MRD testing which platforms which patients Roger Owen



- Slides: 26

MRD testing: which platforms, which patients? Roger Owen St James’s Institute of Oncology Leeds



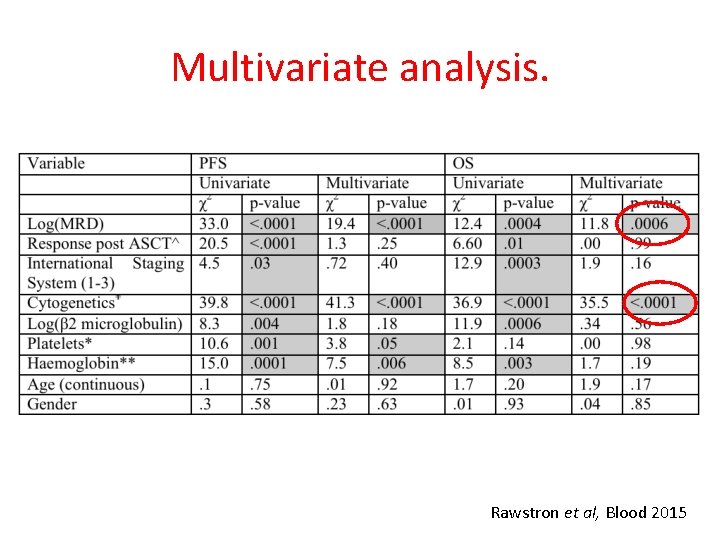
Multivariate analysis. Rawstron et al, Blood 2015

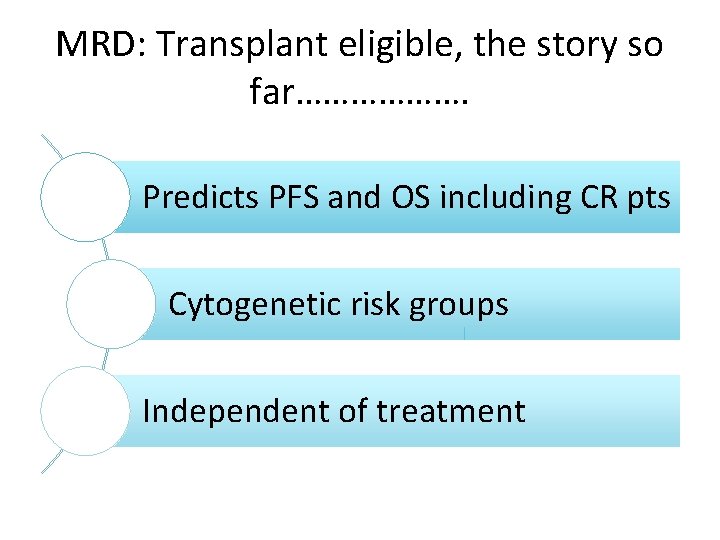
MRD: Transplant eligible, the story so far………………. Predicts PFS and OS including CR pts Cytogenetic risk groups Independent of treatment

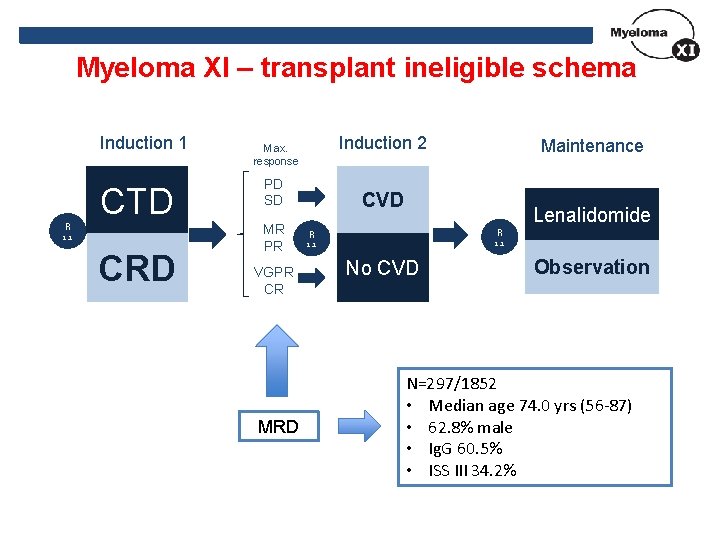
Myeloma XI – transplant ineligible schema Induction 1 R CTD 1: 1 CRD Induction 2 Max. response PD SD MR PR VGPR CR MRD Maintenance CVD R R Lenalidomide 1: 1 No CVD Observation N=297/1852 • Median age 74. 0 yrs (56 -87) • 62. 8% male • Ig. G 60. 5% • ISS III 34. 2%

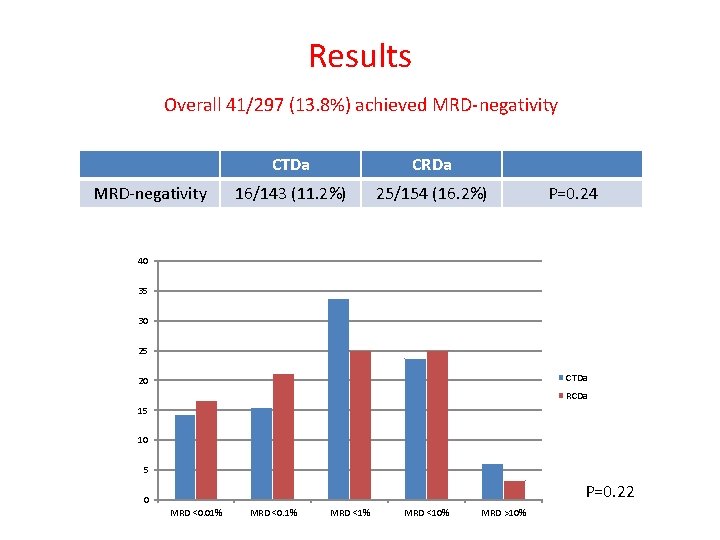
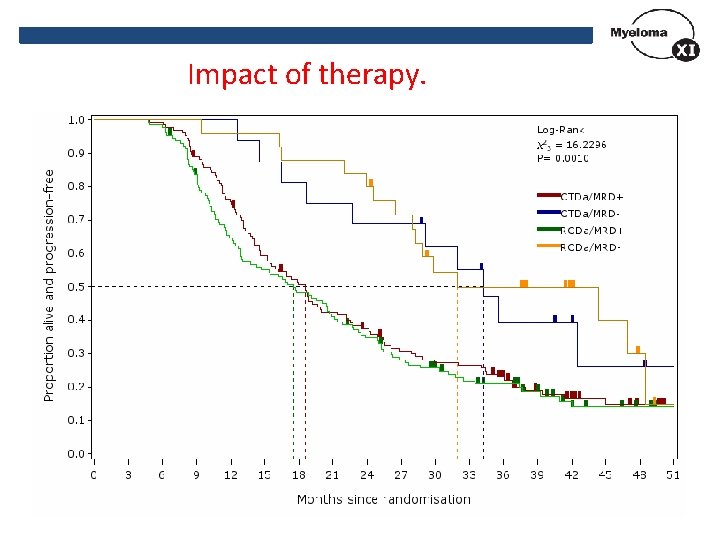
Results Overall 41/297 (13. 8%) achieved MRD-negativity CTDa CRDa 16/143 (11. 2%) 25/154 (16. 2%) P=0. 24 40 35 30 25 CTDa 20 RCDa 15 10 5 P=0. 22 0 MRD <0. 01% MRD <0. 1% MRD <10% MRD >10%

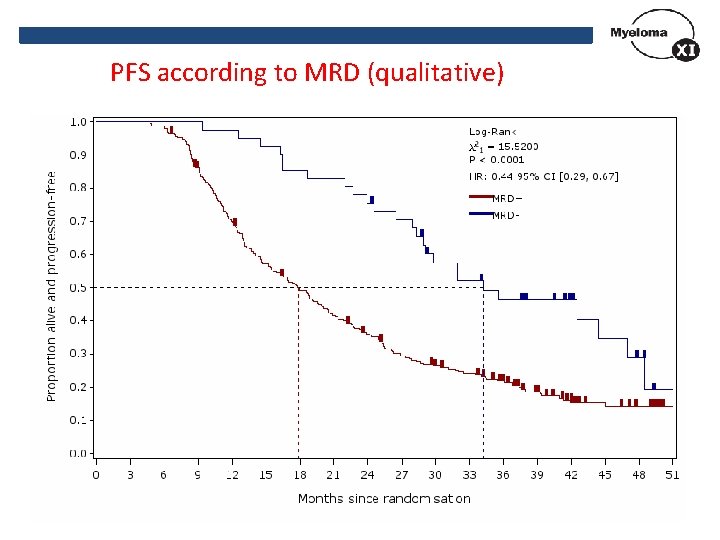
PFS according to MRD (qualitative)

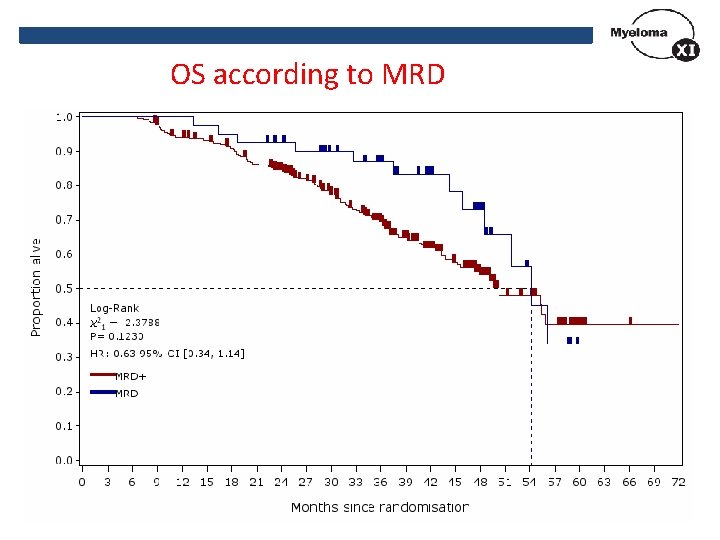
OS according to MRD

Impact of therapy.

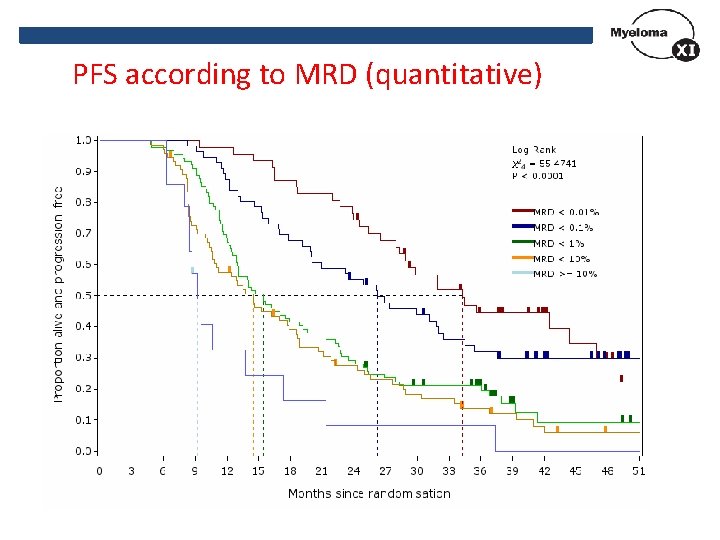
PFS according to MRD (quantitative)

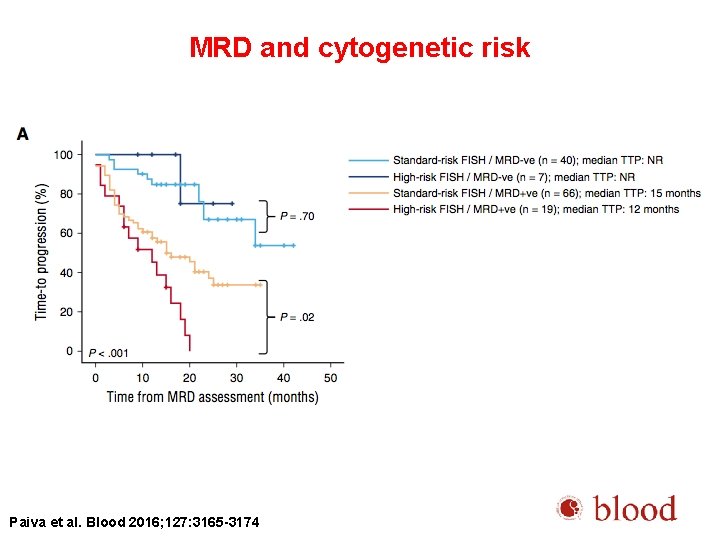
MRD and cytogenetic risk Paiva et al. Blood 2016; 127: 3165 -3174

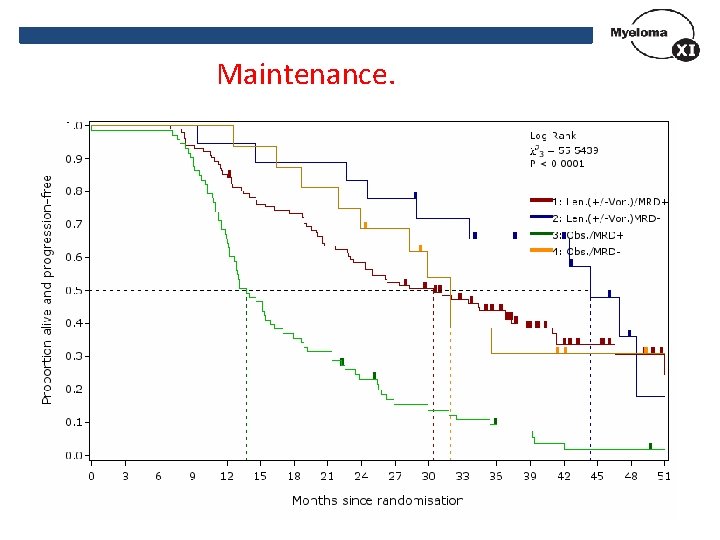
Maintenance.

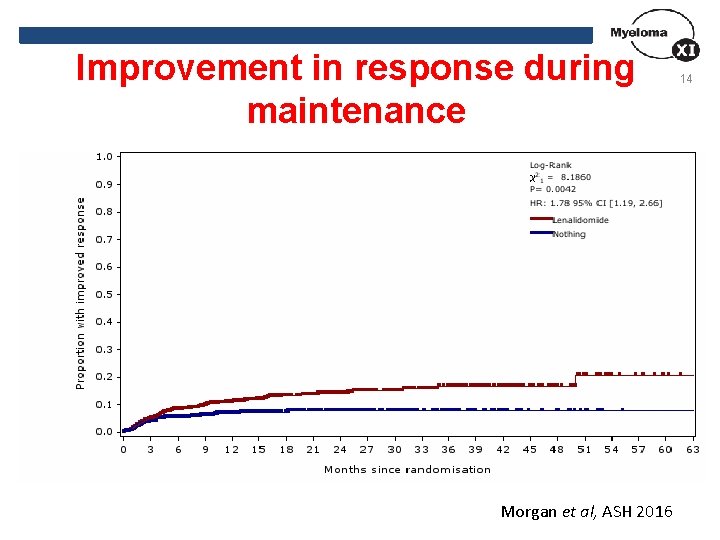
Improvement in response during maintenance Morgan et al, ASH 2016 14

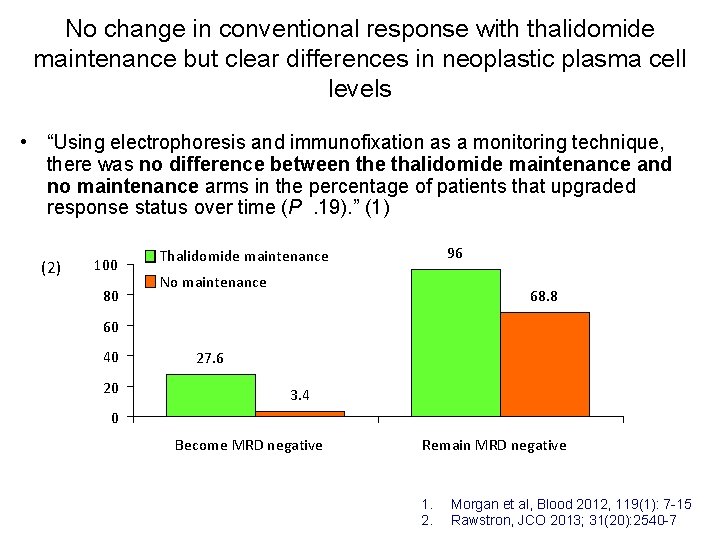
No change in conventional response with thalidomide maintenance but clear differences in neoplastic plasma cell levels • “Using electrophoresis and immunofixation as a monitoring technique, there was no difference between the thalidomide maintenance and no maintenance arms in the percentage of patients that upgraded response status over time (P. 19). ” (1) (2) 100 80 96 Thalidomide maintenance No maintenance 68. 8 60 40 20 27. 6 3. 4 0 Become MRD negative Remain MRD negative 1. 2. Morgan et al, Blood 2012, 119(1): 7 -15 Rawstron, JCO 2013; 31(20): 2540 -7

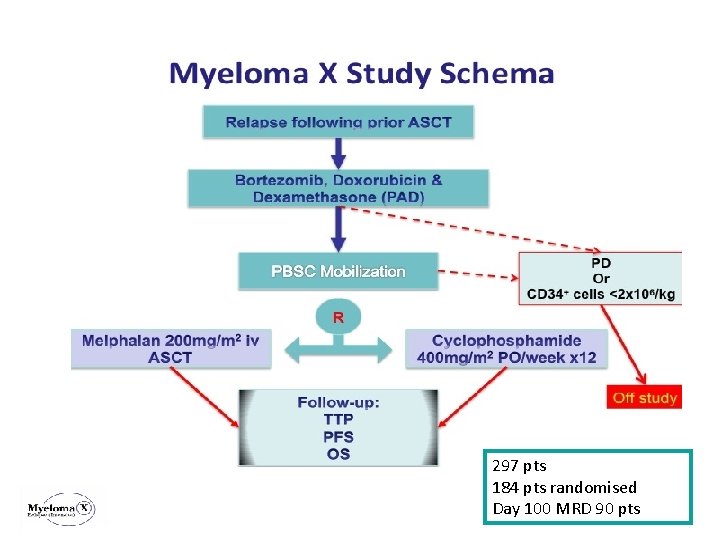
297 pts 184 pts randomised Day 100 MRD 90 pts

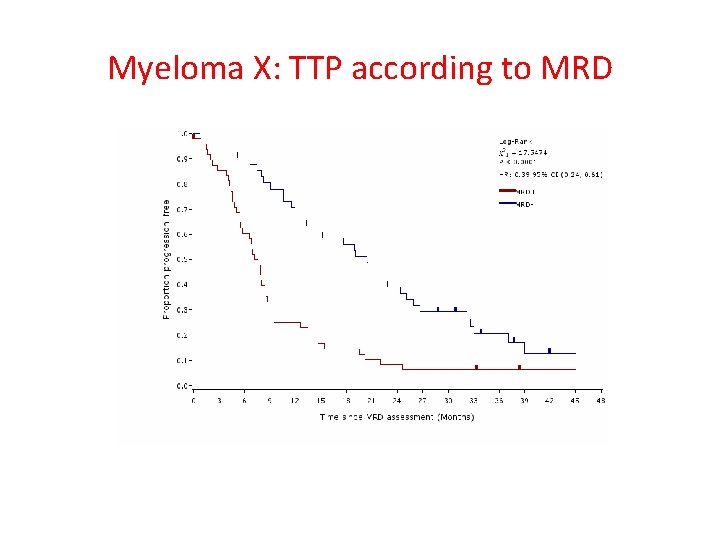
Myeloma X: TTP according to MRD

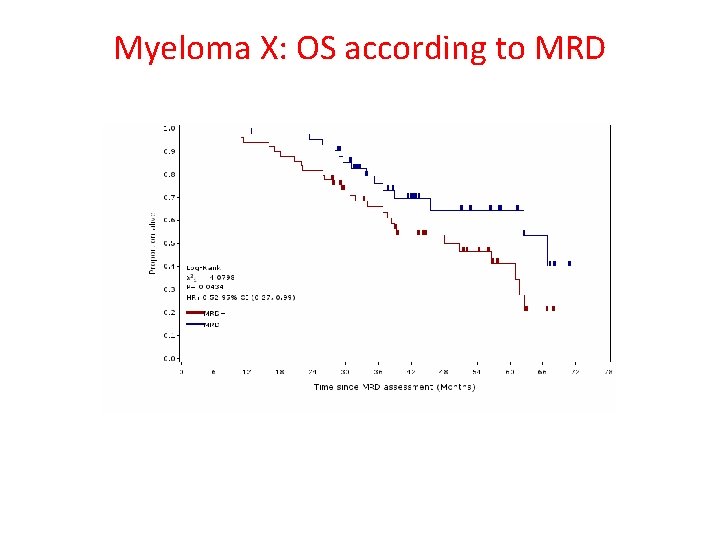
Myeloma X: OS according to MRD

19

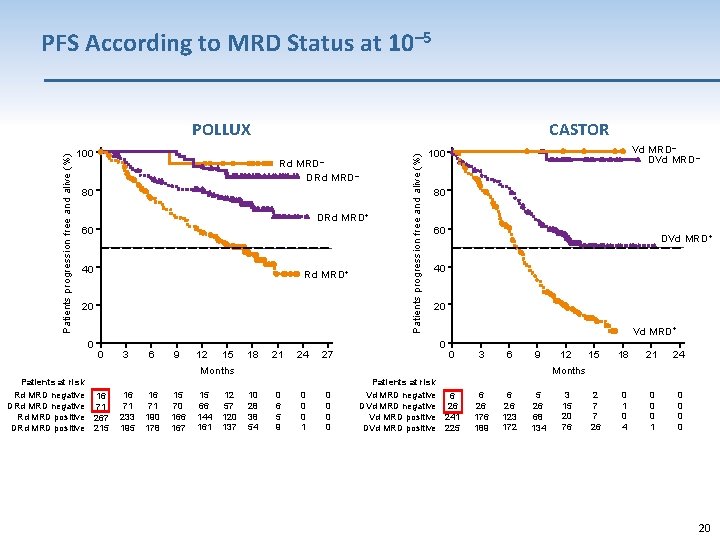
PFS According to MRD Status at 10– 5 100 CASTOR Rd MRD– DRd MRD– 80 DRd MRD+ 60 40 Rd MRD+ 20 0 0 3 6 9 12 15 18 21 24 27 Patients progression free and alive (%) POLLUX Vd MRD– DVd MRD– 100 80 60 DVd MRD+ 40 20 Vd MRD+ 0 0 3 6 9 16 71 233 195 16 71 190 178 15 70 166 167 15 66 144 161 12 57 120 137 15 18 21 24 2 7 7 26 0 1 0 4 0 0 0 1 0 0 Months Patients at risk Rd MRD negative 16 DRd MRD negative 71 Rd MRD positive 267 DRd MRD positive 215 12 10 28 38 54 0 6 5 9 0 0 0 1 0 0 Patients at risk Vd MRD negative 6 DVd MRD negative 26 Vd MRD positive 241 DVd MRD positive 225 6 26 176 189 6 26 123 172 5 26 68 134 3 15 20 76 20

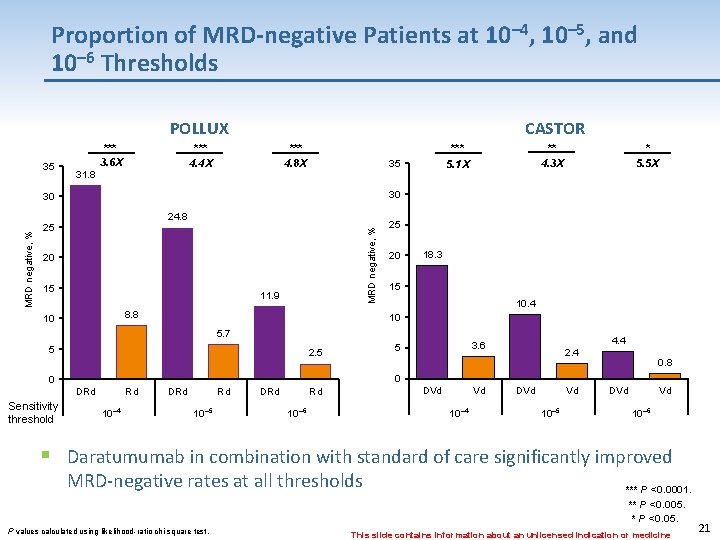
Proportion of MRD-negative Patients at 10– 4, 10– 5, and 10– 6 Thresholds POLLUX 35 *** 3. 6 X CASTOR *** 4. 8 X *** 4. 4 X 31. 8 35 * 5. 5 X 30 30 24. 8 25 MRD negative, % ** 4. 3 X *** 5. 1 X 20 15 11. 9 8. 8 10 25 20 18. 3 15 10. 4 10 5. 7 5 2. 5 DRd § 2. 4 0. 8 0 0 Sensitivity threshold 4. 4 3. 6 5 Rd 10 -4 10– 4 DRd Rd 10 -5 10– 5 DRd Rd 10 -6 10– 6 DVd Vd 10 -4 10– 4 DVd Vd 10 -5 10– 5 DVd Vd 10 -6 – 6 10 Daratumumab in combination with standard of care significantly improved MRD-negative rates at all thresholds *** P <0. 0001. ** P <0. 005. * P <0. 05. P values calculated using likelihood-ratio chi-square test. This slide contains information about an unlicensed indication or medicine 21

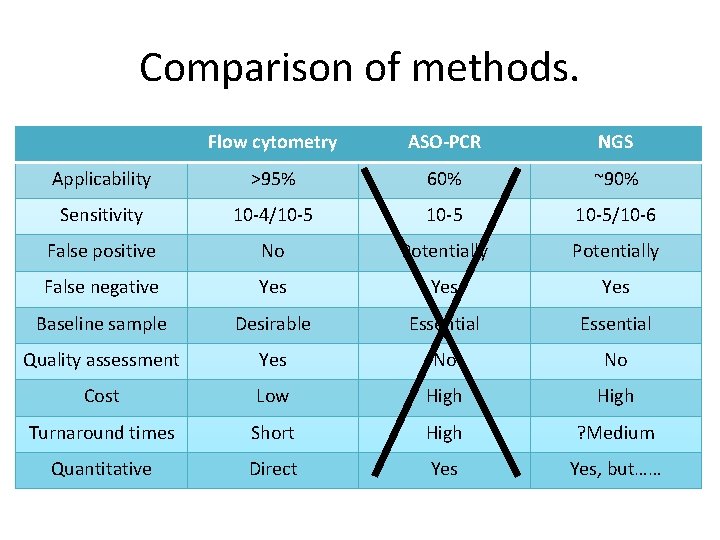
Comparison of methods. Flow cytometry ASO-PCR NGS Applicability >95% 60% ~90% Sensitivity 10 -4/10 -5/10 -6 False positive No Potentially False negative Yes Yes Baseline sample Desirable Essential Quality assessment Yes No No Cost Low High Turnaround times Short High ? Medium Quantitative Direct Yes, but……

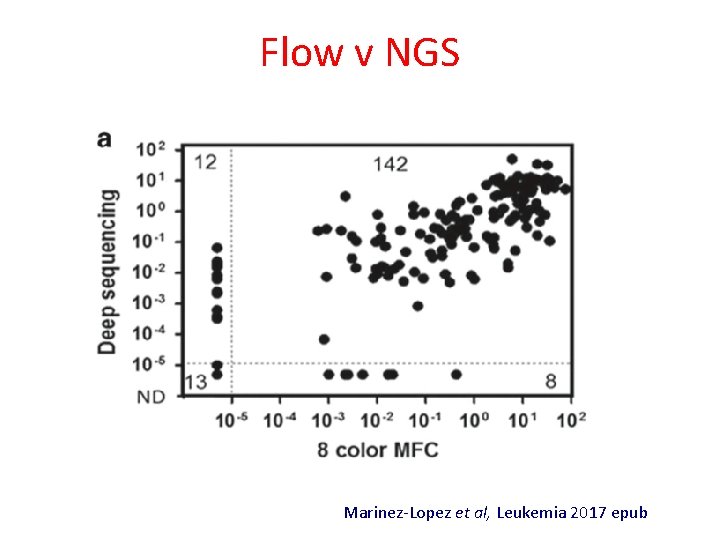
Flow v NGS Marinez-Lopez et al, Leukemia 2017 epub

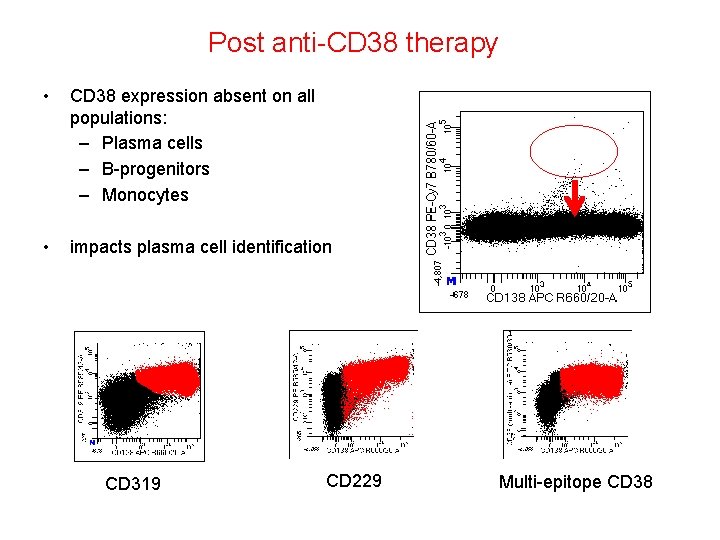
Post anti-CD 38 therapy • CD 38 expression absent on all populations: – Plasma cells – B-progenitors – Monocytes • impacts plasma cell identification CD 319 CD 229 Multi-epitope CD 38

Conclusions. • • Increasing applicability of MRD assessment Trial and regulatory endpoint MFC and NGS both applicable Challenge in daratumumab treated patients
