ENTHALPY Ms Dunishiya de Silva Reaction Energies The







- Slides: 28

ENTHALPY Ms. Dunishiya de Silva

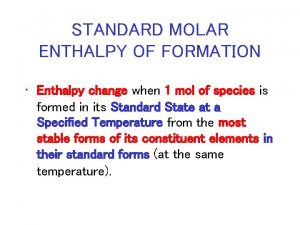
Reaction Energies The energy change associated with a chemical reaction is called the enthalpy of reaction and abbreviated H.

Enthalpy of Reactions There actually a number of different types of enthalpies because enthalpy depends on conditions. THEY ARE ALL JUST SPECIFIC TYPES OF A GENERAL CONCEPT CALLED “ENTHALPY”. H = Hfinal - Hinitial

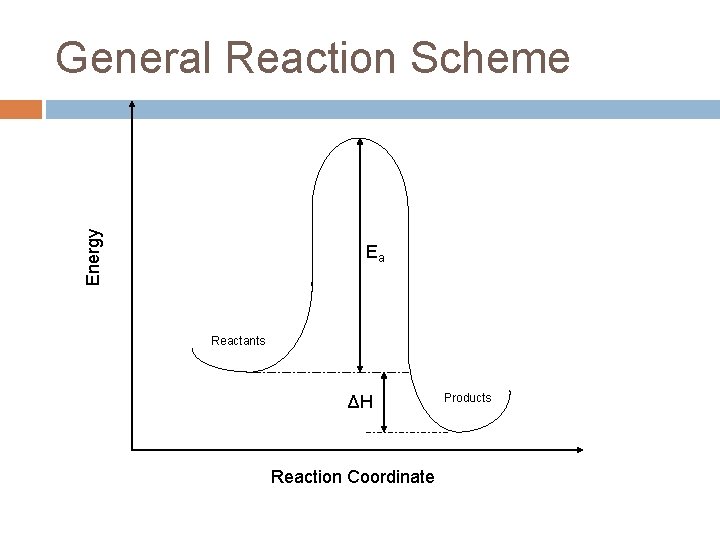
Energy General Reaction Scheme Ea Reactants ΔH Reaction Coordinate Products

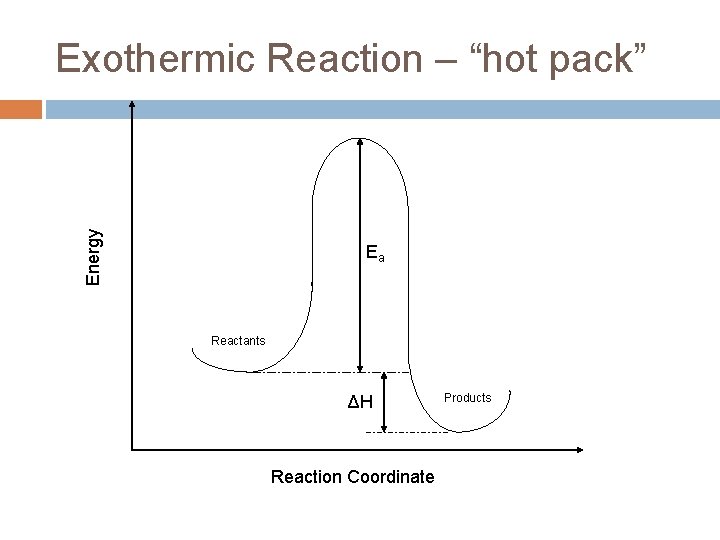
Energy Exothermic Reaction – “hot pack” Ea Reactants ΔH Reaction Coordinate Products

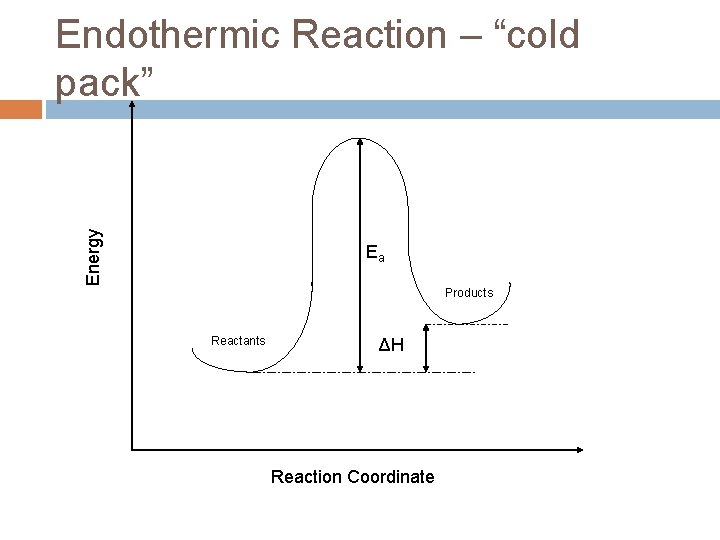
Energy Endothermic Reaction – “cold pack” Ea Products Reactants ΔH Reaction Coordinate

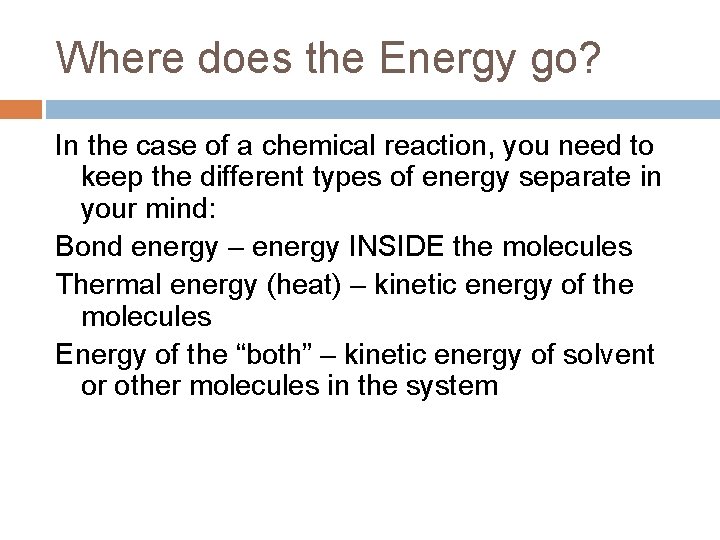
Where does the Energy go? In the case of a chemical reaction, you need to keep the different types of energy separate in your mind: Bond energy – energy INSIDE the molecules Thermal energy (heat) – kinetic energy of the molecules Energy of the “both” – kinetic energy of solvent or other molecules in the system

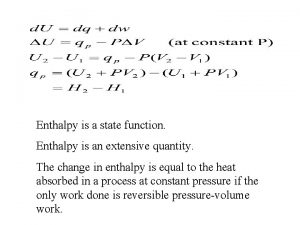
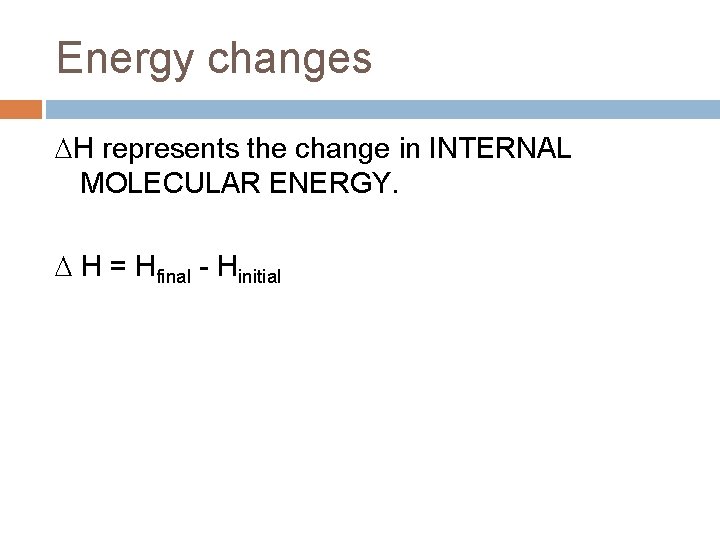
Energy changes H represents the change in INTERNAL MOLECULAR ENERGY. H = Hfinal - Hinitial

Energy Exothermic Reaction – “hot pack” Ea Reactants ΔH Reaction Coordinate Products

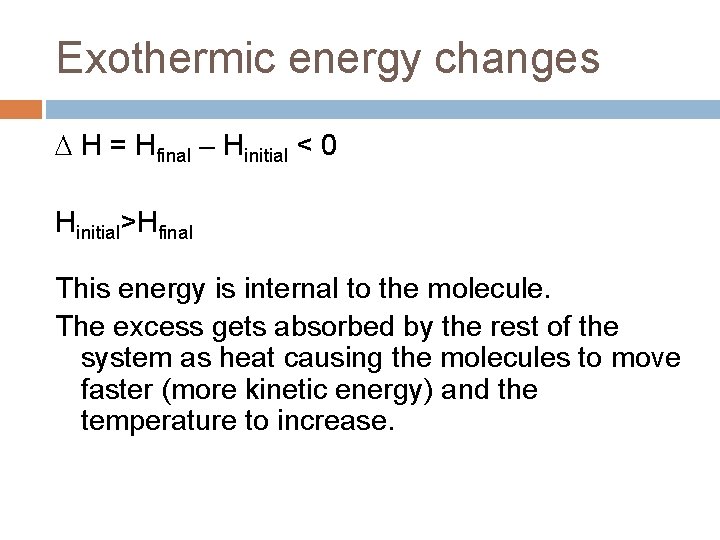
Exothermic energy changes H = Hfinal – Hinitial < 0 Hinitial>Hfinal This energy is internal to the molecule. The excess gets absorbed by the rest of the system as heat causing the molecules to move faster (more kinetic energy) and the temperature to increase.

Energy Endothermic Reaction – “cold pack” Ea Products Reactants ΔH Reaction Coordinate

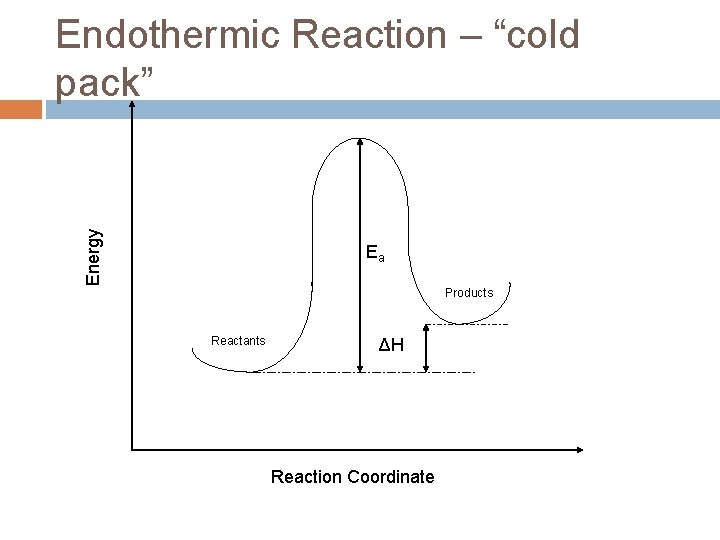
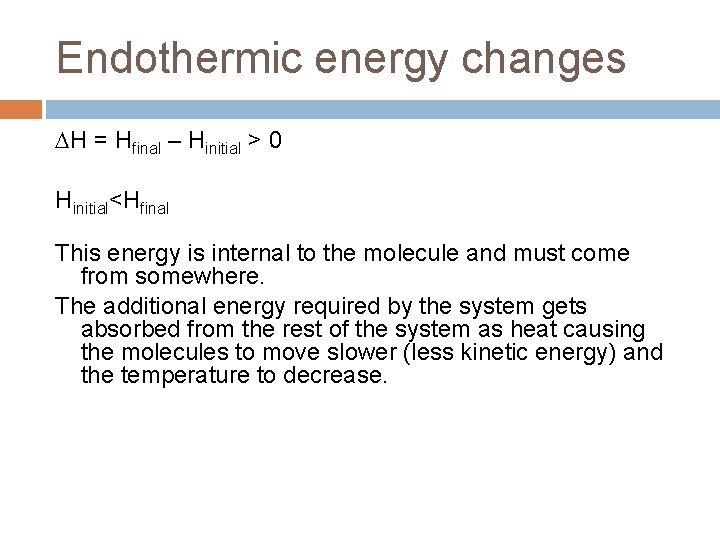
Endothermic energy changes H = Hfinal – Hinitial > 0 Hinitial<Hfinal This energy is internal to the molecule and must come from somewhere. The additional energy required by the system gets absorbed from the rest of the system as heat causing the molecules to move slower (less kinetic energy) and the temperature to decrease.

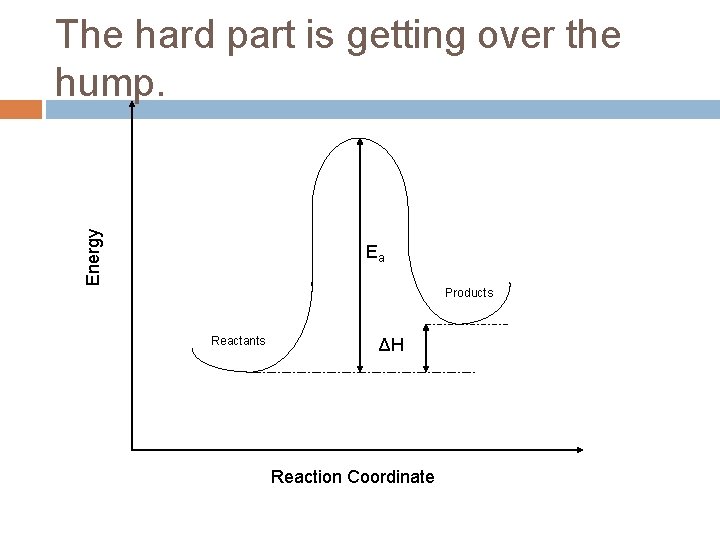
Energy The hard part is getting over the hump. Ea Products Reactants ΔH Reaction Coordinate

Ea = Activation Energy The tale of a reaction is not limited strictly to the identity and energetics of the products and reactants, there is a path (reaction coordinate) that must get followed. The “hump” represents a hurdle that must be overcome to go from reactants to products.

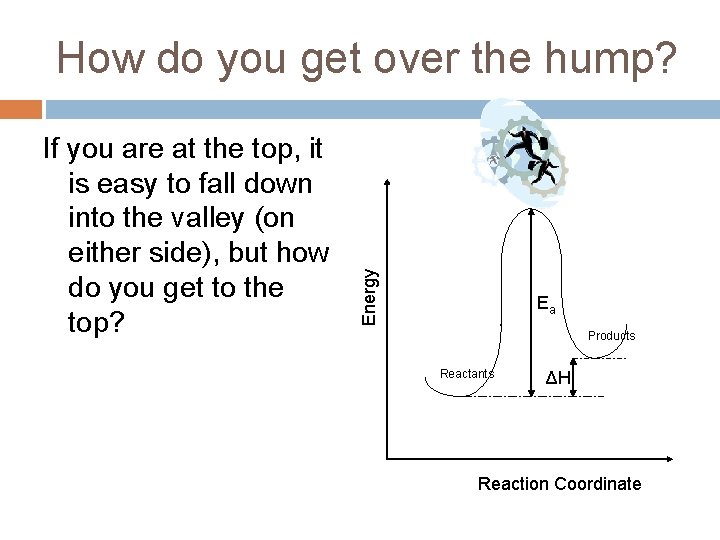
If you are at the top, it is easy to fall down into the valley (on either side), but how do you get to the top? Energy How do you get over the hump? Ea Products Reactants ΔH Reaction Coordinate

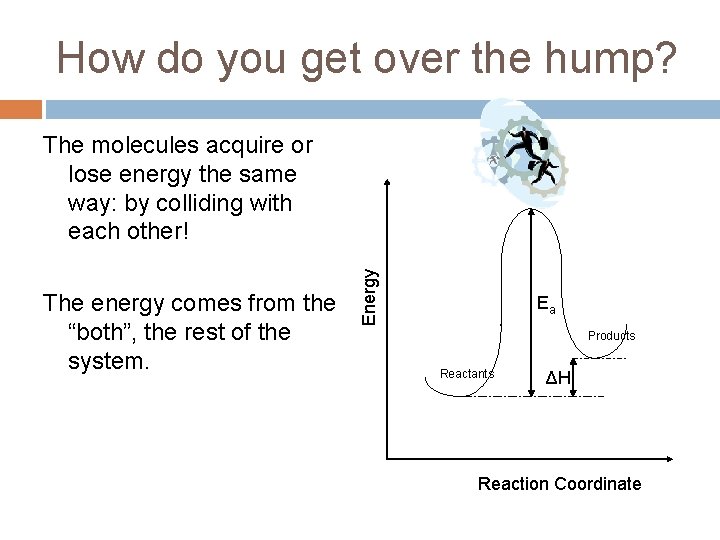
How do you get over the hump? The energy comes from the “both”, the rest of the system. Energy The molecules acquire or lose energy the same way: by colliding with each other! Ea Products Reactants ΔH Reaction Coordinate

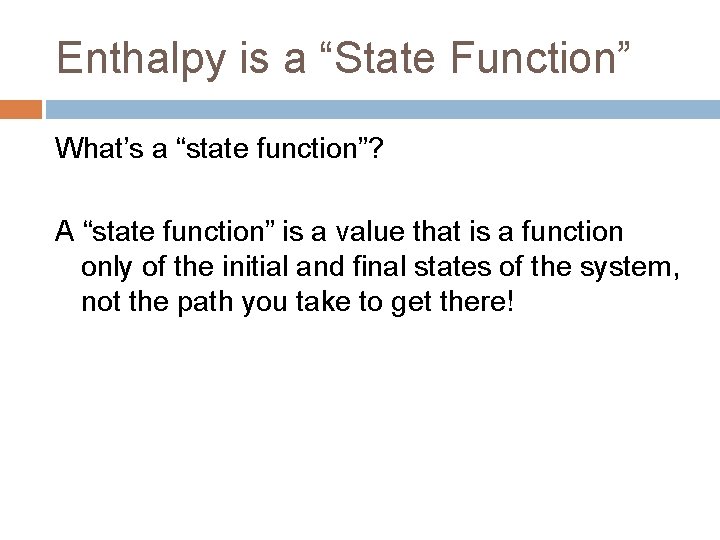
Enthalpy is a “State Function” What’s a “state function”? A “state function” is a value that is a function only of the initial and final states of the system, not the path you take to get there!

Enthalpy as a State Function Enthalpy is like that. It doesn’t care how you got where you are going, it simply looks at the difference from where you started.

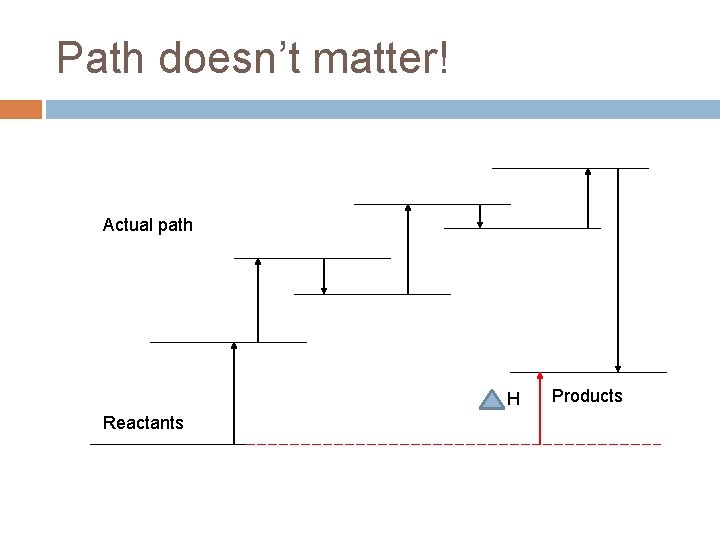
Path doesn’t matter! Actual path H Reactants Products

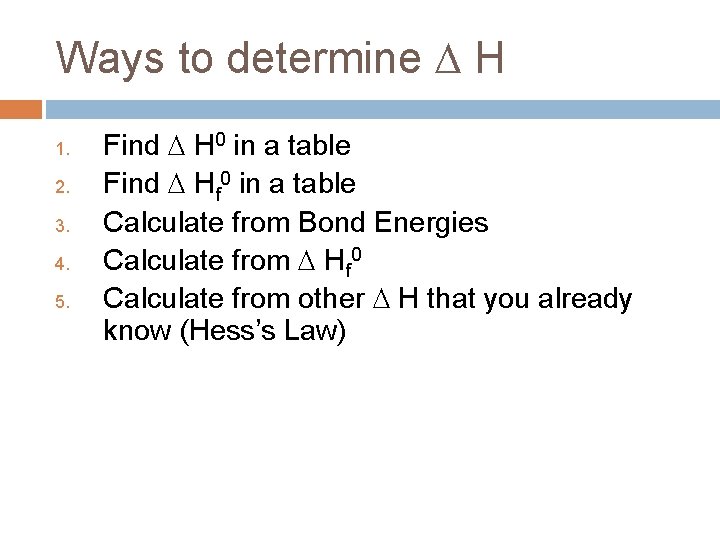
Ways to determine H 1. 2. 3. 4. 5. Find H 0 in a table Find Hf 0 in a table Calculate from Bond Energies Calculate from Hf 0 Calculate from other H that you already know (Hess’s Law)

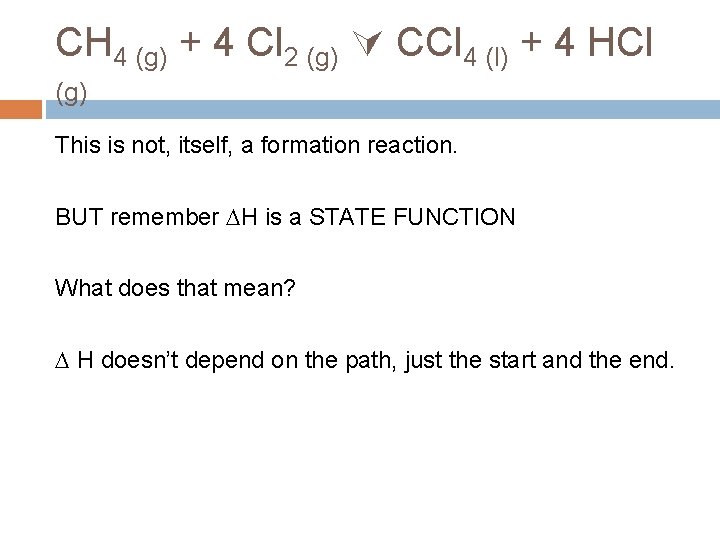
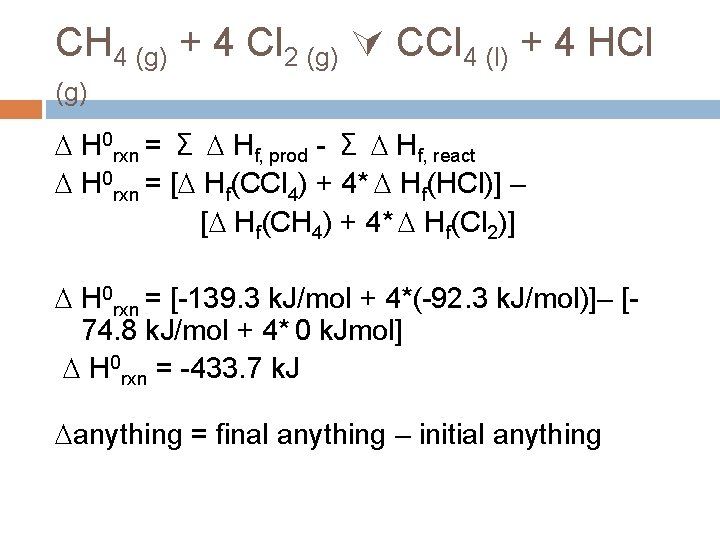
CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) This is not, itself, a formation reaction. BUT remember H is a STATE FUNCTION What does that mean? H doesn’t depend on the path, just the start and the end.

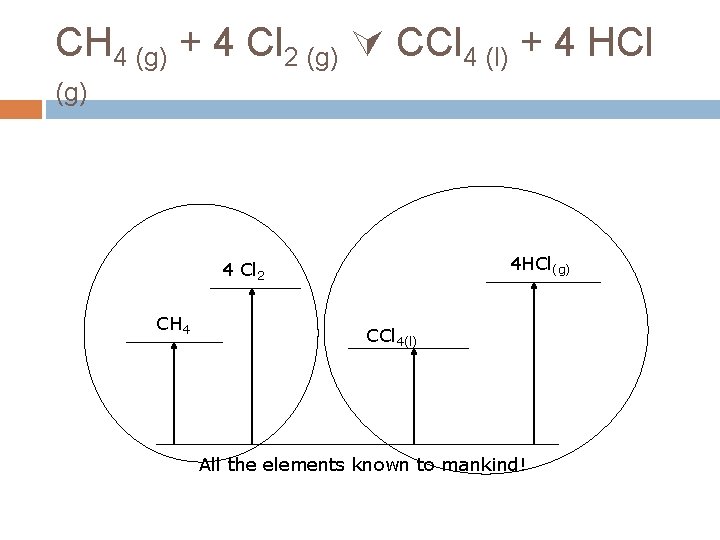
CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) 4 HCl(g) 4 Cl 2 CH 4 CCl 4(l) All the elements known to mankind!

CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) How does that help? You can take the long road. Don’t do the reaction as written, take a convenient path that you know the value of each step.

CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) Can you think of a path where you know the value of each step? Make the products from elements (formation reactions). Make the reactants from elements (formation reactions). The difference between the Hf 0 of the products and the reactants must be the H 0 rxn

CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) 4 HCl(g) 4 Cl 2 CH 4 CCl 4(l) All the elements known to mankind!

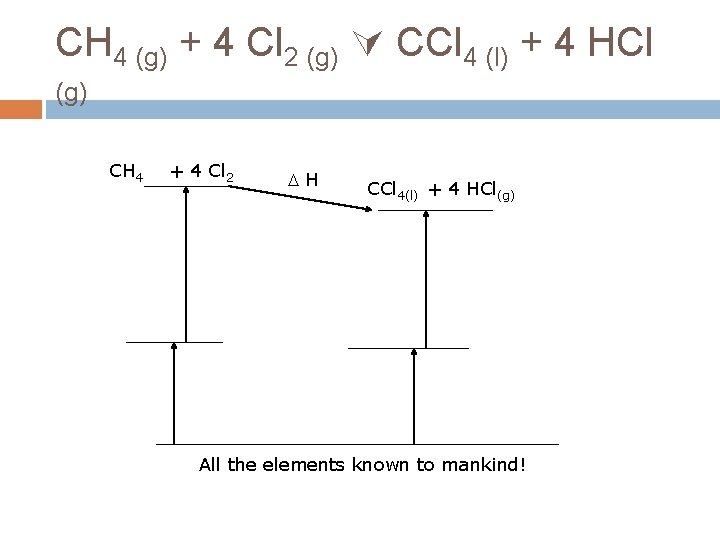
CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) CH 4 + 4 Cl 2 H CCl 4(l) + 4 HCl(g) All the elements known to mankind!

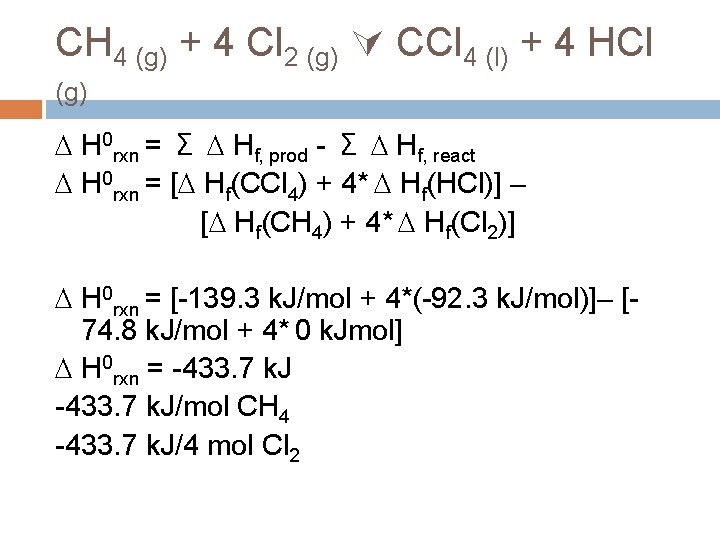
CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) H 0 rxn = Σ Hf, prod - Σ Hf, react H 0 rxn = [ Hf(CCl 4) + 4* Hf(HCl)] – [ Hf(CH 4) + 4* Hf(Cl 2)] H 0 rxn = [-139. 3 k. J/mol + 4*(-92. 3 k. J/mol)]– [74. 8 k. J/mol + 4* 0 k. Jmol] H 0 rxn = -433. 7 k. J anything = final anything – initial anything

CH 4 (g) + 4 Cl 2 (g) CCl 4 (l) + 4 HCl (g) H 0 rxn = Σ Hf, prod - Σ Hf, react H 0 rxn = [ Hf(CCl 4) + 4* Hf(HCl)] – [ Hf(CH 4) + 4* Hf(Cl 2)] H 0 rxn = [-139. 3 k. J/mol + 4*(-92. 3 k. J/mol)]– [74. 8 k. J/mol + 4* 0 k. Jmol] H 0 rxn = -433. 7 k. J/mol CH 4 -433. 7 k. J/4 mol Cl 2
 Finding enthalpy of reaction
Finding enthalpy of reaction Enthalpy change definition a level
Enthalpy change definition a level Enthalpy definition
Enthalpy definition Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Enthalpy calculation
Enthalpy calculation Enthalpy change definition
Enthalpy change definition Enthalpy change for a reaction
Enthalpy change for a reaction Meter installation/removal
Meter installation/removal Reactants minus products bond energies
Reactants minus products bond energies Energy (physics)
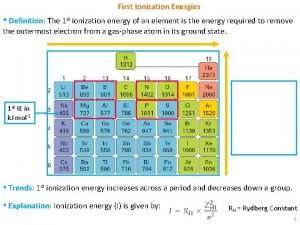
Energy (physics) Successive ionisation energies of calcium
Successive ionisation energies of calcium Four energies
Four energies 1st ionization energy definition
1st ionization energy definition Bonding forces and energies
Bonding forces and energies Energies convencionals
Energies convencionals Is bond breaking always endothermic
Is bond breaking always endothermic European renewable energies federation
European renewable energies federation Negative image
Negative image E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Proton capture equation
Proton capture equation Rate of reaction formula
Rate of reaction formula Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Gấu đi như thế nào
Gấu đi như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi