Flowcytometric quantification of minimal residual disease MRD in





- Slides: 41

Flow-cytometric quantification of minimal residual disease (MRD) in myeloma: independent outcome prediction & sequential survival benefits per log tumour reduction Andy C. Rawstron St James's Institute of Oncology

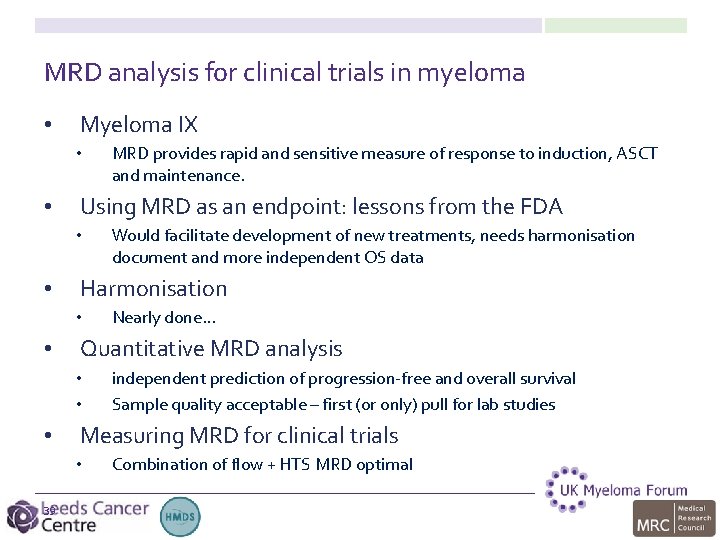
MRD analysis for clinical trials in myeloma • • • 2 Myeloma IX Using MRD as an endpoint: lessons from the FDA Harmonisation Quantitative MRD analysis Measuring MRD for clinical trials

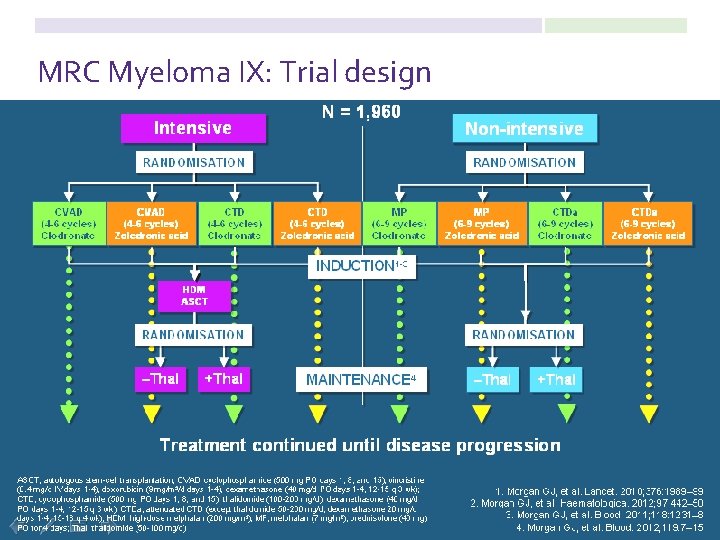
MRC Myeloma IX: Trial design

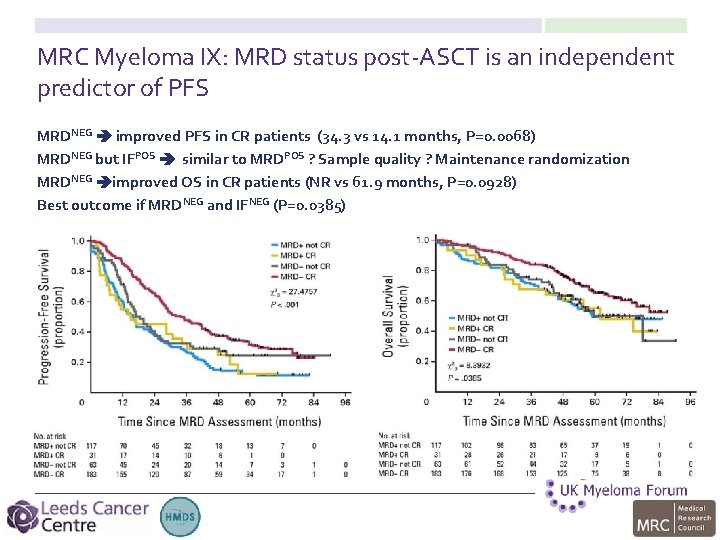
MRC Myeloma IX: MRD status post-ASCT is an independent predictor of PFS MRDNEG improved PFS in CR patients (34. 3 vs 14. 1 months, P=0. 0068) MRDNEG but IFPOS similar to MRDPOS ? Sample quality ? Maintenance randomization MRDNEG improved OS in CR patients (NR vs 61. 9 months, P=0. 0928) Best outcome if MRDNEG and IFNEG (P=0. 0385)

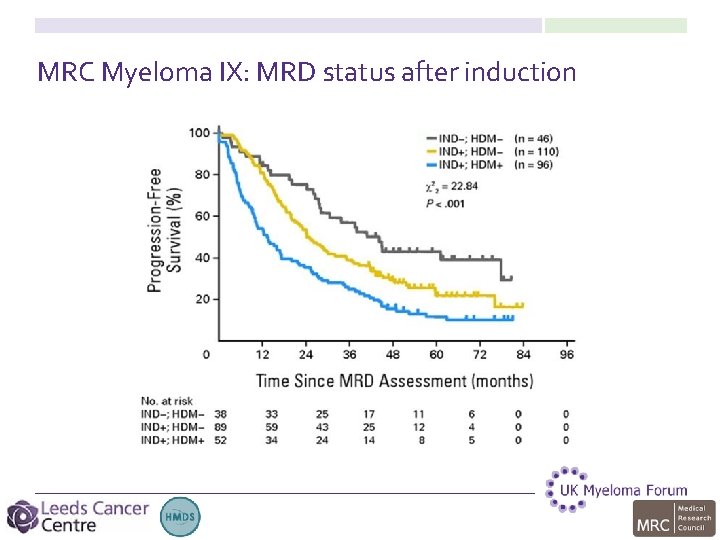
MRC Myeloma IX: MRD status after induction

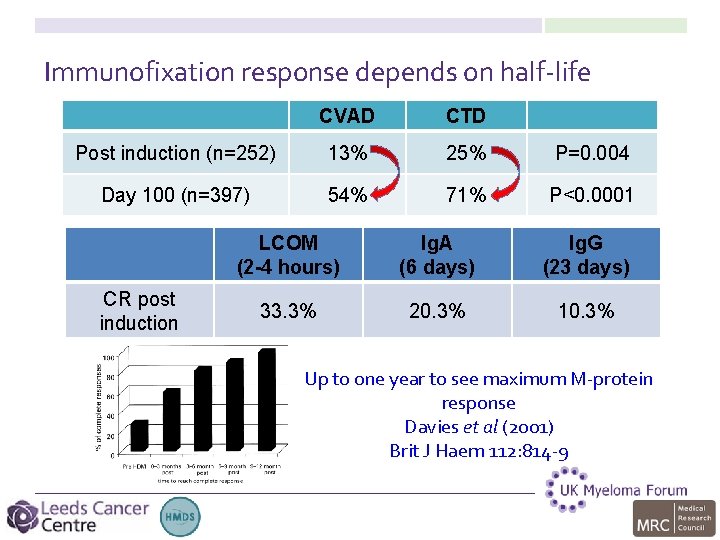
Immunofixation response depends on half-life CVAD CTD Post induction (n=252) 13% 25% P=0. 004 Day 100 (n=397) 54% 71% P<0. 0001 CR post induction LCOM (2 -4 hours) Ig. A (6 days) Ig. G (23 days) 33. 3% 20. 3% 10. 3% Up to one year to see maximum M-protein response Davies et al (2001) Brit J Haem 112: 814 -9

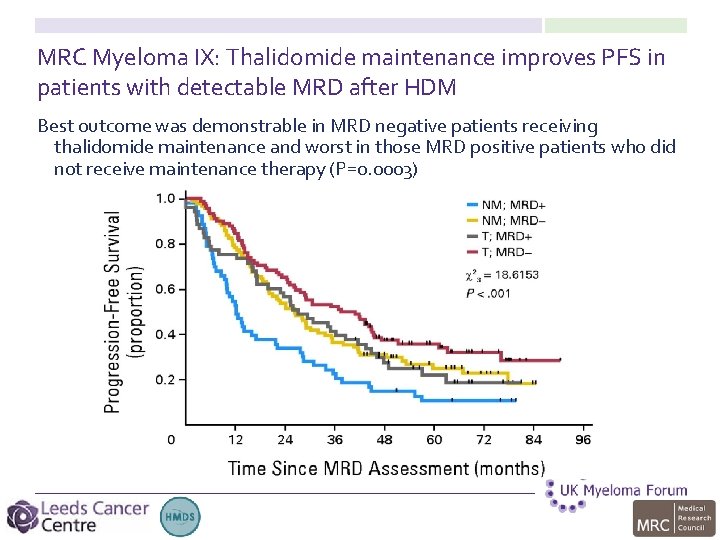
MRC Myeloma IX: Thalidomide maintenance improves PFS in patients with detectable MRD after HDM Best outcome was demonstrable in MRD negative patients receiving thalidomide maintenance and worst in those MRD positive patients who did not receive maintenance therapy (P=0. 0003)

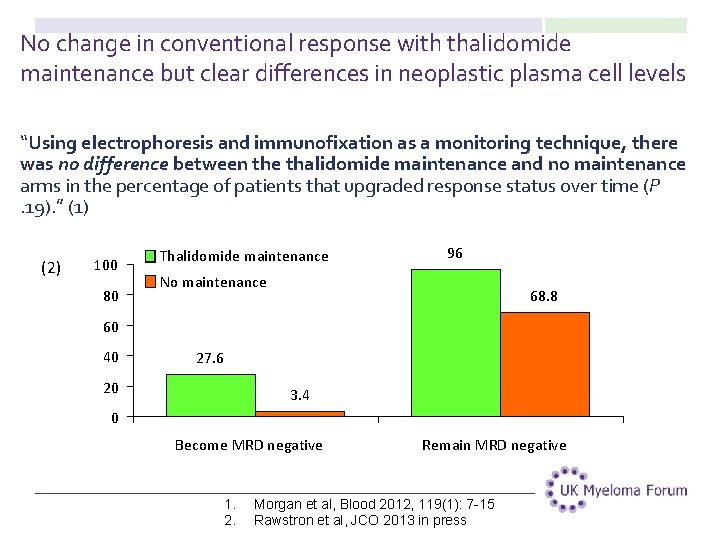
No change in conventional response with thalidomide maintenance but clear differences in neoplastic plasma cell levels “Using electrophoresis and immunofixation as a monitoring technique, there was no difference between the thalidomide maintenance and no maintenance arms in the percentage of patients that upgraded response status over time (P. 19). ” (1) (2) 100 80 Thalidomide maintenance 96 No maintenance 68. 8 60 40 27. 6 20 3. 4 0 Become MRD negative 1. 2. Remain MRD negative Morgan et al, Blood 2012, 119(1): 7 -15 Rawstron et al, JCO 2013 in press

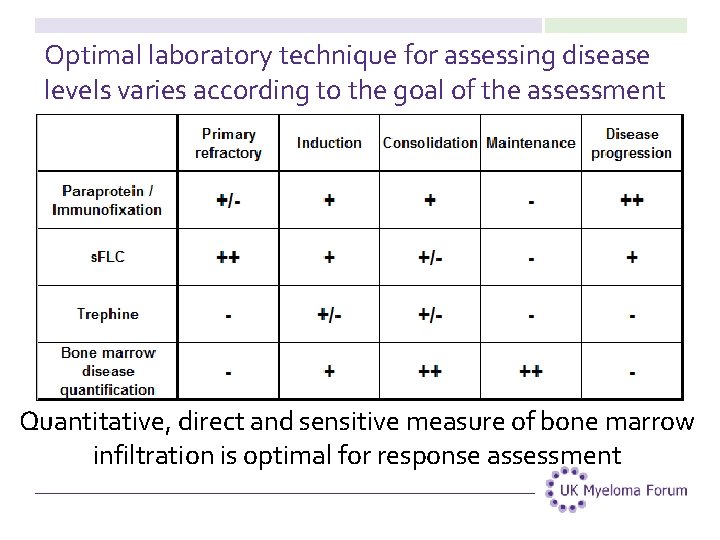
Optimal laboratory technique for assessing disease levels varies according to the goal of the assessment Quantitative, direct and sensitive measure of bone marrow infiltration is optimal for response assessment

MRD analysis for clinical trials in myeloma • Myeloma IX • • • 10 MRD provides rapid and sensitive measure of response to induction, ASCT and maintenance. Using MRD as an endpoint: lessons from the FDA Harmonisation Quantitative MRD analysis Measuring MRD for clinical trials

Is MRD suitable as an end-point for clinical trials in myeloma? • MRD analysis improves assessment of response compared to serum markers alone, particularly in multicomponent strategies • Longer survival with increasing treatment options need for biomarkers that predict clinical benefit and offer a rapid measure of treatment efficacy Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI roundtable symposium Am J Hematol. 2014 Dec; 89(12): 1159 -60

Development of “MRD” as a regulatory end-point • Identify MRD Endpoint in Clinical Trials • • Develop Assay • • Disease-specific flow assay applicable to larger trials Standardization of Assay (NIH Consensus Conference) • • • 5 -10 sub-group analyses, primarily ASO-IGH q. PCR EMN consensus Apply Standardized Assay Prospectively Apply to Regulatory Action

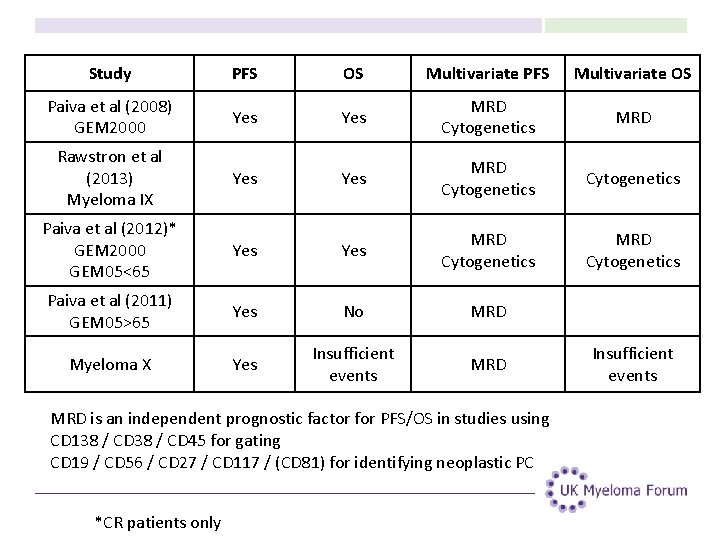
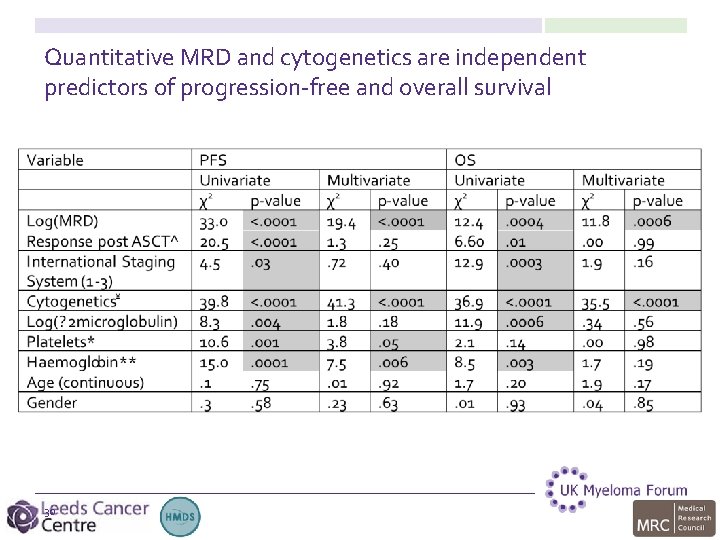
Study PFS OS Multivariate PFS Multivariate OS Paiva et al (2008) GEM 2000 Yes MRD Cytogenetics MRD Rawstron et al (2013) Myeloma IX Yes MRD Cytogenetics Paiva et al (2012)* GEM 2000 GEM 05<65 Yes MRD Cytogenetics Paiva et al (2011) GEM 05>65 Yes No MRD Myeloma X Yes Insufficient events MRD is an independent prognostic factor for PFS/OS in studies using CD 138 / CD 45 for gating CD 19 / CD 56 / CD 27 / CD 117 / (CD 81) for identifying neoplastic PC *CR patients only Insufficient events

MRD analysis for clinical trials in myeloma • Myeloma IX • • Using MRD as an endpoint: lessons from the FDA • • 14 MRD provides rapid and sensitive measure of response to induction, ASCT and maintenance. Would facilitate development of new treatments, needs harmonisation document and more independent OS data Harmonisation Quantitative MRD analysis Measuring MRD for clinical trials

Harmonised assay for MRD detection • Characteristics of assays that predict outcome • • CD 138/CD 45 backbone for gating CD 19/CD 56/CD 27/CD 117/CD 81 for characterization • Reagent specification to permit rapid validation of LDT (lab-developed test) or IVD panels • Backwards compatible • Suitable for prospective studies • targeted acquisition of 3 -5 million events

MRD analysis for clinical trials in myeloma • Myeloma IX • • Using MRD as an endpoint: lessons from the FDA • • 16 Would facilitate development of new treatments, needs harmonisation document and more independent OS data Harmonisation • • • MRD provides rapid and sensitive measure of response to induction, ASCT and maintenance. Nearly done… Quantitative measure of outcome Measuring MRD for clinical trials

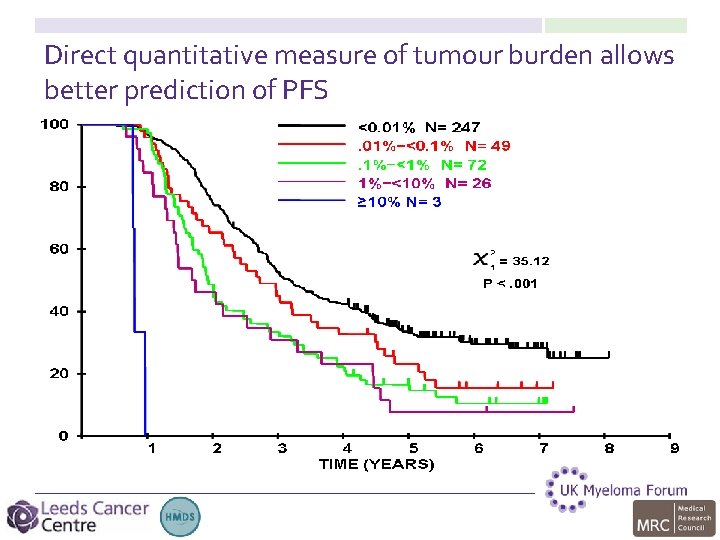
Direct quantitative measure of tumour burden allows better prediction of PFS

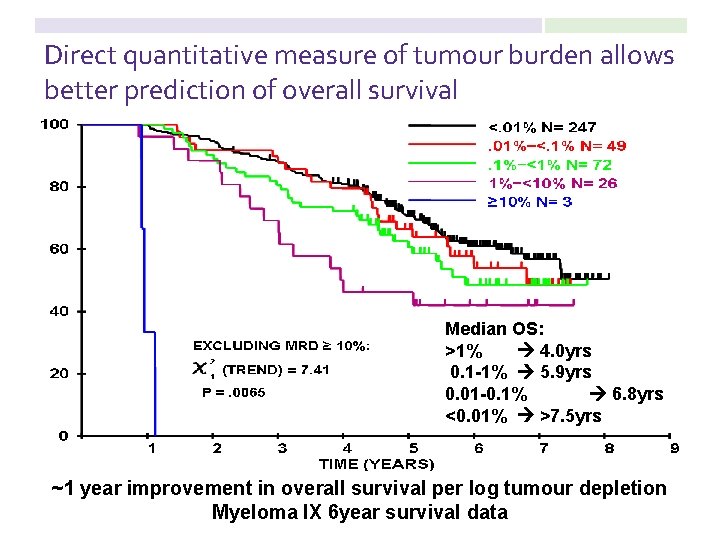
Direct quantitative measure of tumour burden allows better prediction of overall survival Median OS: >1% 4. 0 yrs 0. 1 -1% 5. 9 yrs 0. 01 -0. 1% 6. 8 yrs <0. 01% >7. 5 yrs ~1 year improvement in overall survival per log tumour depletion Myeloma IX 6 year survival data

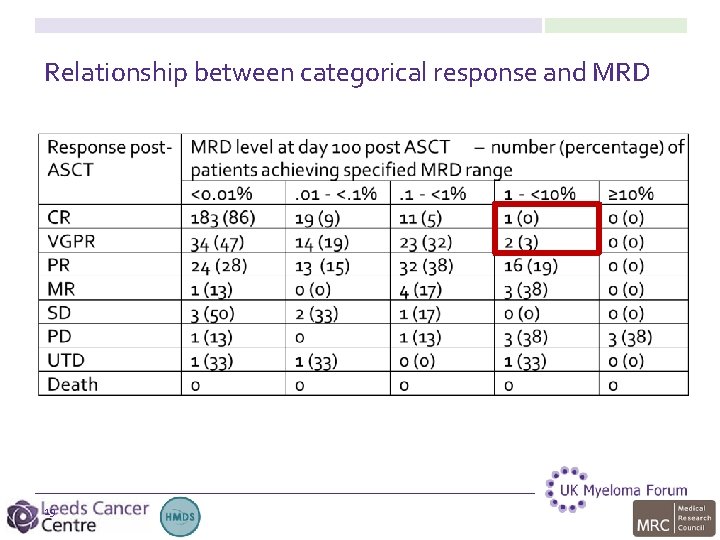
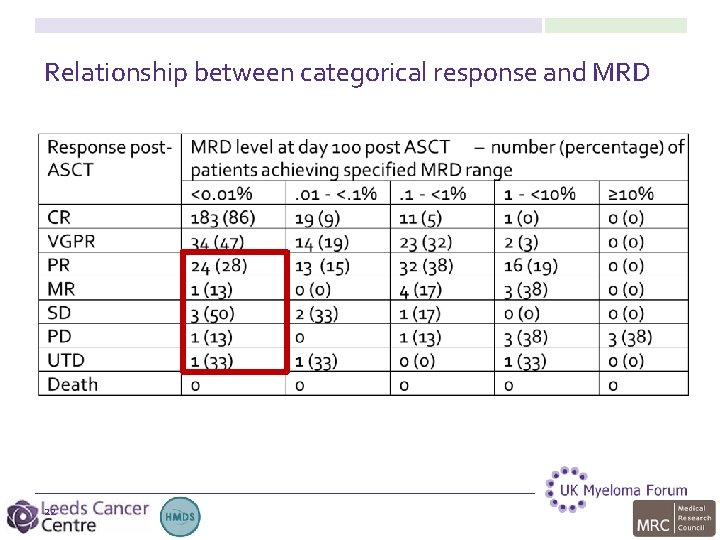
Relationship between categorical response and MRD 19

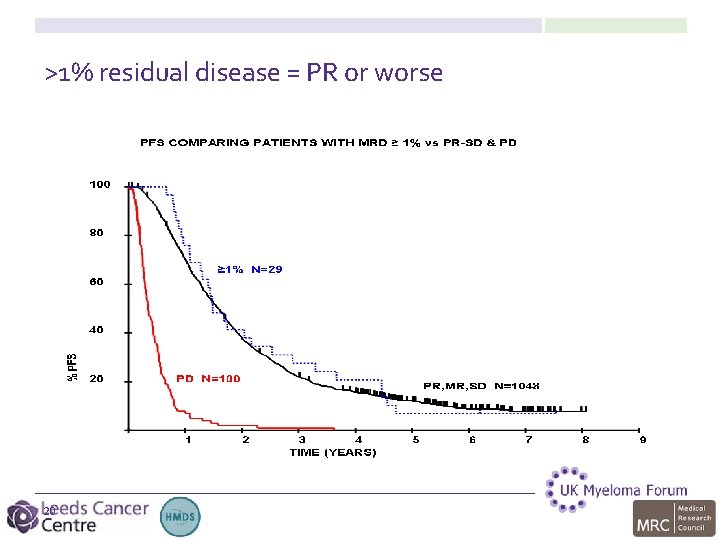
>1% residual disease = PR or worse 20

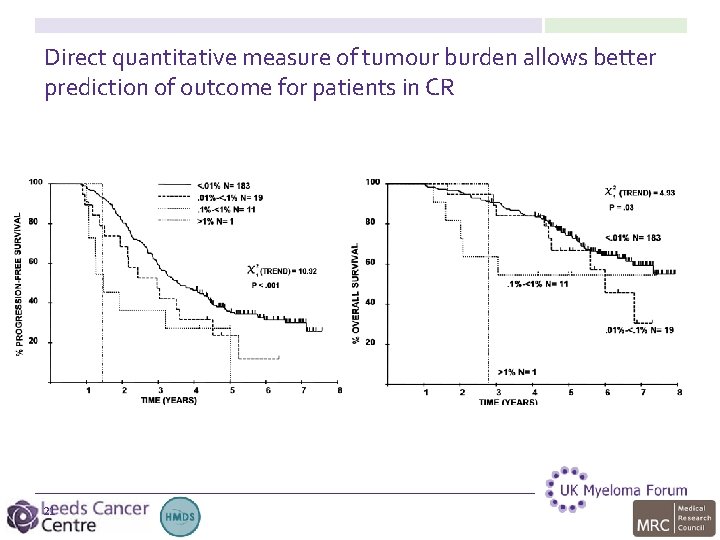
Direct quantitative measure of tumour burden allows better prediction of outcome for patients in CR 21

Relationship between categorical response and MRD 22

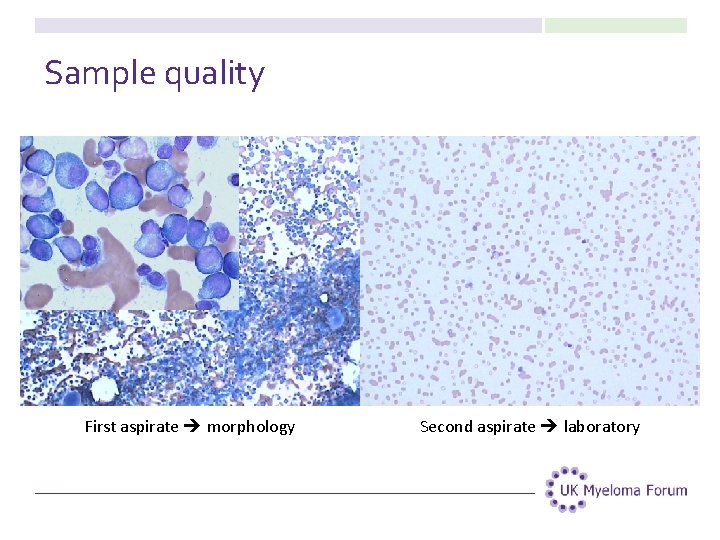
Sample quality First aspirate morphology Second aspirate laboratory

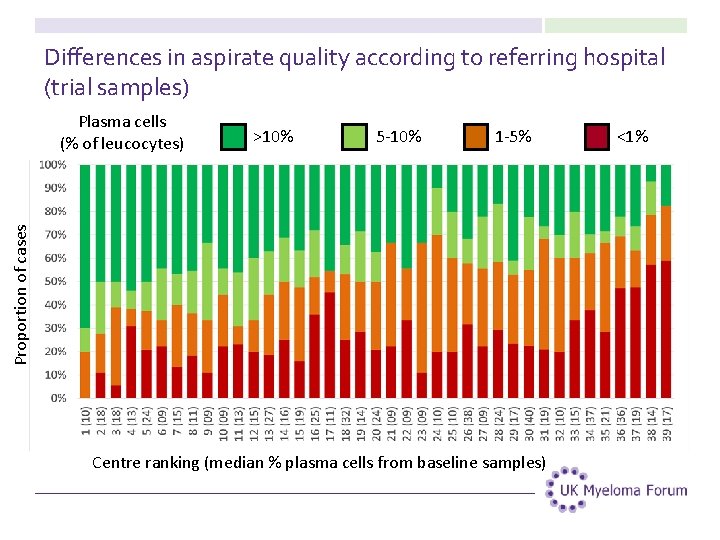
Differences in aspirate quality according to referring hospital (trial samples) >10% 5 -10% 1 -5% Proportion of cases Plasma cells (% of leucocytes) Centre ranking (median % plasma cells from baseline samples) <1%

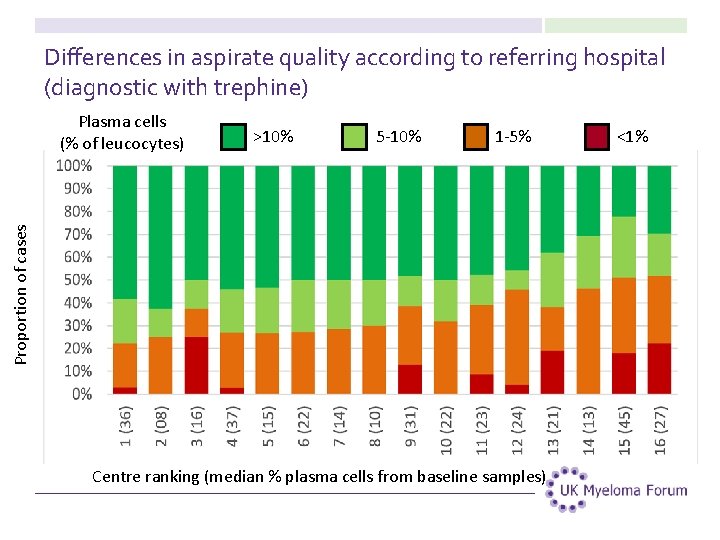
Differences in aspirate quality according to referring hospital (diagnostic with trephine) >10% 5 -10% 1 -5% Proportion of cases Plasma cells (% of leucocytes) Centre ranking (median % plasma cells from baseline samples) <1%

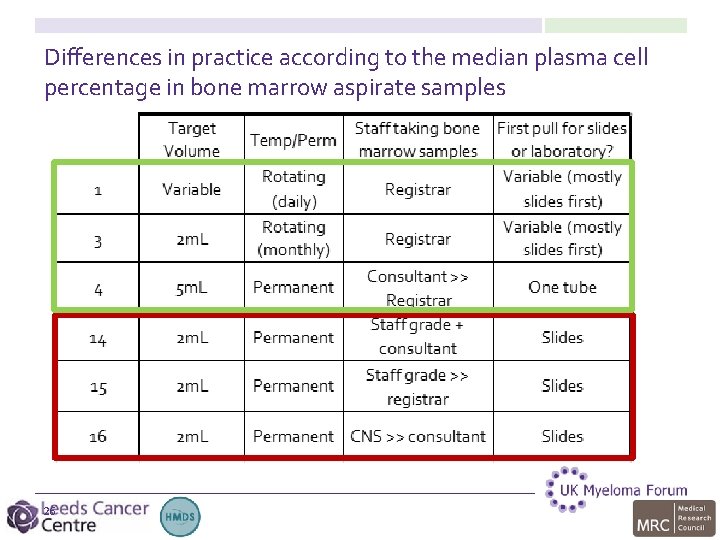
Differences in practice according to the median plasma cell percentage in bone marrow aspirate samples 26

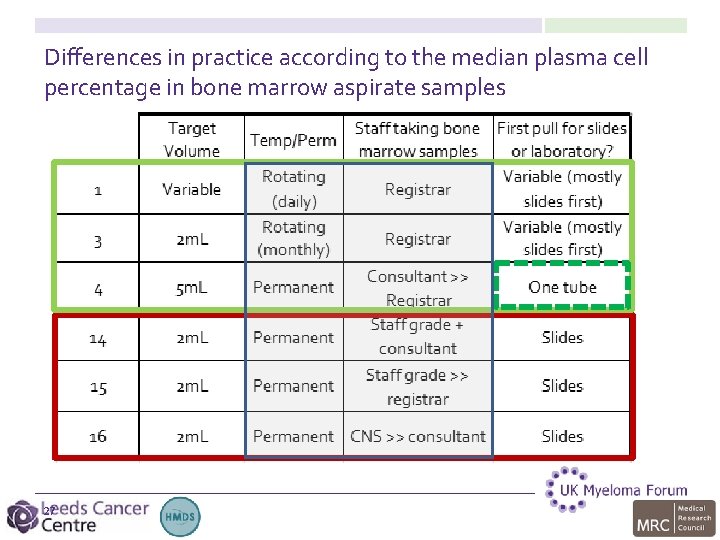
Differences in practice according to the median plasma cell percentage in bone marrow aspirate samples 27

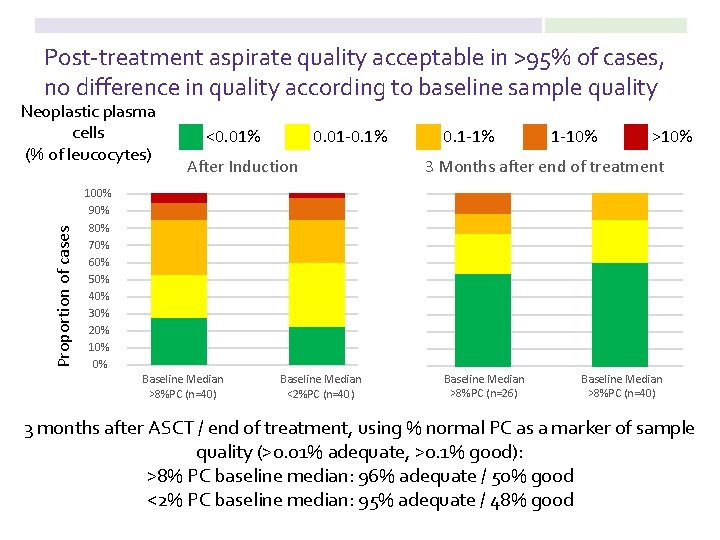
Post-treatment aspirate quality acceptable in >95% of cases, no difference in quality according to baseline sample quality Proportion of cases Neoplastic plasma cells (% of leucocytes) <0. 01% 0. 01 -0. 1% After Induction 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Baseline Median >8%PC (n=40) Baseline Median <2%PC (n=40) 0. 1 -1% 1 -10% >10% 3 Months after end of treatment Baseline Median >8%PC (n=26) Baseline Median >8%PC (n=40) 3 months after ASCT / end of treatment, using % normal PC as a marker of sample quality (>0. 01% adequate, >0. 1% good): >8% PC baseline median: 96% adequate / 50% good <2% PC baseline median: 95% adequate / 48% good

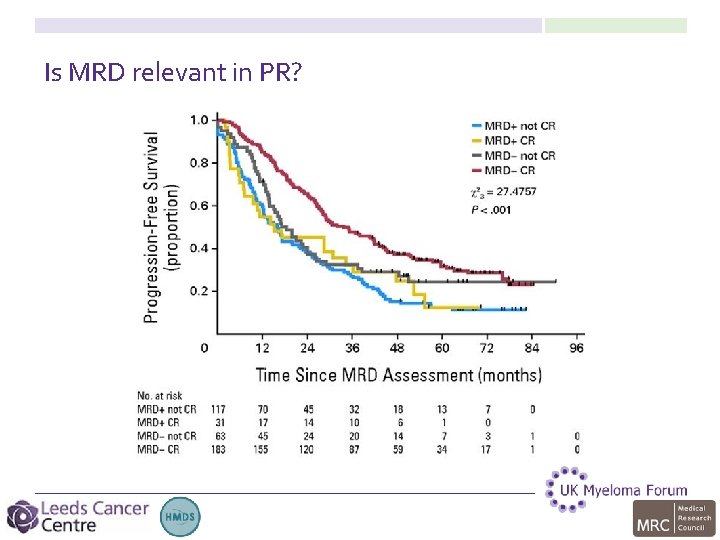
Is MRD relevant in PR?

Quantitative MRD and cytogenetics are independent predictors of progression-free and overall survival 30

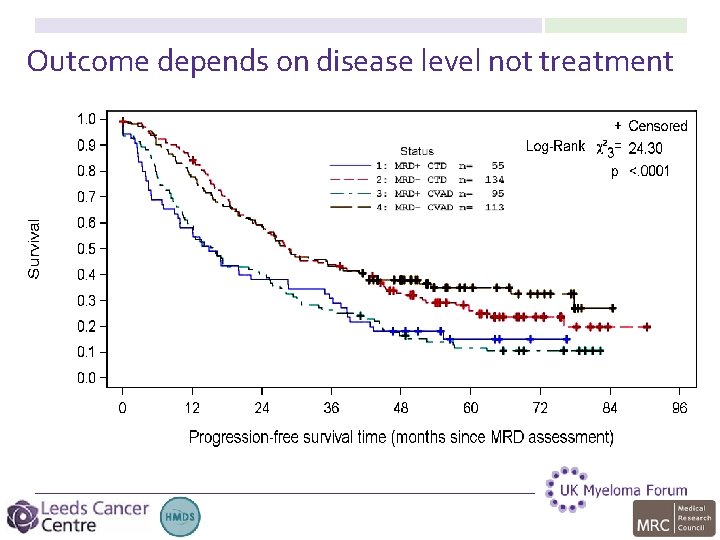
Outcome depends on disease level not treatment

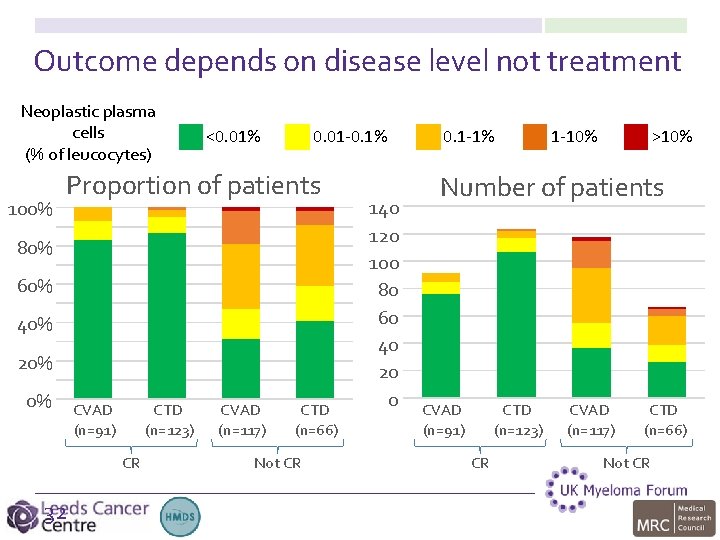
Outcome depends on disease level not treatment Neoplastic plasma cells (% of leucocytes) 100% <0. 01% 0. 01 -0. 1% Proportion of patients 80% 60% 40% 20% 0% CVAD (n=91) CTD (n=123) CR 32 CVAD (n=117) CTD (n=66) Not CR 140 120 100 80 60 40 20 0 0. 1 -1% 1 -10% >10% Number of patients CVAD (n=91) CTD (n=123) CR CVAD (n=117) CTD (n=66) Not CR

Achieving <0. 01% MRD: impact of ASCT • Median (range) % neoplastic plasma cells at end of induction, data available in 253/397 cases, of which 47/253 had <0. 01% MRD after induction and ASCT ≥ 0. 01% MRD after ASCT (n=96) 1. 5% (0. 02 – 25%) <0. 01% MRD after ASCT (n=110) 0. 02% (0. 02 – 14%) • ≥ 0. 01% MRD after induction & ASCT Median 0. 67 log tumour depletion (range -1. 4 – 2. 6) • ≥ 0. 01% MRD after induction & <0. 01% MRD after ASCT Median >1. 7 log tumour depletion • Responsive patients achieve ~2 log depletion to ASCT • >1% MRD after induction unlikely to respond optimally • • 33

MRD analysis for clinical trials in myeloma • Myeloma IX • • Using MRD as an endpoint: lessons from the FDA • • 34 Nearly done… Quantitative measure of outcome • • • Would facilitate development of new treatments, needs harmonisation document and more independent OS data Harmonisation • • MRD provides rapid and sensitive measure of response to induction, ASCT and maintenance. independent predictors of progression-free and overall survival Sample quality acceptable – first (or only) pull for lab studies Measuring MRD for clinical trials

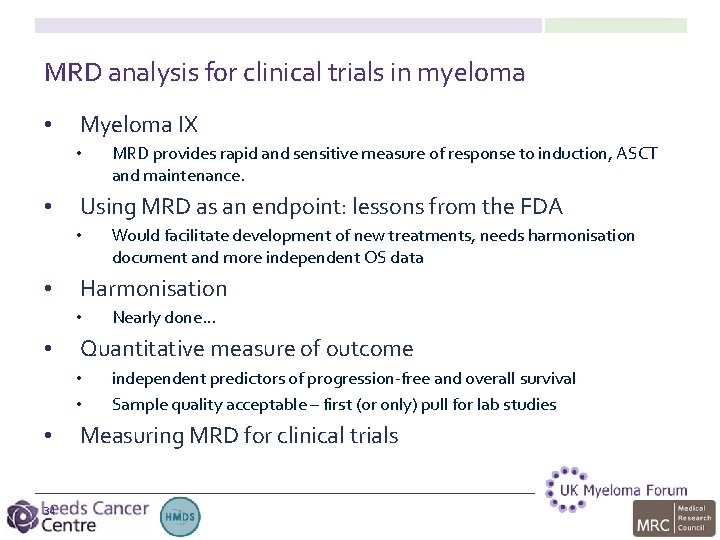
High-throughput sequencing: >1 log errors ERIC harmonised approach Leukemia 2007, 21(5): & 2013, 27(1) Logan et al. Leukemia 12 March 2013; doi: 10. 1038/leu. 2013. 52:

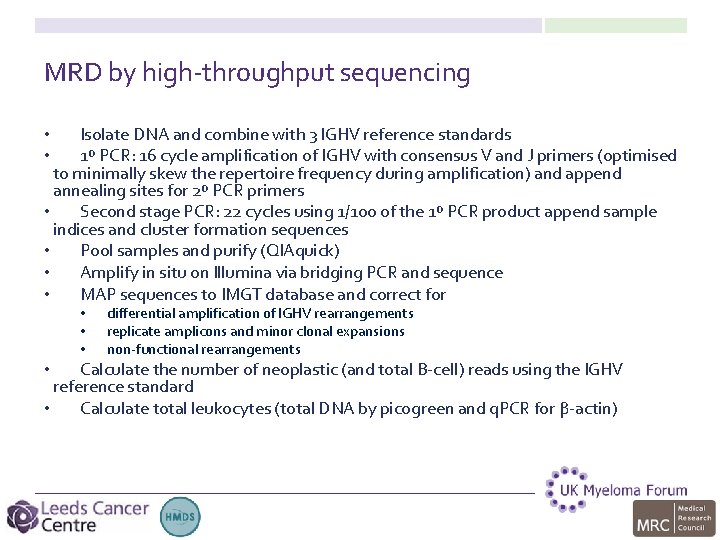
MRD by high-throughput sequencing Isolate DNA and combine with 3 IGHV reference standards 1º PCR: 16 cycle amplification of IGHV with consensus V and J primers (optimised to minimally skew the repertoire frequency during amplification) and append annealing sites for 2º PCR primers • Second stage PCR: 22 cycles using 1/100 of the 1º PCR product append sample indices and cluster formation sequences • Pool samples and purify (QIAquick) • Amplify in situ on Illumina via bridging PCR and sequence • MAP sequences to IMGT database and correct for • • • differential amplification of IGHV rearrangements replicate amplicons and minor clonal expansions non-functional rearrangements Calculate the number of neoplastic (and total B-cell) reads using the IGHV reference standard • Calculate total leukocytes (total DNA by picogreen and q. PCR for β-actin) •

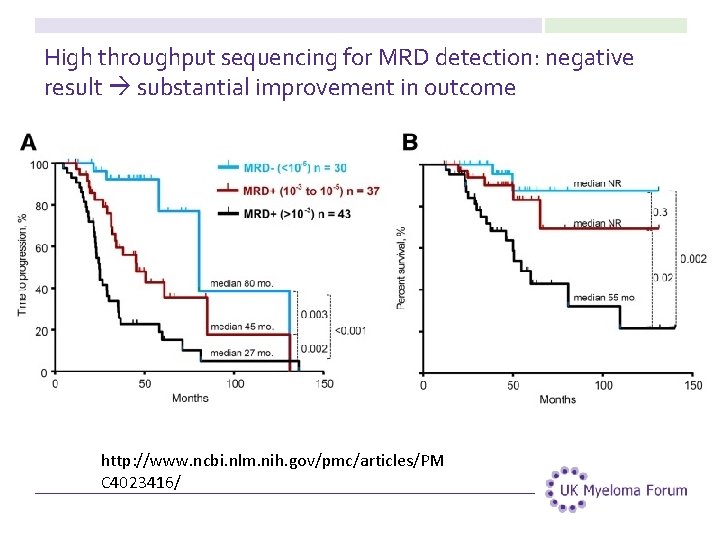
High throughput sequencing for MRD detection: negative result substantial improvement in outcome http: //www. ncbi. nlm. nih. gov/pmc/articles/PM C 4023416/

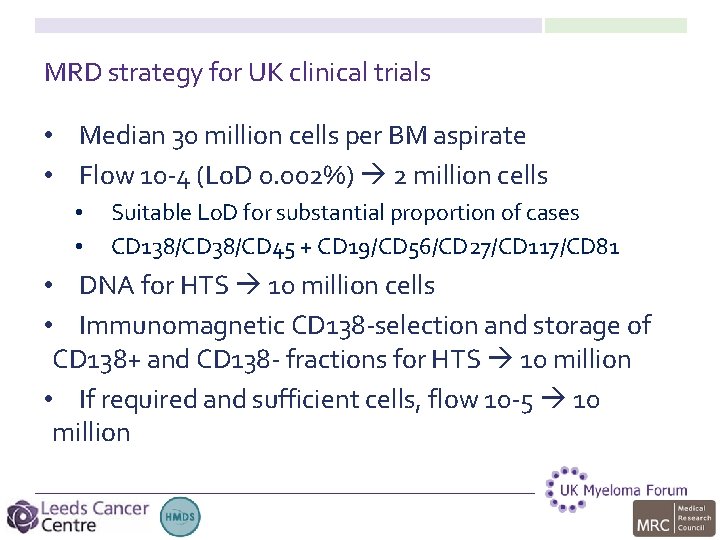
MRD strategy for UK clinical trials • Median 30 million cells per BM aspirate • Flow 10 -4 (Lo. D 0. 002%) 2 million cells • • Suitable Lo. D for substantial proportion of cases CD 138/CD 45 + CD 19/CD 56/CD 27/CD 117/CD 81 • DNA for HTS 10 million cells • Immunomagnetic CD 138 -selection and storage of CD 138+ and CD 138 - fractions for HTS 10 million • If required and sufficient cells, flow 10 -5 10 million

MRD analysis for clinical trials in myeloma • Myeloma IX • • Using MRD as an endpoint: lessons from the FDA • • independent prediction of progression-free and overall survival Sample quality acceptable – first (or only) pull for lab studies Measuring MRD for clinical trials • 39 Nearly done… Quantitative MRD analysis • • • Would facilitate development of new treatments, needs harmonisation document and more independent OS data Harmonisation • • MRD provides rapid and sensitive measure of response to induction, ASCT and maintenance. Combination of flow + HTS MRD optimal

Acknowledgements Chief Investigators JA Child GJ Morgan GH Jackson CTRU, Leeds K Cocks W Gregory A Szubert S Bell N Navarro Coy University of Birmingham MT Drayson K Walker A Adkins N Newnham Wessex Regional Genetics Laboratory, Salisbury F Ross L Chieccio LTHT, Leeds G Cook S Feyler D Bowen HMDS, Leeds RG Owen AC Rawstron R de Tute M Dewar S Denman ICR, London FE Davies M Jenner B Walker D Johnson D Gonzalez N Dickens K Boyd P Leone L Brito A Avridromou MRC Leukaemia Trial Steering Committee MRC Leukaemia Data Monitoring and Ethics Committee NCRI Haematological Oncology Clinical Studies Group NIHR, through the National Cancer Research Network UK Myeloma Forum Clinical Trials Committee Myeloma UK Funding Medical Research Council Pharmion Novartis Chugai Pharma Bayer Schering Pharma Ortho. Biotech Celgene Kay Kendall Leukaemia Fund

41 Thanks!
 Minimal residual disease
Minimal residual disease Analisis residual
Analisis residual Vacuous quantification
Vacuous quantification Universal quantification examples
Universal quantification examples Highly multiplexed protein quantification
Highly multiplexed protein quantification Kriti mohan abnormal
Kriti mohan abnormal Dr shahzad ahmad
Dr shahzad ahmad Minimal change disease
Minimal change disease Goodpasture syndrome
Goodpasture syndrome Mrd
Mrd Bee
Bee Mrd supply chain
Mrd supply chain What is mrd in product management
What is mrd in product management Mrd kx1
Mrd kx1 Ngs mrd
Ngs mrd Mandatory removal date army regulation
Mandatory removal date army regulation Mrd
Mrd Bharathi viswanathan
Bharathi viswanathan Residual properties in thermodynamics
Residual properties in thermodynamics Cultura residual dibujos
Cultura residual dibujos Section 10 topic 5 residuals and residual plots
Section 10 topic 5 residuals and residual plots Residual plot shapes
Residual plot shapes Residual standard error
Residual standard error Residual blockade
Residual blockade Residual mix
Residual mix Calculate residual income
Calculate residual income Modulación de banda lateral vestigial
Modulación de banda lateral vestigial Residual properties in thermodynamics
Residual properties in thermodynamics Residual method of valuation pros and cons
Residual method of valuation pros and cons Acn compensation plan
Acn compensation plan Types of leases
Types of leases Tax preference theory
Tax preference theory Deep residual learning for image recognition
Deep residual learning for image recognition Functional residual capacity
Functional residual capacity Residual cyst
Residual cyst Ppr dentomucosoportada
Ppr dentomucosoportada Entropia residual
Entropia residual Llnet
Llnet Residual titration คือ
Residual titration คือ Radiacion residual rayos x
Radiacion residual rayos x Advanced thermodynamics
Advanced thermodynamics Ilsvrc
Ilsvrc