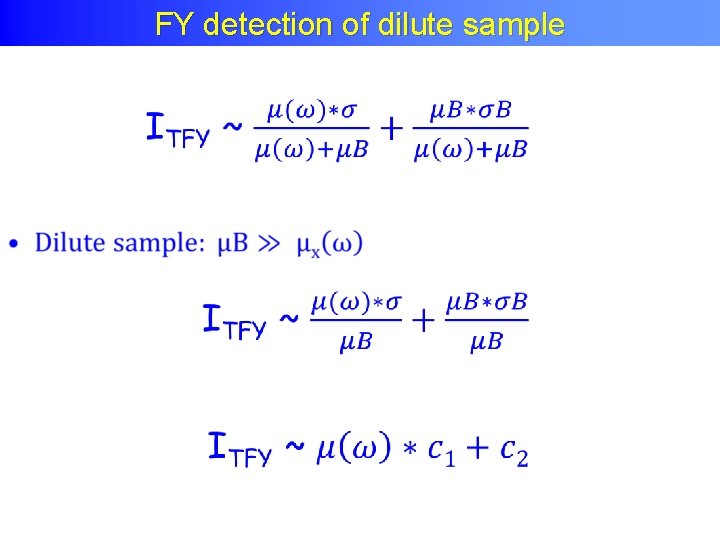
FY detection of dilute sample

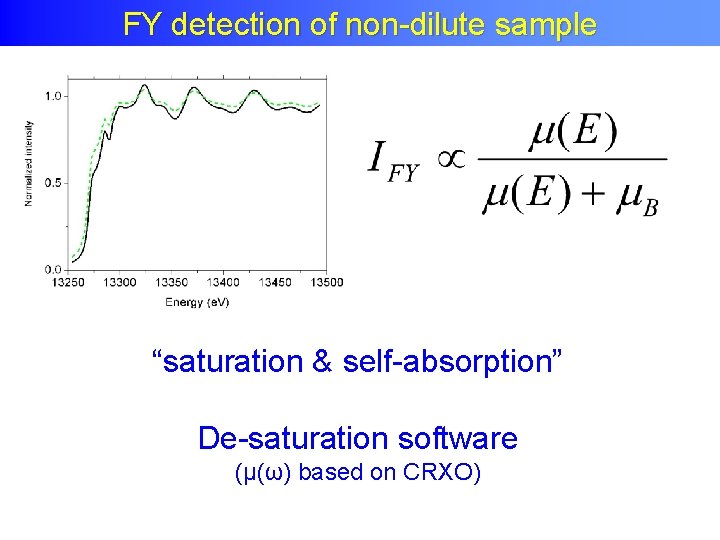
Fluorescence Yield FY detection of non-dilute sample “saturation & self-absorption” De-saturation software (μ(ω) based on CRXO)

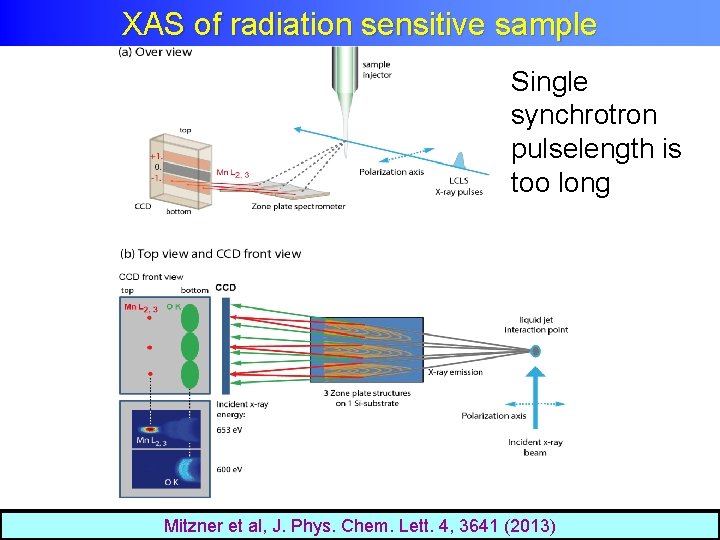
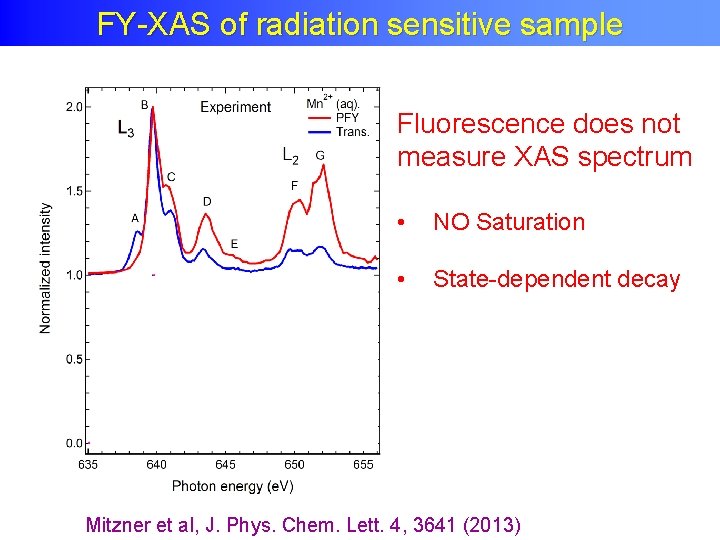
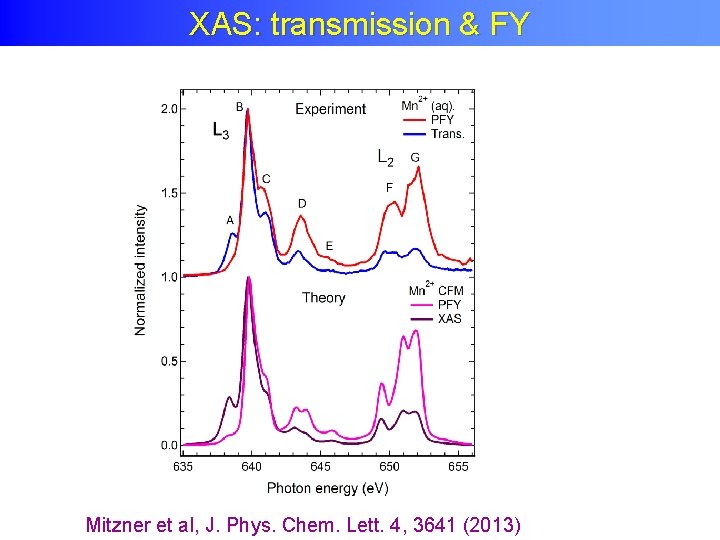
XAS of radiation sensitive sample Single synchrotron pulselength is too long Mitzner et al, J. Phys. Chem. Lett. 4, 3641 (2013)

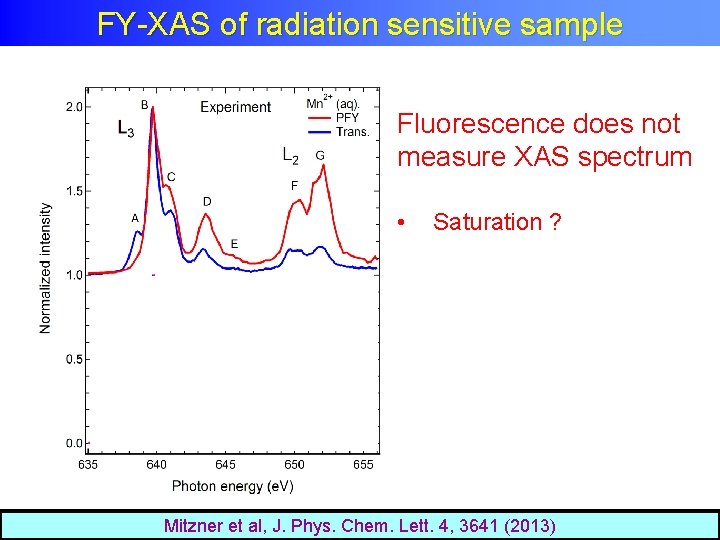
FY-XAS of radiation sensitive sample Fluorescence does not measure XAS spectrum • Saturation ? Mitzner et al, J. Phys. Chem. Lett. 4, 3641 (2013)

FY-XAS of radiation sensitive sample Fluorescence does not measure XAS spectrum • NO Saturation • State-dependent decay Mitzner et al, J. Phys. Chem. Lett. 4, 3641 (2013)

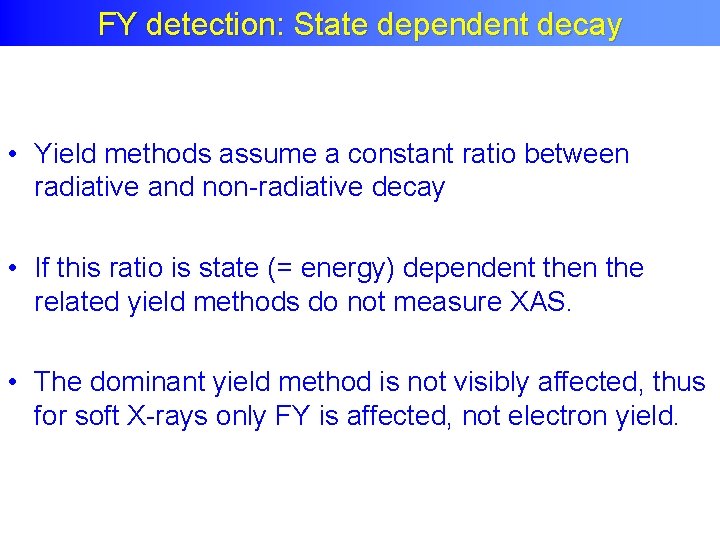
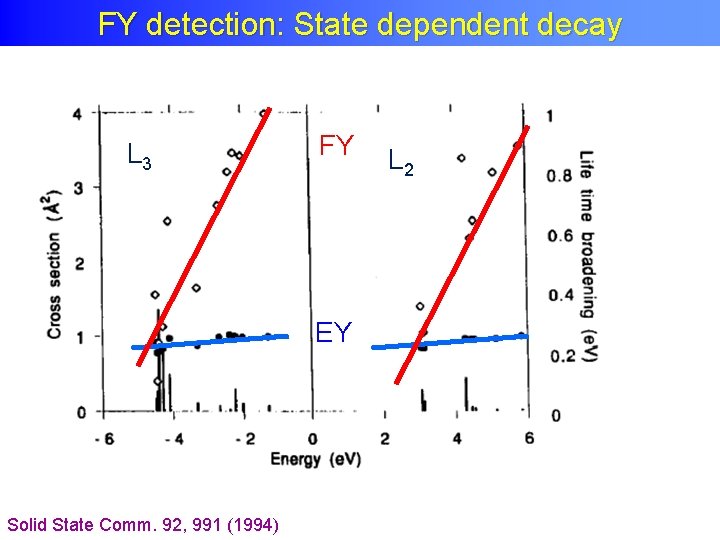
FY detection: State dependent decay • Yield methods assume a constant ratio between radiative and non-radiative decay • If this ratio is state (= energy) dependent then the related yield methods do not measure XAS. • The dominant yield method is not visibly affected, thus for soft X-rays only FY is affected, not electron yield.

FY detection: State dependent decay L 3 FY EY Solid State Comm. 92, 991 (1994) L 2

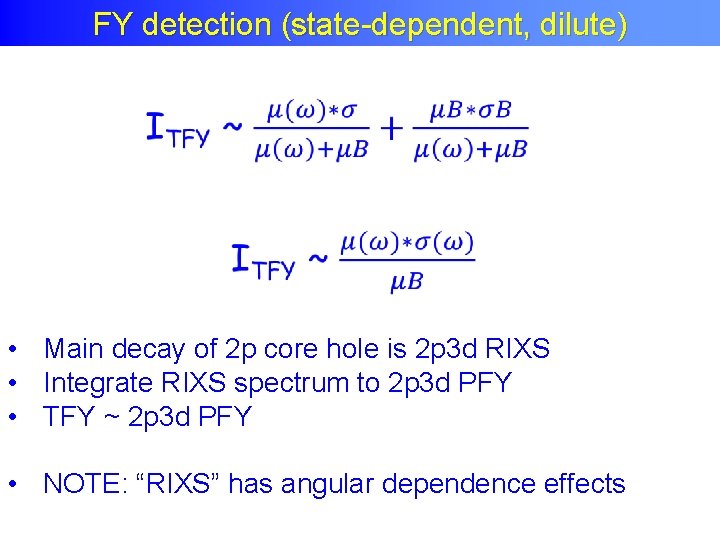
FY detection (state-dependent, dilute) • Main decay of 2 p core hole is 2 p 3 d RIXS • Integrate RIXS spectrum to 2 p 3 d PFY • TFY ~ 2 p 3 d PFY • NOTE: “RIXS” has angular dependence effects

XAS: transmission & FY Mitzner et al, J. Phys. Chem. Lett. 4, 3641 (2013)

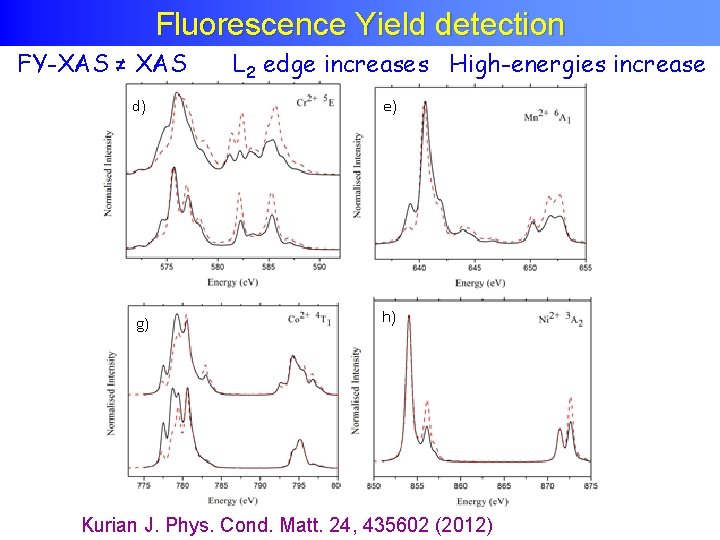
Fluorescence Yield detection FY-XAS ≠ XAS L 2 edge increases High-energies increase Kurian J. Phys. Cond. Matt. 24, 435602 (2012)

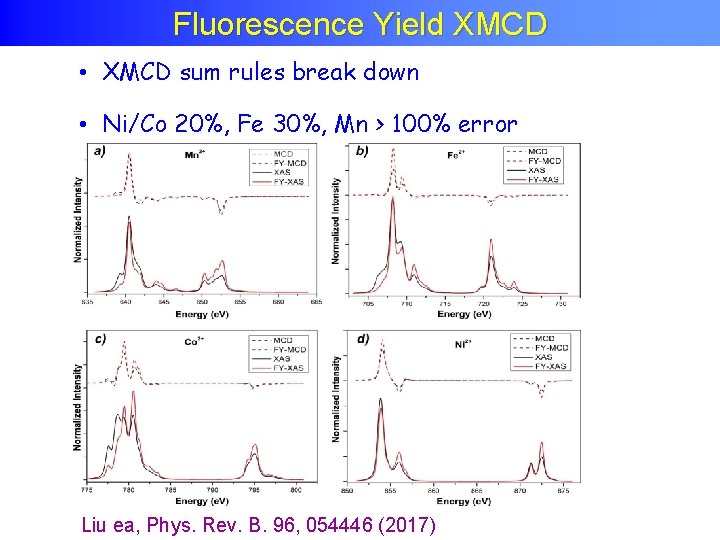
Fluorescence Yield XMCD • XMCD sum rules break down • Ni/Co 20%, Fe 30%, Mn > 100% error Liu ea, Phys. Rev. B. 96, 054446 (2017)

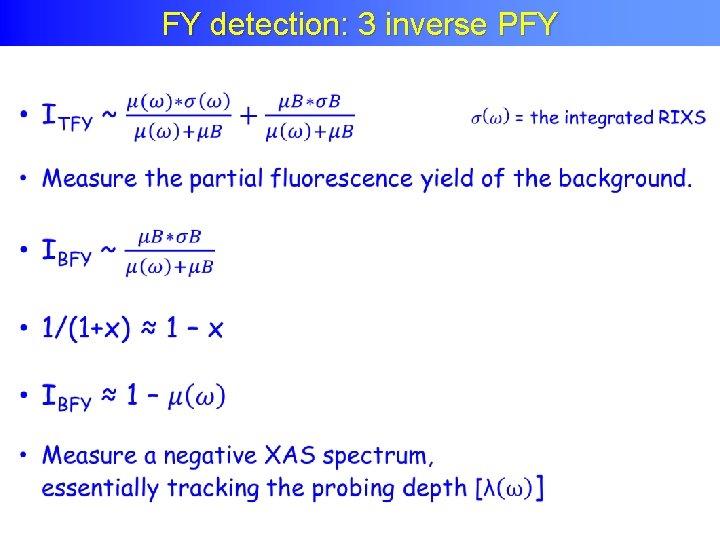
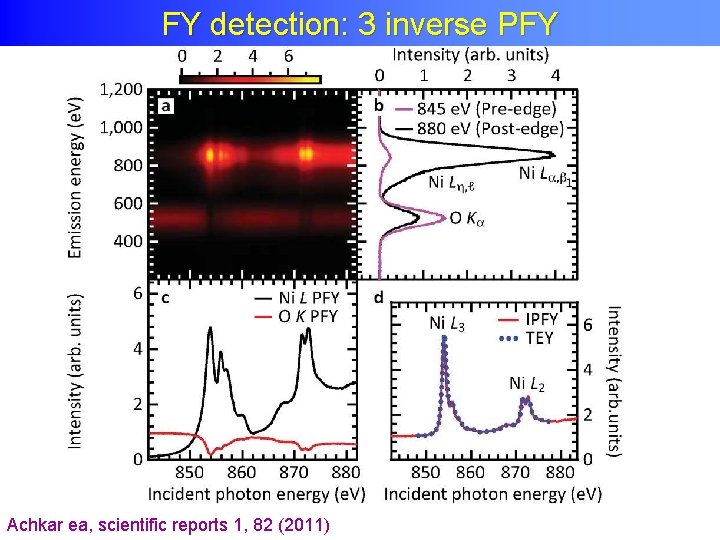
FY detection: 3 inverse PFY

FY detection: 3 inverse PFY Achkar ea, scientific reports 1, 82 (2011)

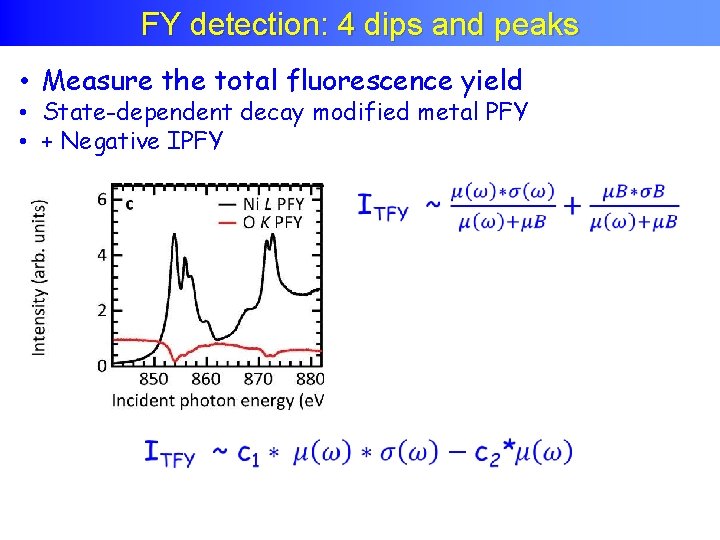
FY detection: 4 dips and peaks • Measure the total fluorescence yield • State-dependent decay modified metal PFY • + Negative IPFY

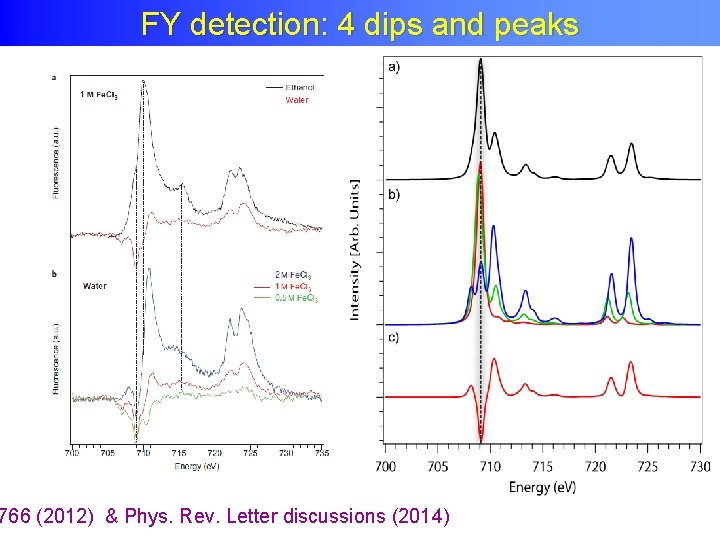
FY detection: 4 dips and peaks 766 (2012) & Phys. Rev. Letter discussions (2014)

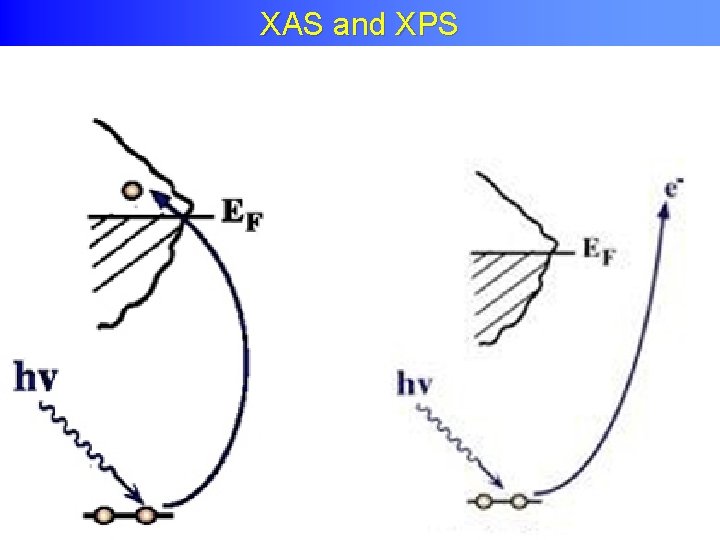
XPS

X-ray absorption and X-ray XAS and XPS photoemission I( FIXED)

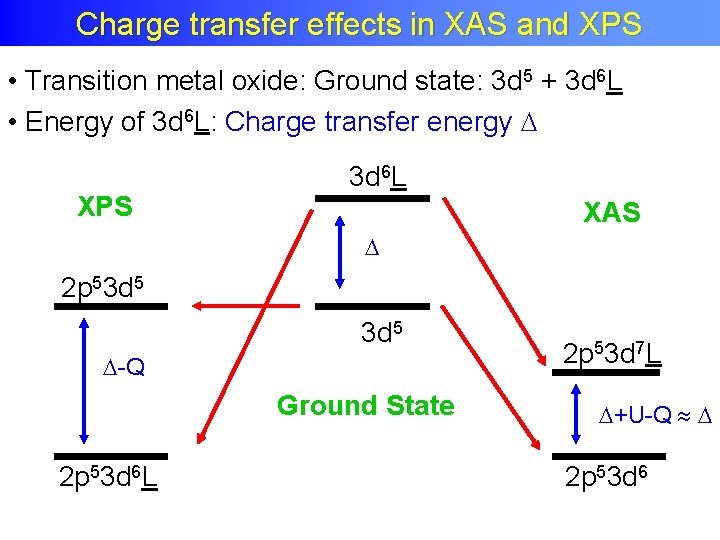
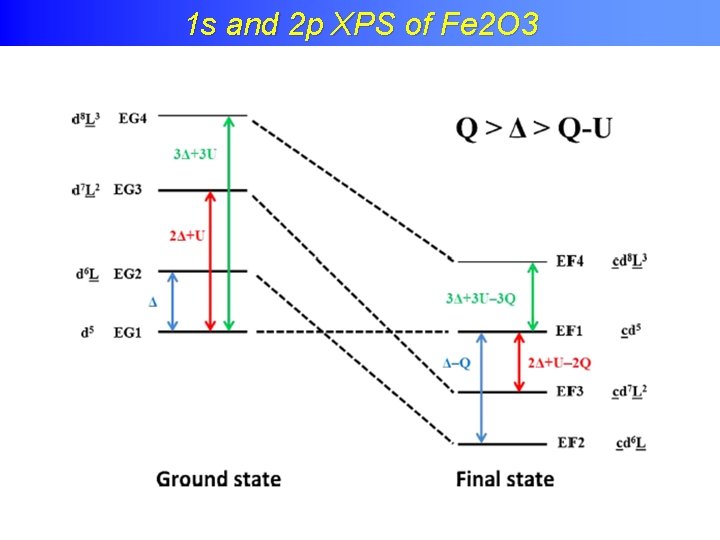
Charge transfer XAS and XPS Charge transfer effects inin. XAS and XPS • Transition metal oxide: Ground state: 3 d 5 + 3 d 6 L • Energy of 3 d 6 L: Charge transfer energy XPS 3 d 6 L XAS 2 p 53 d 5 -Q Ground State 2 p 53 d 6 L 2 p 53 d 7 L +U-Q 2 p 53 d 6

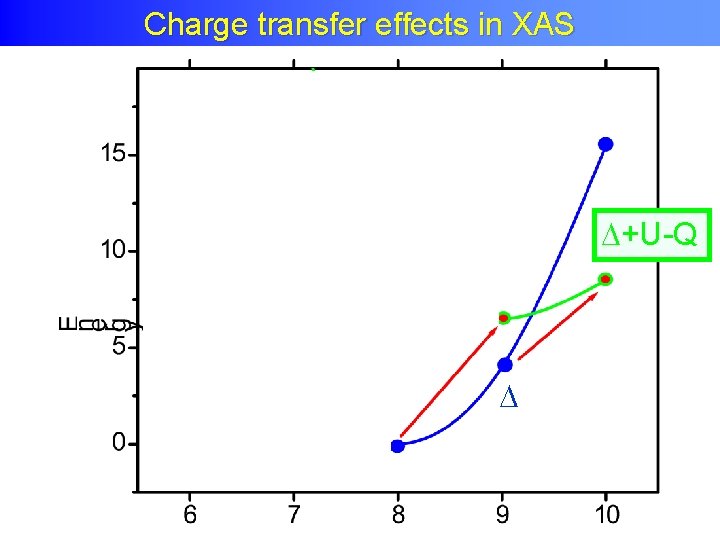
Charge Transfer Effects XAS Charge transfer effects in in XAS +U-Q

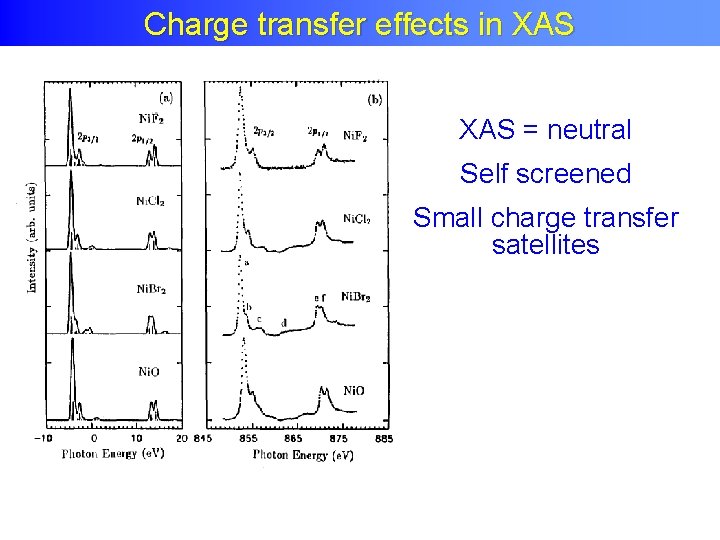
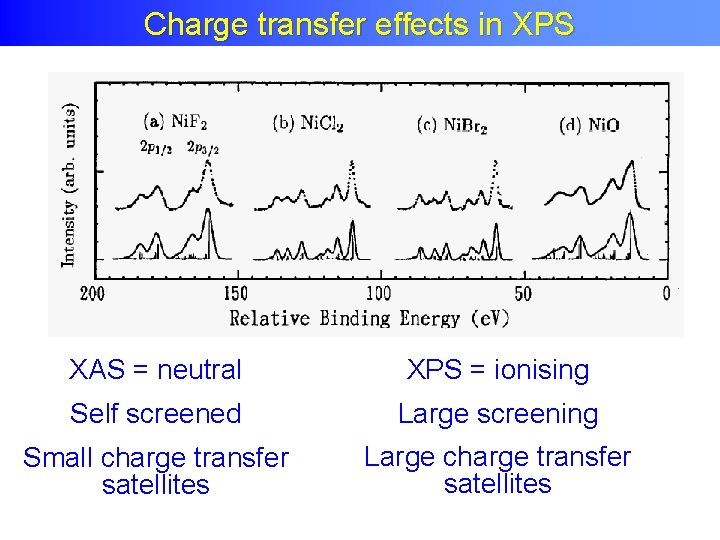
Charge transfer effects in XAS = neutral Self screened Small charge transfer satellites

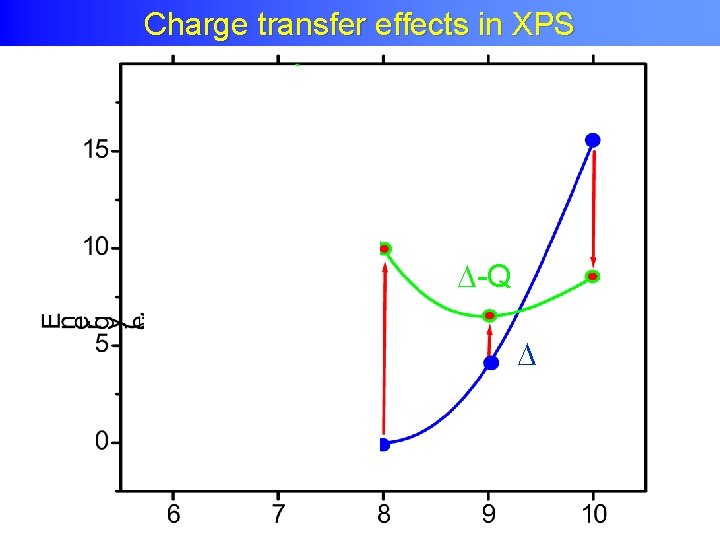
Charge Transfer Effects XPS Charge transfer effects in in XPS -Q

Charge effectsin in XPS Chargetransfer effects XPS XAS = neutral XPS = ionising Self screened Large screening Small charge transfer satellites Large charge transfer satellites

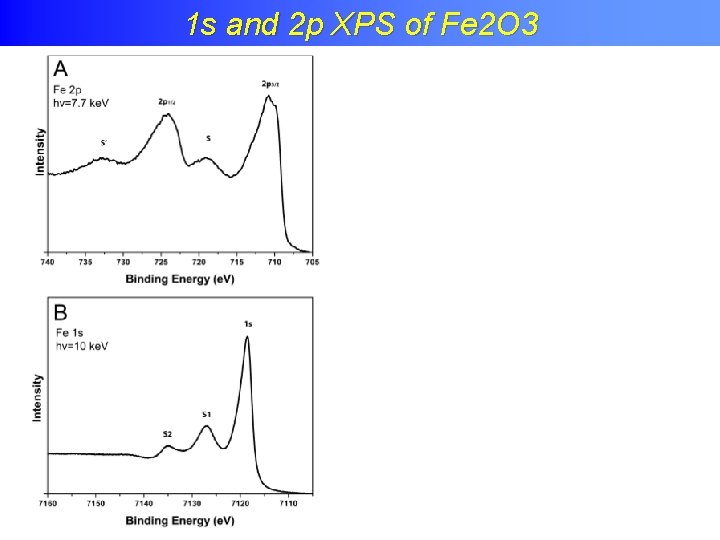
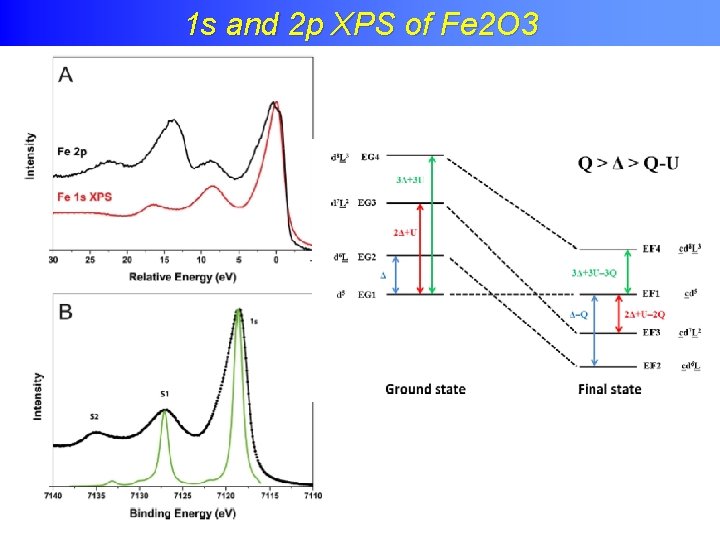
Charge transfer effects in XPS 1 s and 2 p XPS of Fe 2 O 3

Charge transfer effects in XPS 1 s and 2 p XPS of Fe 2 O 3

Charge transfer effects in XPS 1 s and 2 p XPS of Fe 2 O 3

Charge transfer effects in XPS 1 s and 2 p XPS of Fe 2 O 3

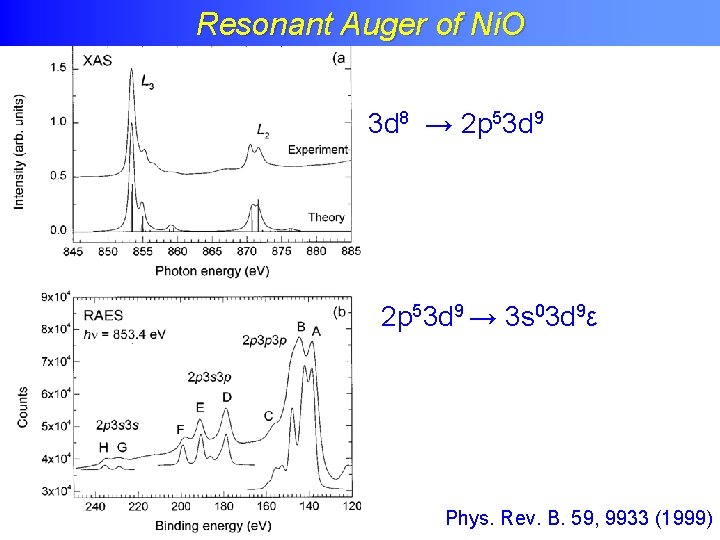
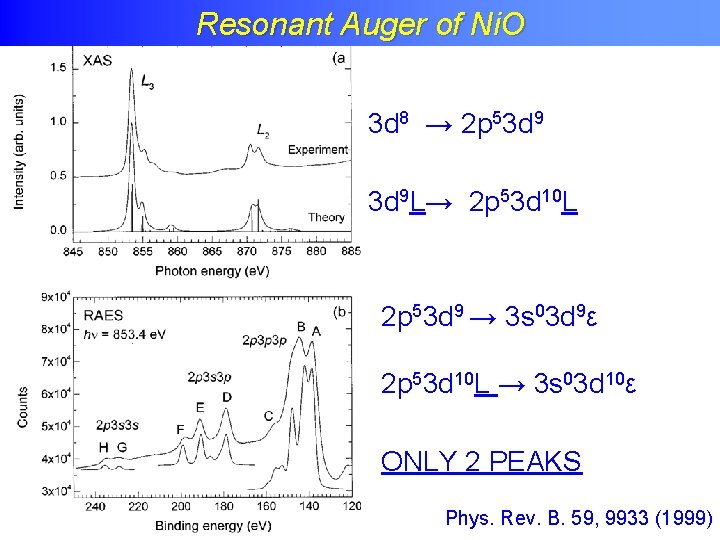
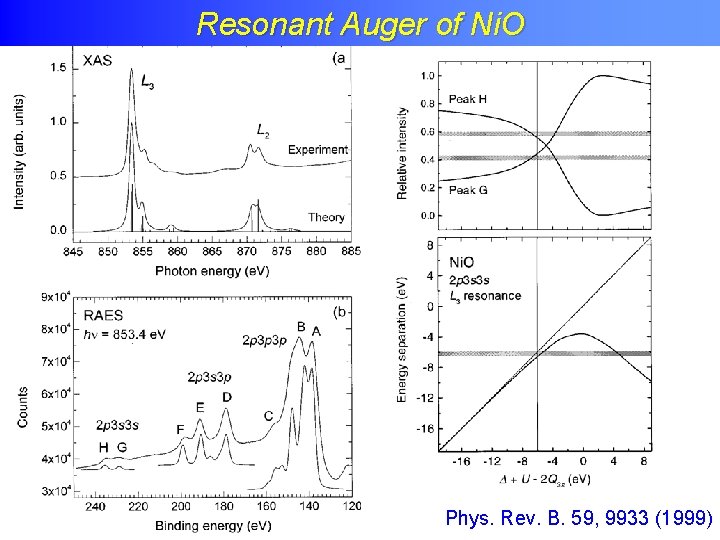
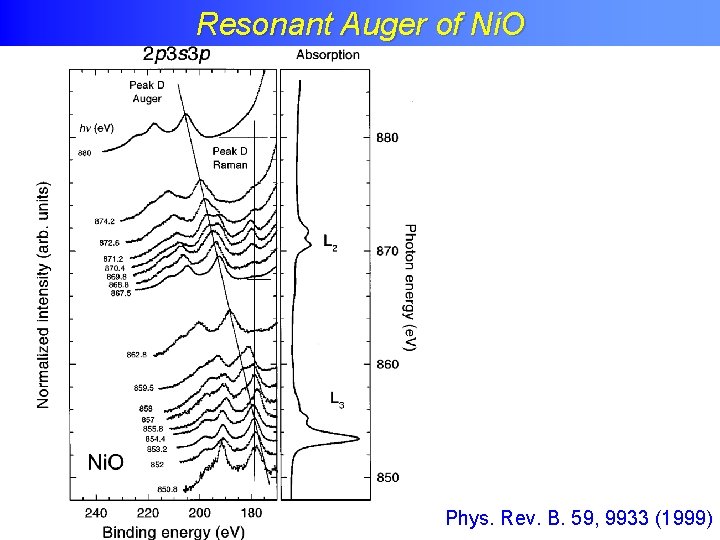
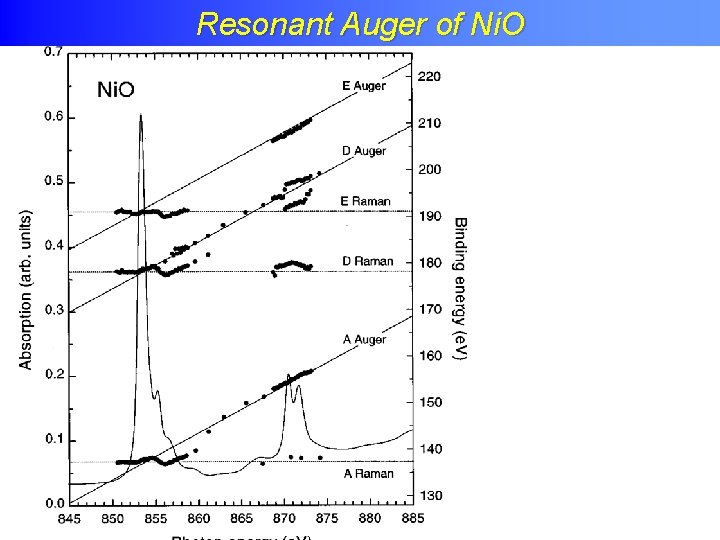
Resonant Auger of Ni. O

Resonant Auger of Ni. O Phys. Rev. B. 59, 9933 (1999)

Resonant Auger of Ni. O 3 d 8 → 2 p 53 d 9 → 3 s 03 d 9ε Phys. Rev. B. 59, 9933 (1999)

Resonant Auger of Ni. O 3 d 8 → 2 p 53 d 9 L→ 2 p 53 d 10 L 2 p 53 d 9 → 3 s 03 d 9ε 2 p 53 d 10 L → 3 s 03 d 10ε ONLY 2 PEAKS Phys. Rev. B. 59, 9933 (1999)

Resonant Auger of Ni. O Phys. Rev. B. 59, 9933 (1999)

Resonant Auger of Ni. O Phys. Rev. B. 59, 9933 (1999)

Resonant Auger of Ni. O

Ground state analysis

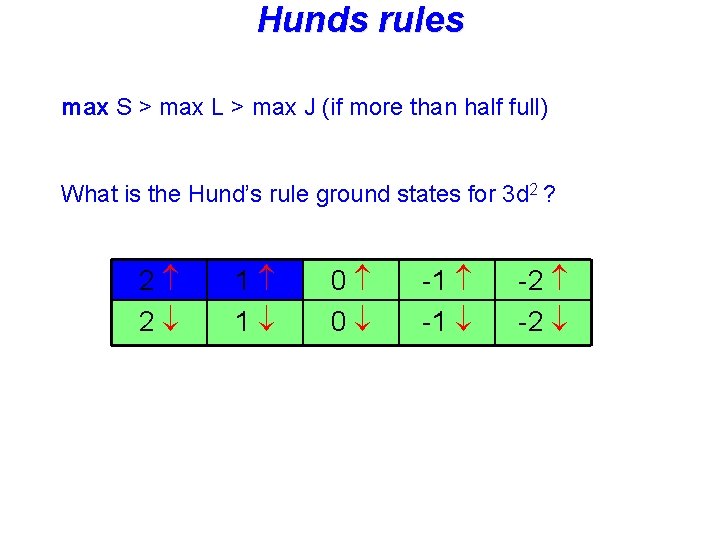
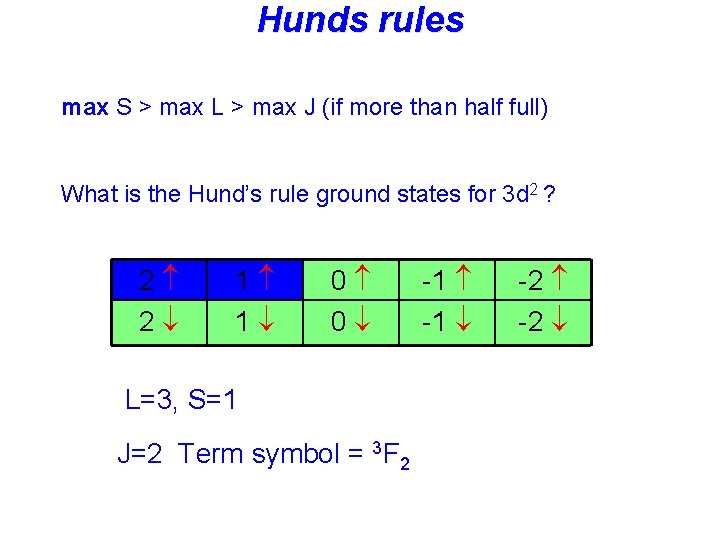
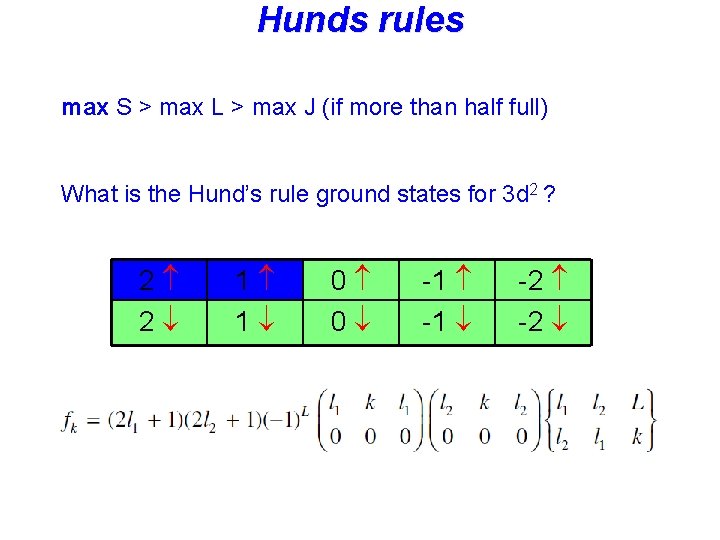
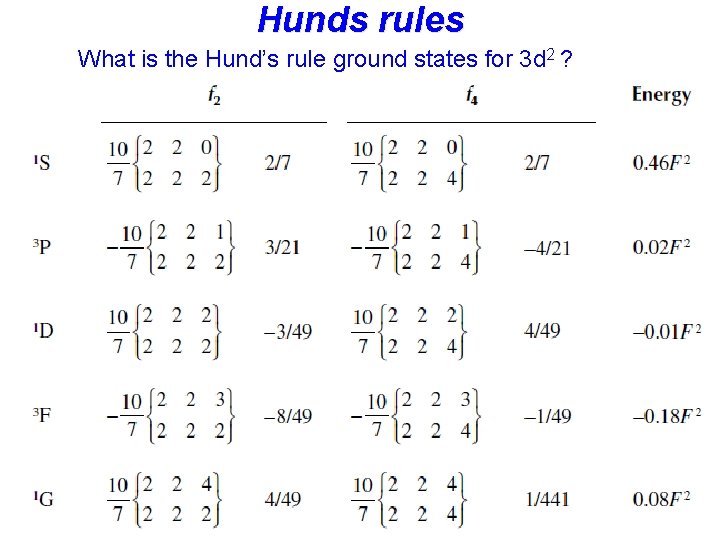
Hunds rules • Term symbols with maximum spin S are lowest in energy, • Among these terms: Term symbols with maximum L are lowest in energy • In the presence of spin-orbit coupling, the lowest term has • J = |L-S| if the shell is less than half full • J = L+S if the shell is more than half full

Hunds rules max S > max L > max J (if more than half full) What is the Hund’s rule ground states for 3 d 2 ? 2 2 1 1 0 0 -1 -2

Hunds rules max S > max L > max J (if more than half full) What is the Hund’s rule ground states for 3 d 2 ? 2 2 1 1 0 0 L=3, S=1 J=2 Term symbol = 3 F 2 -1 -2

Hunds rules max S > max L > max J (if more than half full) What is the Hund’s rule ground states for 3 d 2 ? 2 2 1 1 0 0 -1 -2

Hunds rules What is the Hund’s rule ground states for 3 d 2 ?

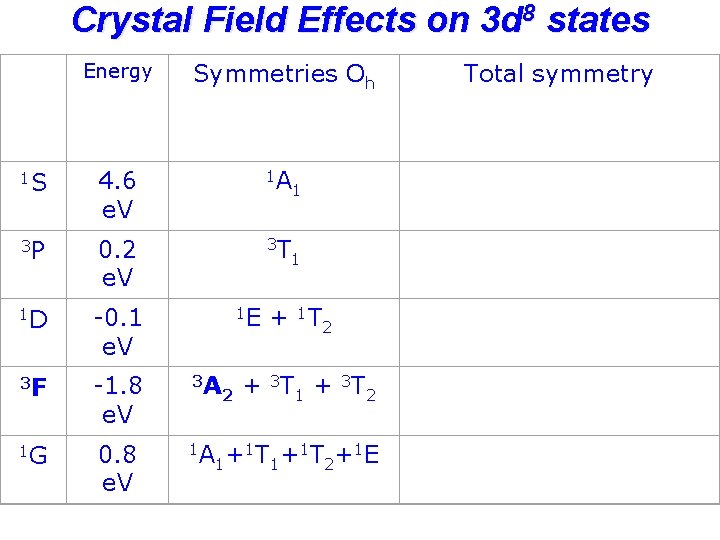
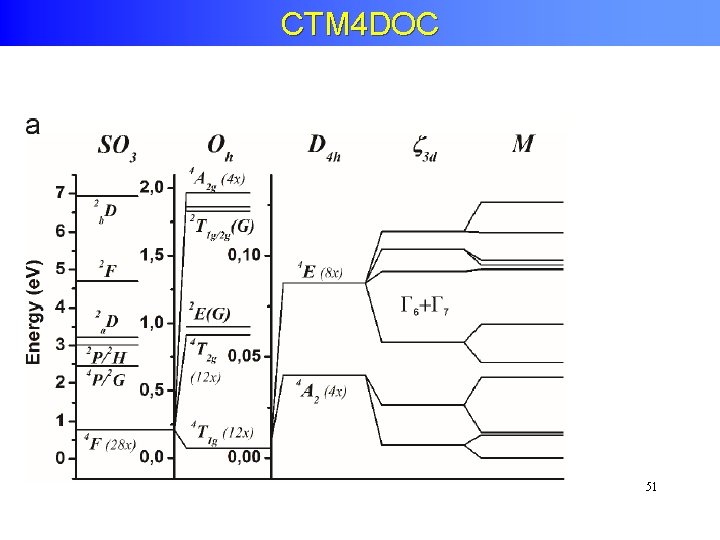
Crystal Field Effects on 3 d 8 states Energy Symmetries Oh 1 S 4. 6 e. V 1 A 3 P 0. 2 e. V 3 T 1 D -0. 1 e. V 3 F -1. 8 e. V 1 G 0. 8 e. V 1 E 3 A 1 A 2 1 1 + 1 T 2 + 3 T 1 + 3 T 2 1 T +1 E + 1 1 2 Total symmetry

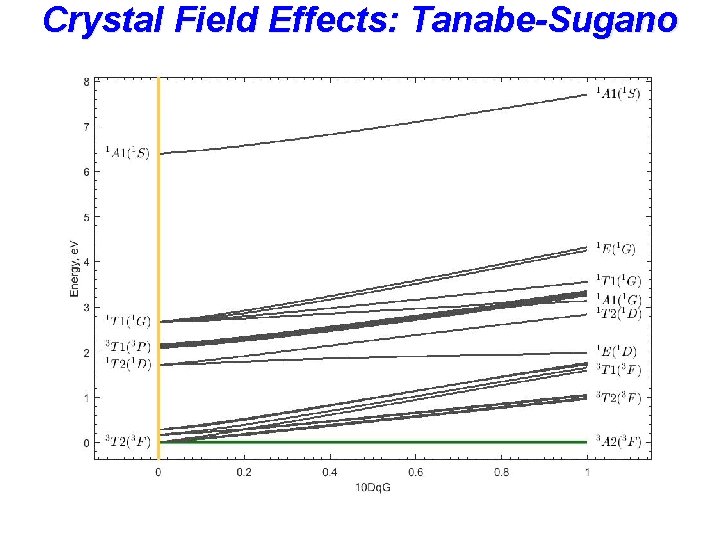
Crystal Field Effects: Tanabe-Sugano

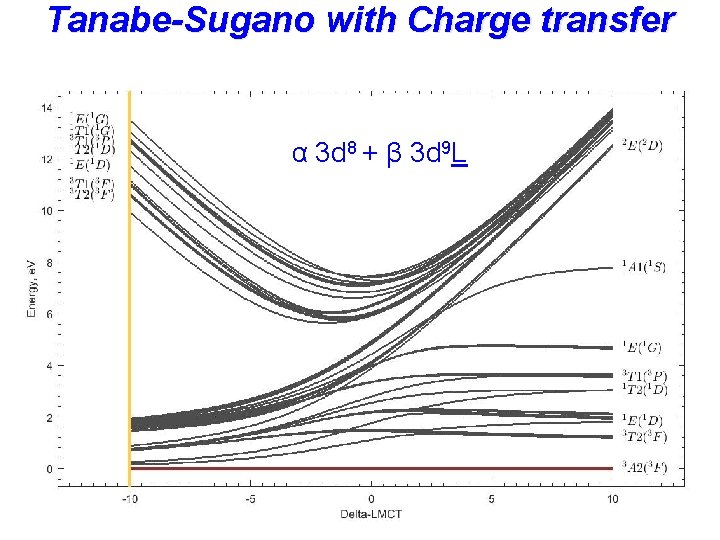
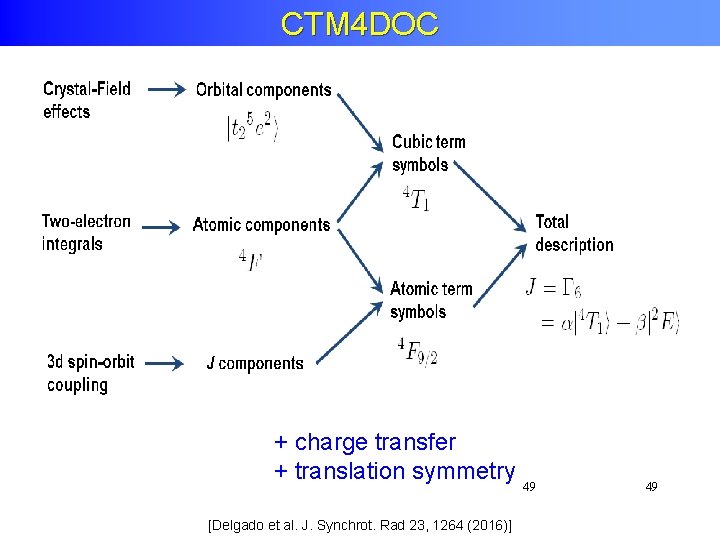
Tanabe-Sugano with Charge transfer α 3 d 8 + β 3 d 9 L
![CTM 4 DOC 48 Delgado et al J Synchrot Rad 23 1264 2016 48 CTM 4 DOC 48 [Delgado et al. J. Synchrot. Rad 23, 1264 (2016)] 48](https://slidetodoc.com/presentation_image_h2/7a76a7e78bb3153d31ed4811a94ad9fe/image-48.jpg)
CTM 4 DOC 48 [Delgado et al. J. Synchrot. Rad 23, 1264 (2016)] 48

CTM 4 DOC + charge transfer + translation symmetry 49 [Delgado et al. J. Synchrot. Rad 23, 1264 (2016)] 49

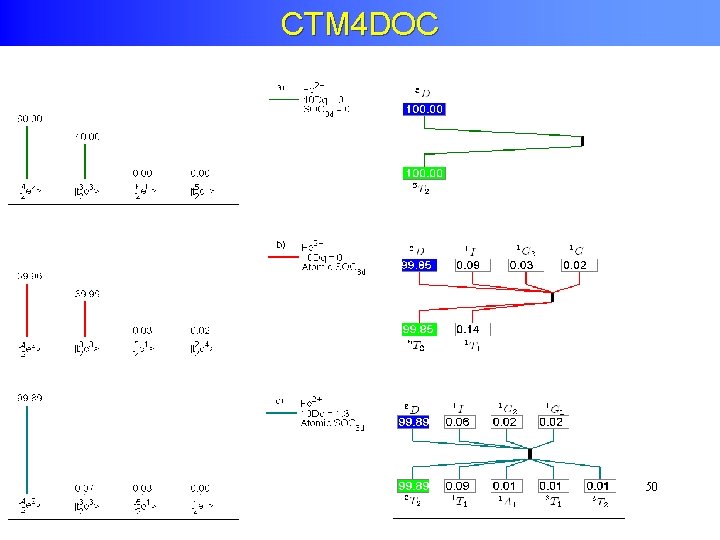
CTM 4 DOC 50 50

CTM 4 DOC 51 51