Epitaxial Electrodeposition of Metal Oxide Thin Films and

![Tilted Zn. O Nanospears on Si(001) Epitaxial relationships: Zn. O(203)[010] // Si(100)[010], Zn. O(203)[010] Tilted Zn. O Nanospears on Si(001) Epitaxial relationships: Zn. O(203)[010] // Si(100)[010], Zn. O(203)[010]](https://slidetodoc.com/presentation_image_h2/b88f6d95405f17be2168186ba9080e81/image-7.jpg)



- Slides: 22

Epitaxial Electrodeposition of Metal Oxide Thin Films and Superlattices for Energy Conversion and Storage Jay A. Switzer, Elizabeth Kulp, Rakesh Gudavarthy, and Guojun Mu Department of Chemistry Materials Research Center Missouri University of Science & Technology Rolla, MO 65409, USA Email: jswitzer@mst. edu

Outline • Electrodeposition of ceramic films • Tilted Zn. O nanospears on Si(001) – photovoltaics & solid-state lighting • Superlattices based on Fe 3 O 4 – sensors & RRAM memory – Defect-chemistry superlattices based on Fe 3 O 4 – Zn-doped Fe 3 O 4 superlattices • Electrodeposition of nanostructured lithium battery materials

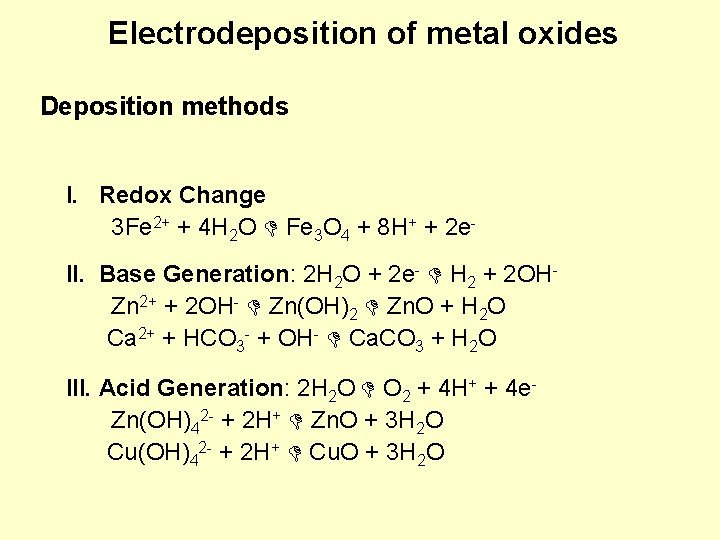
Electrodeposition of metal oxides Deposition methods I. Redox Change 3 Fe 2+ + 4 H 2 O Fe 3 O 4 + 8 H+ + 2 e. II. Base Generation: 2 H 2 O + 2 e- H 2 + 2 OHZn 2+ + 2 OH- Zn(OH)2 Zn. O + H 2 O Ca 2+ + HCO 3 - + OH- Ca. CO 3 + H 2 O III. Acid Generation: 2 H 2 O O 2 + 4 H+ + 4 e. Zn(OH)42 - + 2 H+ Zn. O + 3 H 2 O Cu(OH)42 - + 2 H+ Cu. O + 3 H 2 O

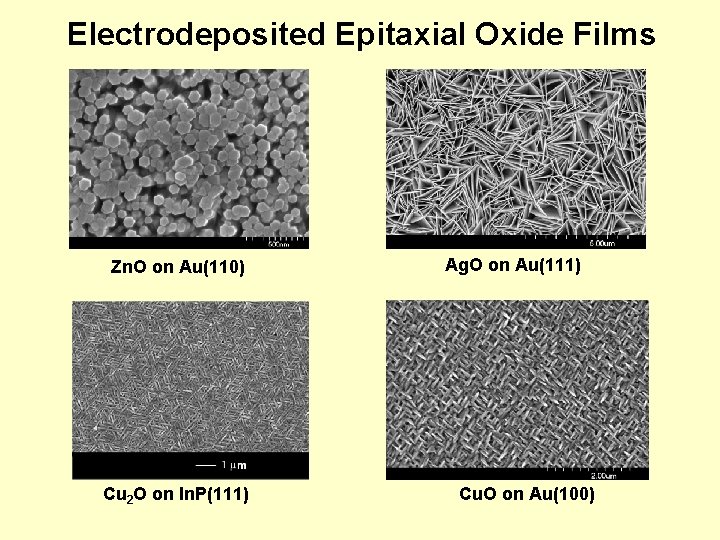
Electrodeposited Epitaxial Oxide Films Zn. O on Au(110) Cu 2 O on In. P(111) Ag. O on Au(111) Cu. O on Au(100)

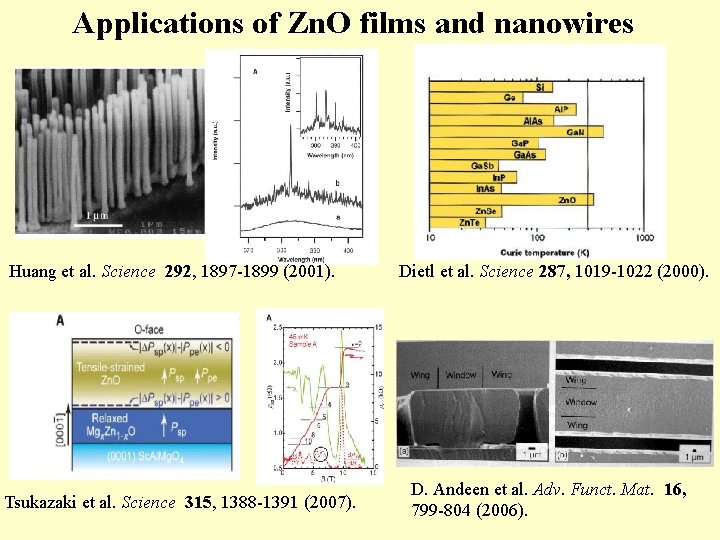
Applications of Zn. O films and nanowires Huang et al. Science 292, 1897 -1899 (2001). Tsukazaki et al. Science 315, 1388 -1391 (2007). Dietl et al. Science 287, 1019 -1022 (2000). D. Andeen et al. Adv. Funct. Mat. 16, 799 -804 (2006).

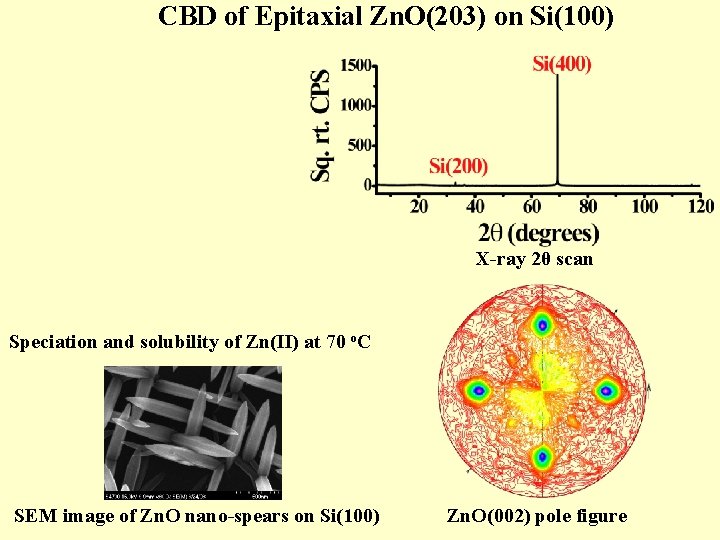
CBD of Epitaxial Zn. O(203) on Si(100) X-ray 2θ scan Speciation and solubility of Zn(II) at 70 o. C SEM image of Zn. O nano-spears on Si(100) Zn. O(002) pole figure
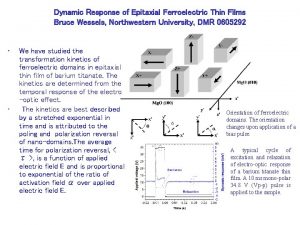
![Tilted Zn O Nanospears on Si001 Epitaxial relationships Zn O203010 Si100010 Zn O203010 Tilted Zn. O Nanospears on Si(001) Epitaxial relationships: Zn. O(203)[010] // Si(100)[010], Zn. O(203)[010]](https://slidetodoc.com/presentation_image_h2/b88f6d95405f17be2168186ba9080e81/image-7.jpg)
Tilted Zn. O Nanospears on Si(001) Epitaxial relationships: Zn. O(203)[010] // Si(100)[010], Zn. O(203)[010] // Si(100)[001], Zn. O(203)[010] // Si(100)[010], and Zn. O(203)[010] //Si(100)[001]

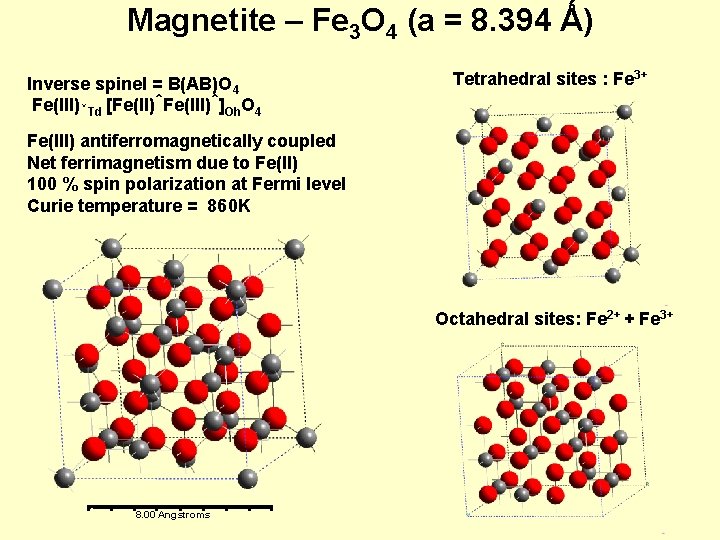
Magnetite – Fe 3 O 4 (a = 8. 394 Ǻ) Inverse spinel = B(AB)O 4 Fe(III)↓Td [Fe(II)↑Fe(III)↑]Oh. O 4 Tetrahedral sites : Fe 3+ Fe(III) antiferromagnetically coupled Net ferrimagnetism due to Fe(II) 100 % spin polarization at Fermi level Curie temperature = 860 K Octahedral sites: Fe 2+ + Fe 3+ 8. 00 Angstroms

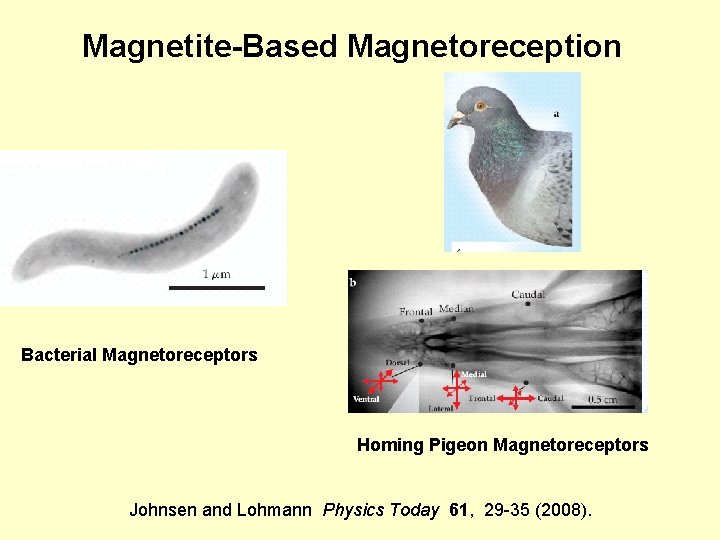
Magnetite-Based Magnetoreception Bacterial Magnetoreceptors Homing Pigeon Magnetoreceptors Johnsen and Lohmann Physics Today 61, 29 -35 (2008).

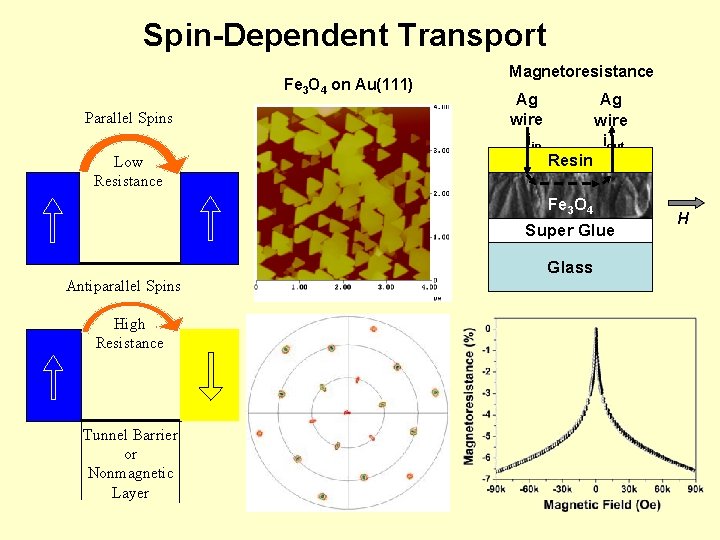
Spin-Dependent Transport Fe 3 O 4 on Au(111) Parallel Spins Low Resistance Magnetoresistance Ag wire iin Resin Ag wire iout Fe 3 O 4 Super Glue Glass Antiparallel Spins High Resistance Tunnel Barrier or Nonmagnetic Layer H

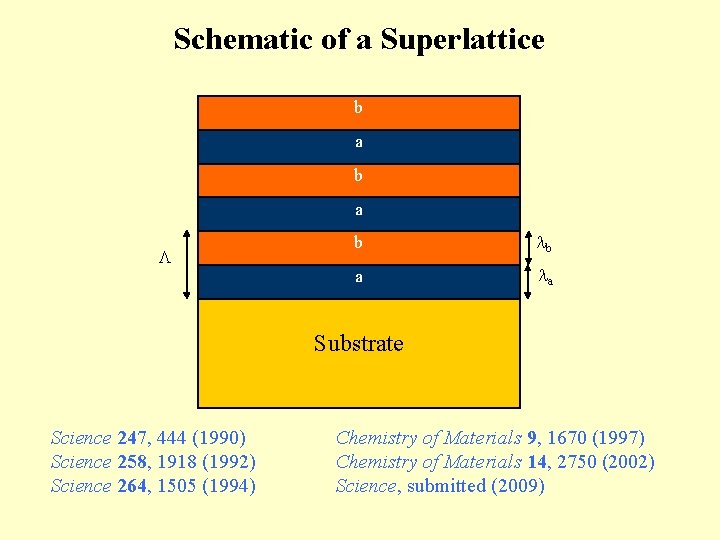
Schematic of a Superlattice b a b b a a Substrate Science 247, 444 (1990) Science 258, 1918 (1992) Science 264, 1505 (1994) Chemistry of Materials 9, 1670 (1997) Chemistry of Materials 14, 2750 (2002) Science, submitted (2009)

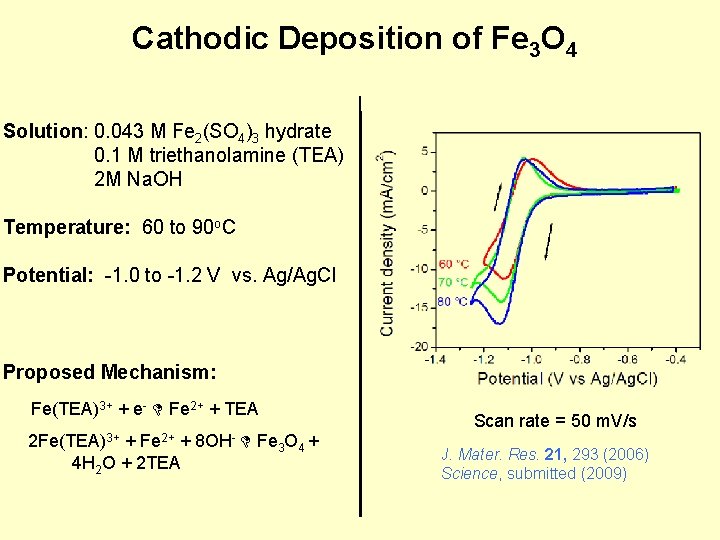
Cathodic Deposition of Fe 3 O 4 Solution: 0. 043 M Fe 2(SO 4)3 hydrate 0. 1 M triethanolamine (TEA) 2 M Na. OH Temperature: 60 to 90 o. C Potential: -1. 0 to -1. 2 V vs. Ag/Ag. Cl Proposed Mechanism: Fe(TEA)3+ + e- Fe 2+ + TEA 2 Fe(TEA)3+ + Fe 2+ + 8 OH- Fe 3 O 4 + 4 H 2 O + 2 TEA Scan rate = 50 m. V/s J. Mater. Res. 21, 293 (2006) Science, submitted (2009)

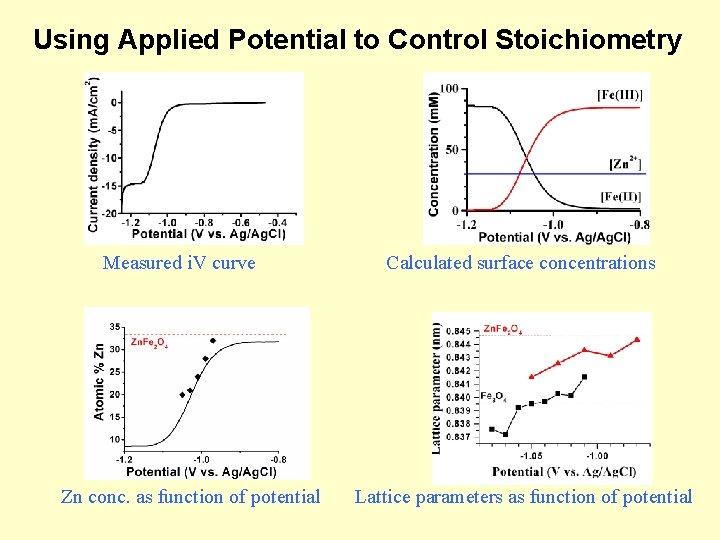
Using Applied Potential to Control Stoichiometry Measured i. V curve Zn conc. as function of potential Calculated surface concentrations Lattice parameters as function of potential

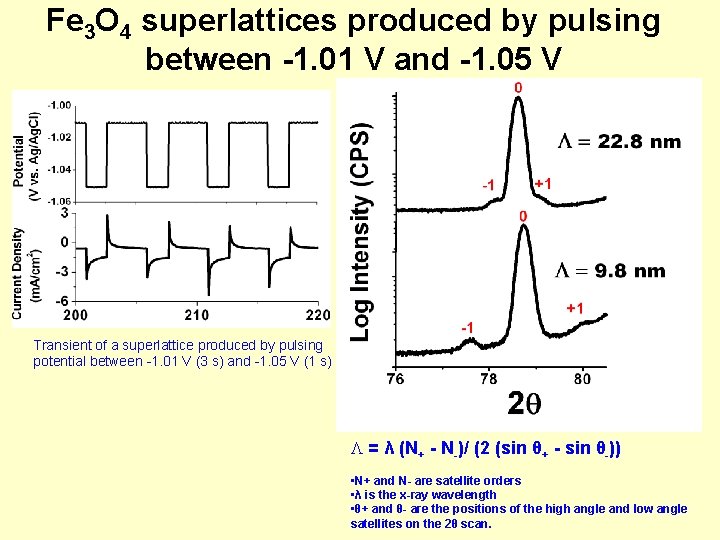
Fe 3 O 4 superlattices produced by pulsing between -1. 01 V and -1. 05 V Transient of a superlattice produced by pulsing potential between -1. 01 V (3 s) and -1. 05 V (1 s) = λ (N+ - N-)/ (2 (sin θ+ - sin θ-)) • N+ and N- are satellite orders • λ is the x-ray wavelength • θ+ and θ- are the positions of the high angle and low angle satellites on the 2θ scan.

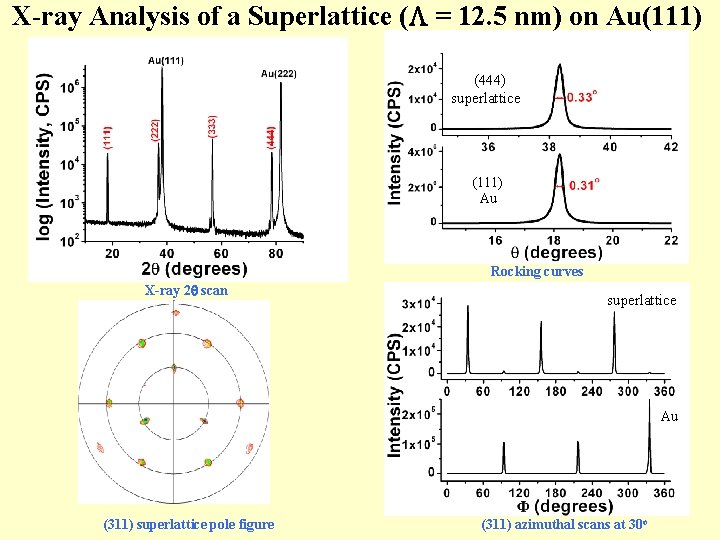
X-ray Analysis of a Superlattice ( = 12. 5 nm) on Au(111) (444) superlattice (111) Au Rocking curves X-ray 2 scan superlattice Au (311) superlattice pole figure (311) azimuthal scans at 30 o

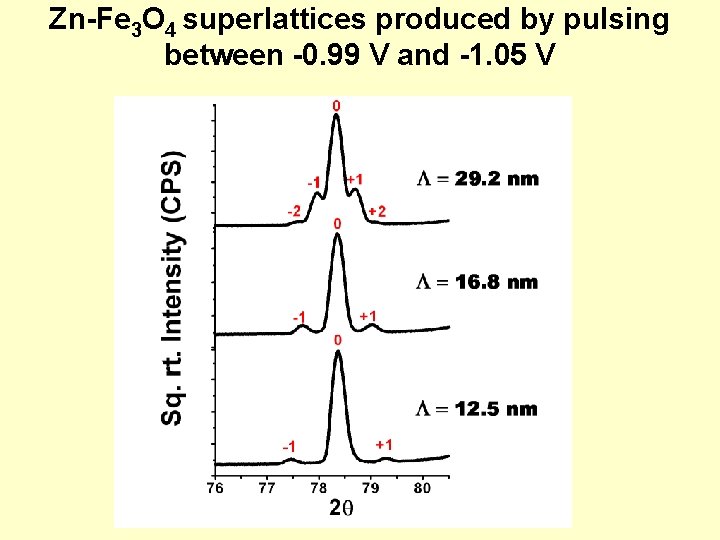
Zn-Fe 3 O 4 superlattices produced by pulsing between -0. 99 V and -1. 05 V

FIB Cross Section of a Znx. Fe 3 -x. O 4 Superlattice = 78 nm

Magnetoresistance of Zinc Ferrite Superlattice (12. 2 nm) at 45 K

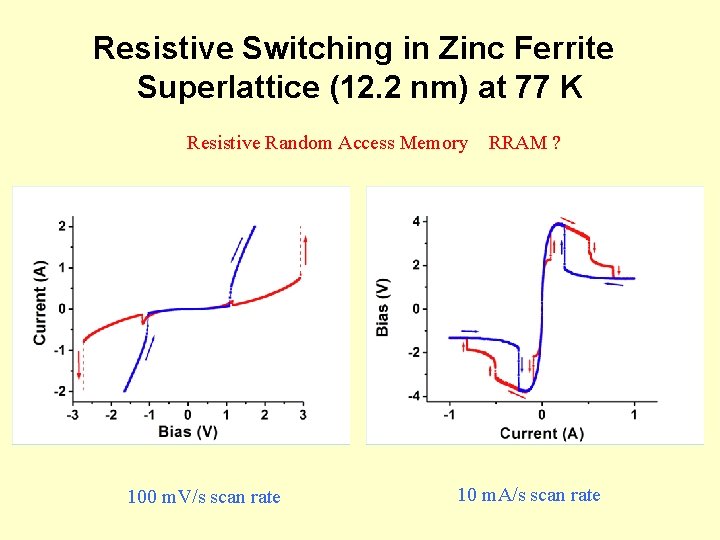
Resistive Switching in Zinc Ferrite Superlattice (12. 2 nm) at 77 K Resistive Random Access Memory 100 m. V/s scan rate RRAM ? 10 m. A/s scan rate

Electrodeposition of Nanostructured Na. Mn. O 2 for Li Battery Cathodes ØElectrodeposition of Na. Mn. O 2 from Mn(II)-TEA in strong base ØNa. Mn. O 2 is a precursor to Li. Mn. O 2 ØElectrical continuity ØNanostructured material has high surface area ØPotentially high capacity ØDoes not revert to Li. Mn 2 O 4 spinel on cycling ØFunctions as supercapacitor

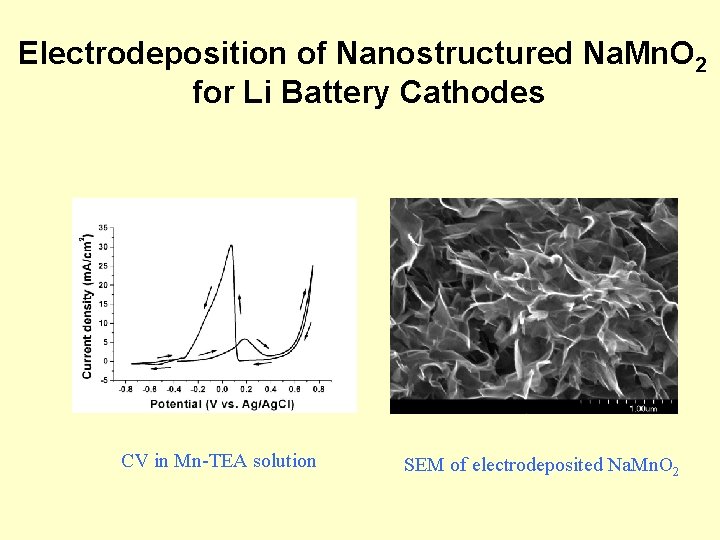
Electrodeposition of Nanostructured Na. Mn. O 2 for Li Battery Cathodes CV in Mn-TEA solution SEM of electrodeposited Na. Mn. O 2

Acknowledgements Students/Postdocs Elizabeth Kulp Rakesh Gudavarthy Eric Bohannan Hiten Kothari Steven Limmer Shuji Nakanishi Shaibal Sarkar Niharika Burla Guojun Mu Financial Support National Science Foundation CHE-0437346 CHE-0243424 DMR-0504715 DMR-0076338 Department of Energy DE-FG 02 -08 ER 46518
 Working title production company
Working title production company Crescimento epitaxial
Crescimento epitaxial Acid oxides examples
Acid oxides examples Thin films testing
Thin films testing Bulge test thin films
Bulge test thin films Electrodeposition equation
Electrodeposition equation Electrodeposition equation
Electrodeposition equation Thin gate oxide
Thin gate oxide Metal oxide and water
Metal oxide and water Mixed metal oxide tubular anodes
Mixed metal oxide tubular anodes Apex metal oxide coating
Apex metal oxide coating Metal–oxide–semiconductor
Metal–oxide–semiconductor Darlington kapcsolás
Darlington kapcsolás Metal oxide semiconductor field effect transistor
Metal oxide semiconductor field effect transistor Dp periodic table
Dp periodic table Compare the properties of metals and nonmetals
Compare the properties of metals and nonmetals Metal and nonmetal
Metal and nonmetal Liquid elements at room temperature
Liquid elements at room temperature Blanch def
Blanch def Physical properties of metals
Physical properties of metals Reactivity periodic table
Reactivity periodic table 3 states of matter venn diagram
3 states of matter venn diagram Metal 1 metal 2
Metal 1 metal 2