Endometrial and other cancers David W Sturdee Endometrial













- Slides: 50

Endometrial and other cancers David W. Sturdee

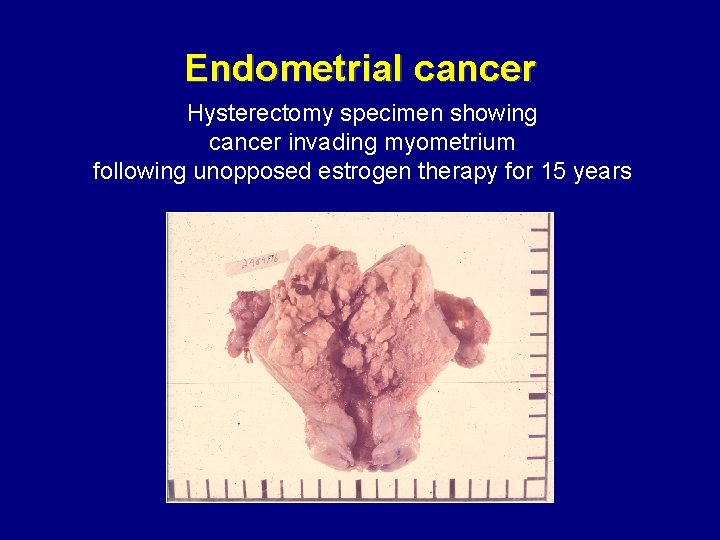
Endometrial cancer Hysterectomy specimen showing cancer invading myometrium following unopposed estrogen therapy for 15 years

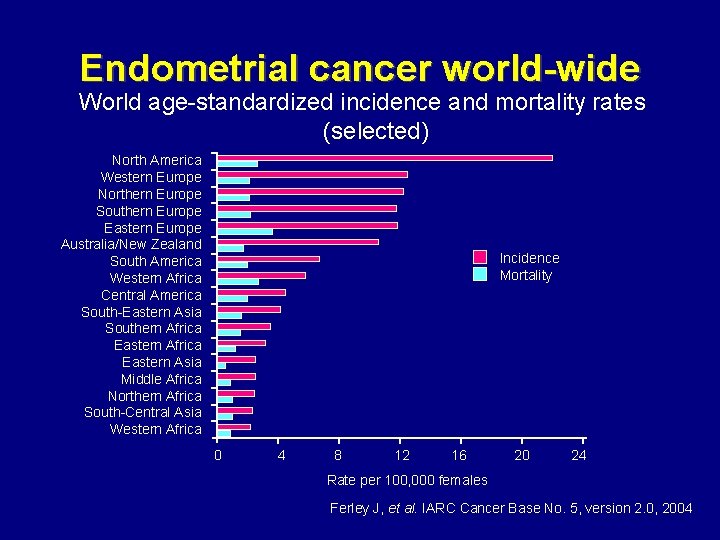
Endometrial cancer world-wide World age-standardized incidence and mortality rates (selected) North America Western Europe Northern Europe Southern Europe Eastern Europe Australia/New Zealand South America Western Africa Central America South-Eastern Asia Southern Africa Eastern Asia Middle Africa Northern Africa South-Central Asia Western Africa Incidence Mortality 0 4 8 12 16 20 24 Rate per 100, 000 females Ferley J, et al. IARC Cancer Base No. 5, version 2. 0, 2004

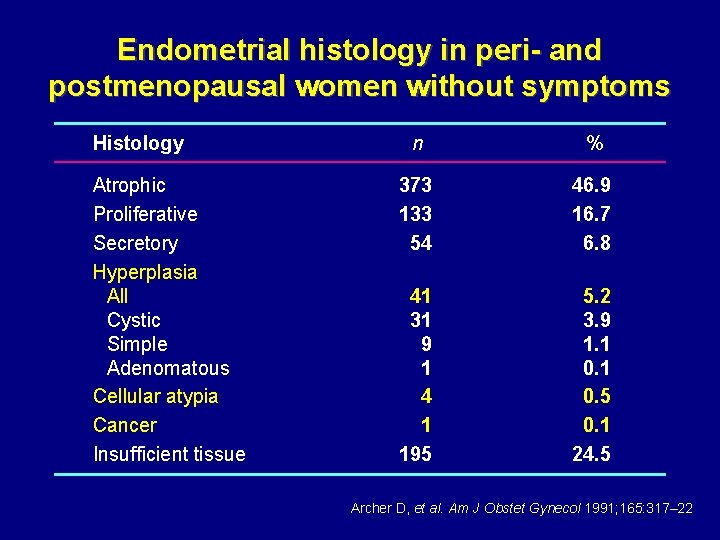
Endometrial histology in peri- and postmenopausal women without symptoms Histology Atrophic Proliferative Secretory Hyperplasia All Cystic Simple Adenomatous Cellular atypia Cancer Insufficient tissue n % 373 133 54 46. 9 16. 7 6. 8 41 31 9 1 4 1 195 5. 2 3. 9 1. 1 0. 5 0. 1 24. 5 Archer D, et al. Am J Obstet Gynecol 1991; 165: 317– 22

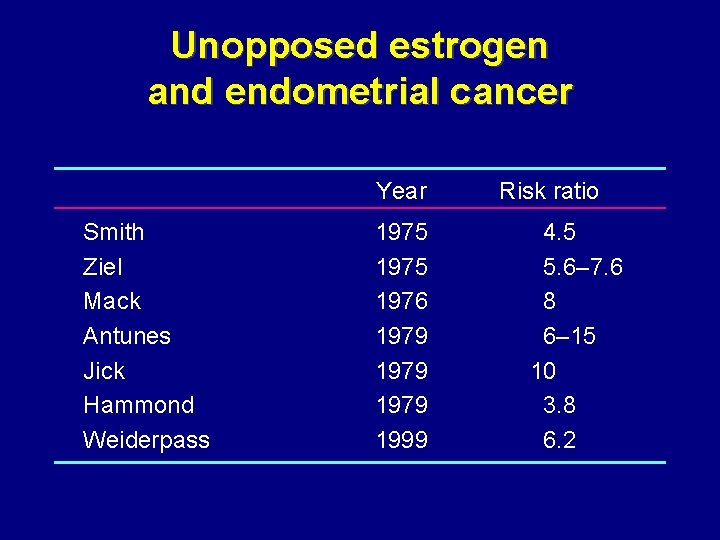
Unopposed estrogen and endometrial cancer Year Smith Ziel Mack Antunes Jick Hammond Weiderpass 1975 1976 1979 1999 Risk ratio 4. 5 5. 6– 7. 6 8 6– 15 10 3. 8 6. 2

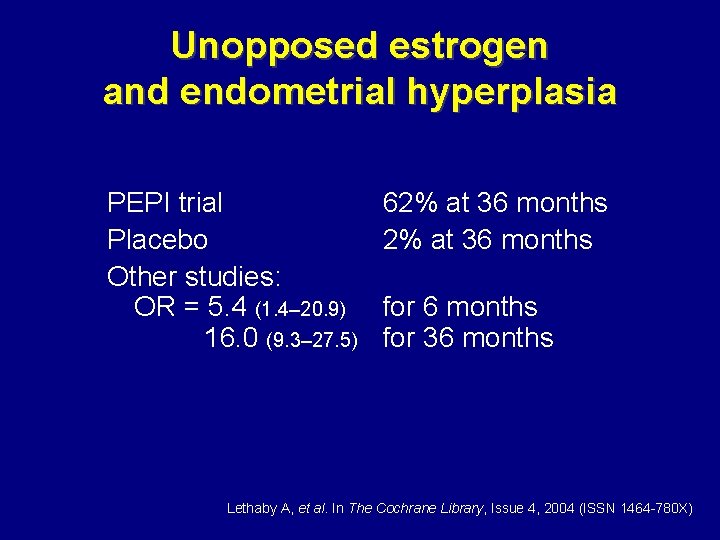
Unopposed estrogen and endometrial hyperplasia PEPI trial Placebo Other studies: OR = 5. 4 (1. 4– 20. 9) 16. 0 (9. 3– 27. 5) 62% at 36 months for 36 months Lethaby A, et al. In The Cochrane Library, Issue 4, 2004 (ISSN 1464 -780 X)

HRT in postmenopausal women: endometrial hyperplasia and irregular bleeding (Cochrane Review) 30 randomized controlled trials were suitable for review • Unopposed estrogen significantly increases • • • hyperplasia Oral progestogen reduces rate of hyperplasia Suggestion that continuous therapy over long duration is more protective from hyperplasia Hyperplasia is more likely when progestogen is given every 3 months Lethaby A, et al. In The Cochrane Library, Issue 4, 2004 (ISSN 1464 -780 X)

HT: the addition of progestogen • For at least 10 days in each cycle to • prevent endometrial hyperplasia and carcinoma In sequential regimens to imitate the ovarian cycle and to promote a regular and predictable bleed

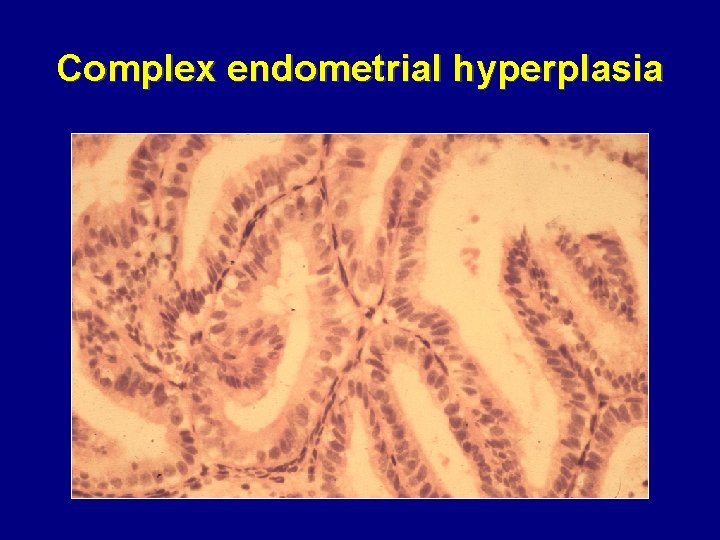
Complex endometrial hyperplasia

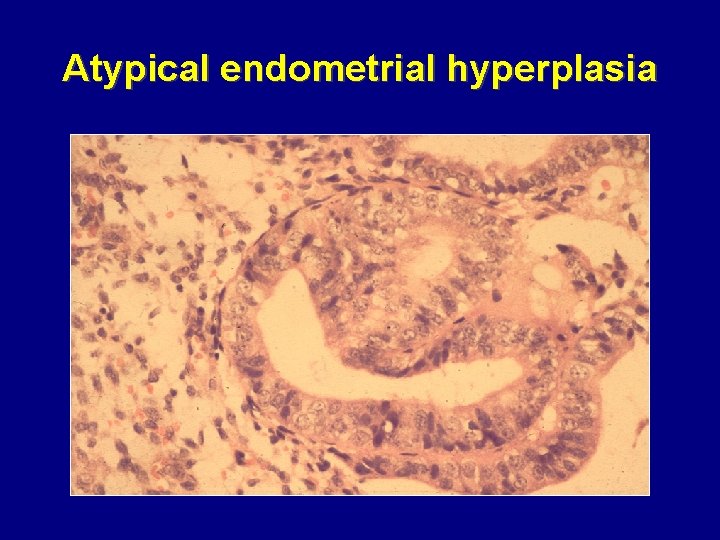
Atypical endometrial hyperplasia

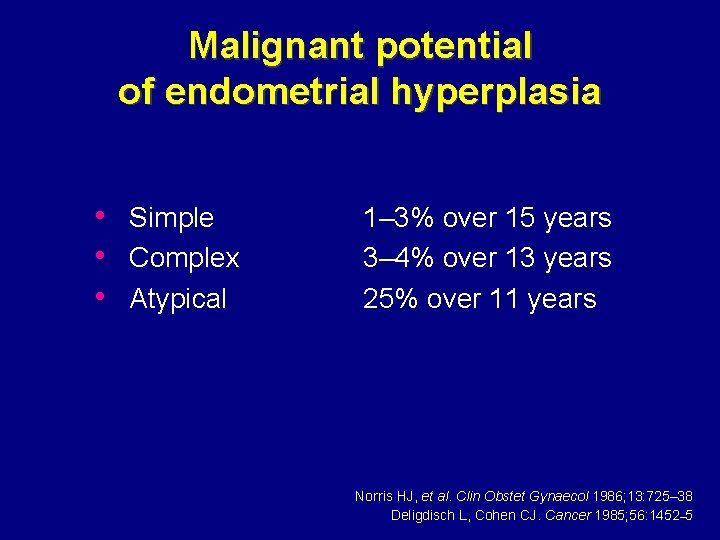
Malignant potential of endometrial hyperplasia • Simple • Complex • Atypical 1– 3% over 15 years 3– 4% over 13 years 25% over 11 years Norris HJ, et al. Clin Obstet Gynaecol 1986; 13: 725– 38 Deligdisch L, Cohen CJ. Cancer 1985; 56: 1452– 5

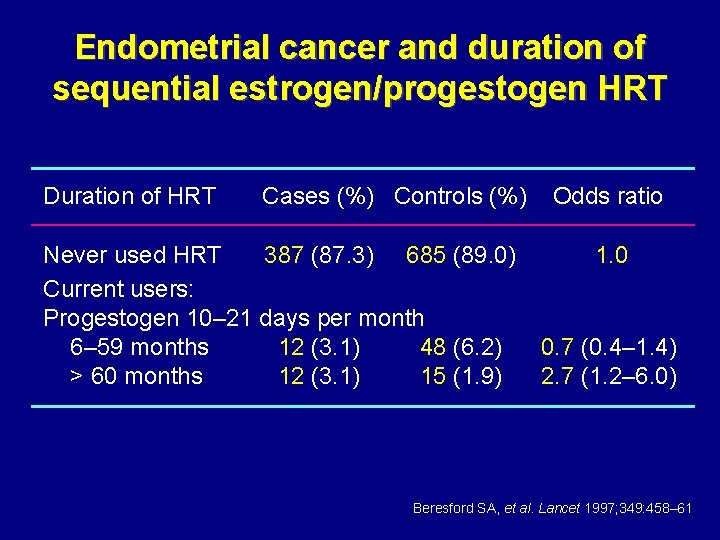
Endometrial cancer and duration of sequential estrogen/progestogen HRT Duration of HRT Cases (%) Controls (%) Never used HRT 387 (87. 3) 685 (89. 0) Current users: Progestogen 10– 21 days per month 6– 59 months 12 (3. 1) 48 (6. 2) > 60 months 12 (3. 1) 15 (1. 9) Odds ratio 1. 0 0. 7 (0. 4– 1. 4) 2. 7 (1. 2– 6. 0) Beresford SA, et al. Lancet 1997; 349: 458– 61

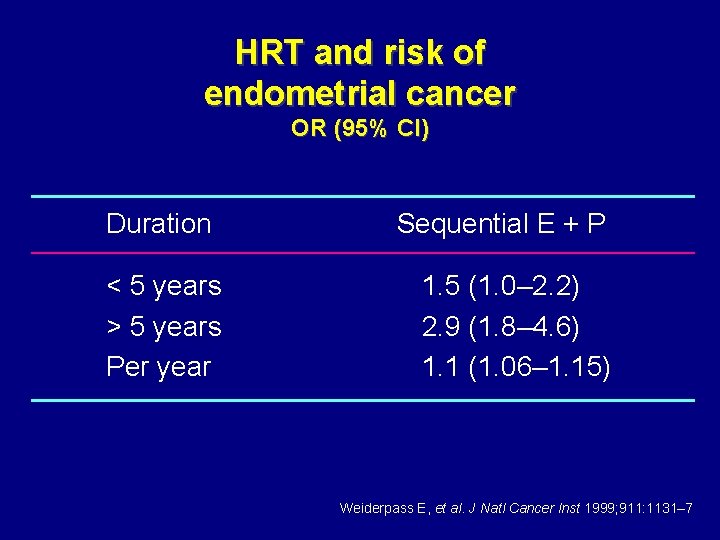
HRT and risk of endometrial cancer OR (95% CI) Duration < 5 years > 5 years Per year Sequential E + P 1. 5 (1. 0– 2. 2) 2. 9 (1. 8– 4. 6) 1. 1 (1. 06– 1. 15) Weiderpass E, et al. J Natl Cancer Inst 1999; 911: 1131– 7

Period-free therapy Continuous combined therapy: estrogen + low-dose progestogen every day (CCEPT) or tibolone • • Same benefits as sequential No cycle Period-free Atrophic endometrium

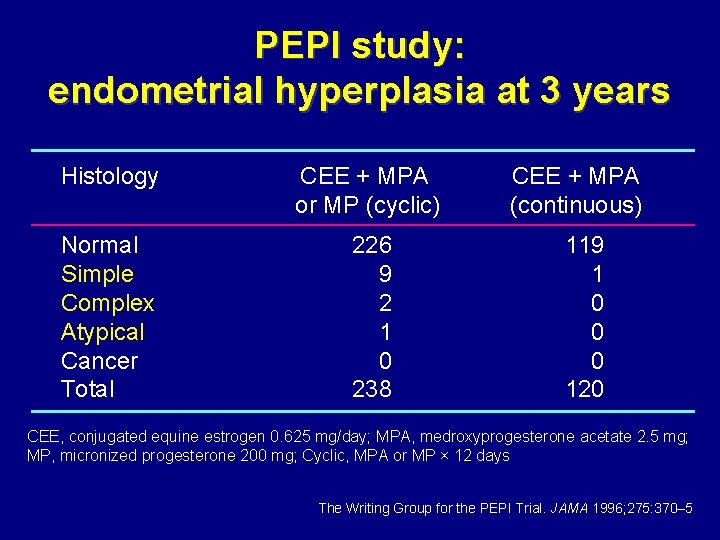
PEPI study: endometrial hyperplasia at 3 years Histology CEE + MPA or MP (cyclic) Normal Simple Complex Atypical Cancer Total 226 9 2 1 0 238 CEE + MPA (continuous) 119 1 0 0 0 120 CEE, conjugated equine estrogen 0. 625 mg/day; MPA, medroxyprogesterone acetate 2. 5 mg; MP, micronized progesterone 200 mg; Cyclic, MPA or MP × 12 days The Writing Group for the PEPI Trial. JAMA 1996; 275: 370– 5

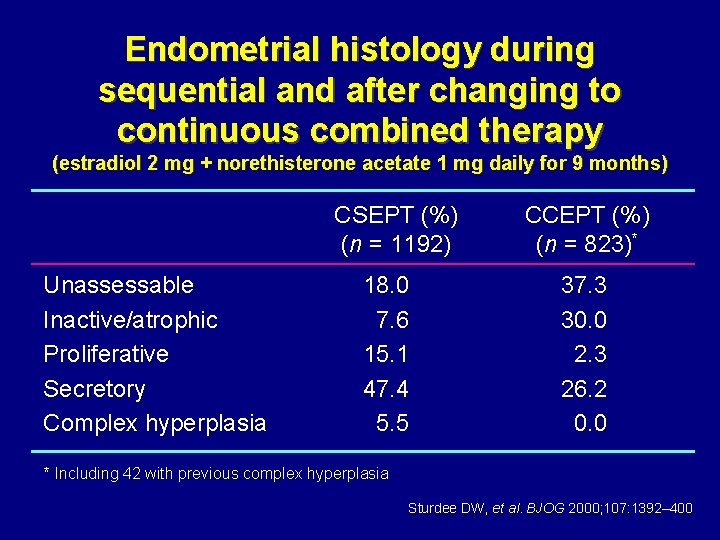
Endometrial histology during sequential and after changing to continuous combined therapy (estradiol 2 mg + norethisterone acetate 1 mg daily for 9 months) CSEPT (%) (n = 1192) Unassessable Inactive/atrophic Proliferative Secretory Complex hyperplasia 18. 0 7. 6 15. 1 47. 4 5. 5 CCEPT (%) (n = 823)* 37. 3 30. 0 2. 3 26. 2 0. 0 * Including 42 with previous complex hyperplasia Sturdee DW, et al. BJOG 2000; 107: 1392– 400

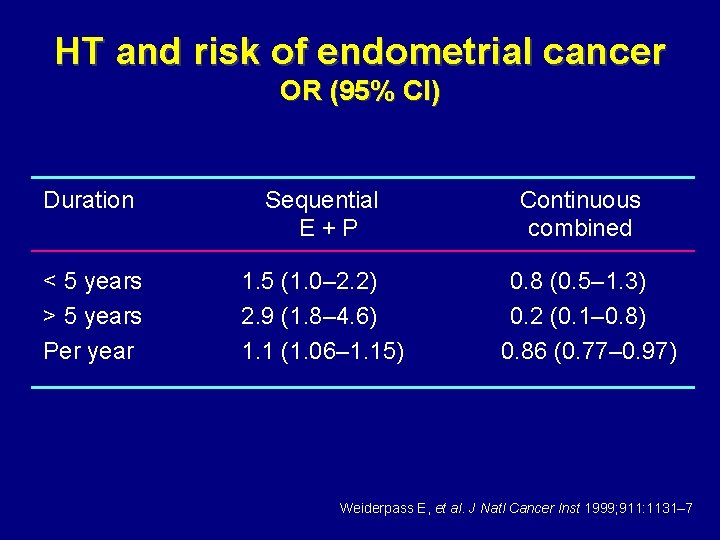
HT and risk of endometrial cancer OR (95% CI) Duration Sequential E+P < 5 years > 5 years Per year 1. 5 (1. 0– 2. 2) 2. 9 (1. 8– 4. 6) 1. 1 (1. 06– 1. 15) Continuous combined 0. 8 (0. 5– 1. 3) 0. 2 (0. 1– 0. 8) 0. 86 (0. 77– 0. 97) Weiderpass E, et al. J Natl Cancer Inst 1999; 911: 1131– 7

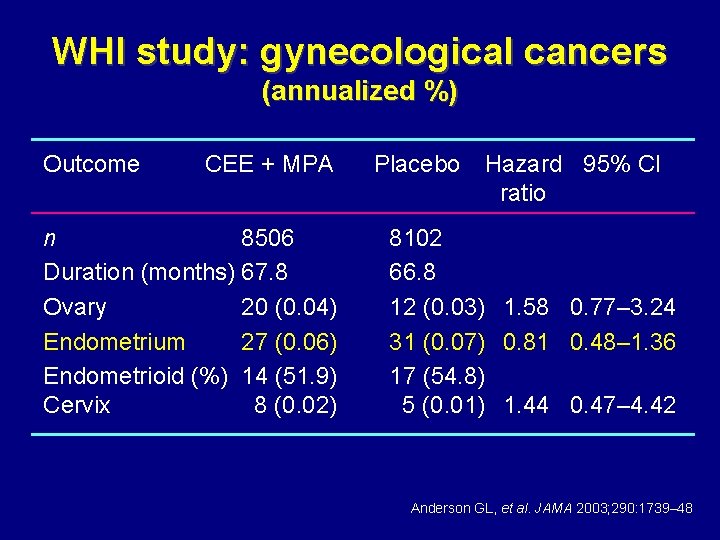
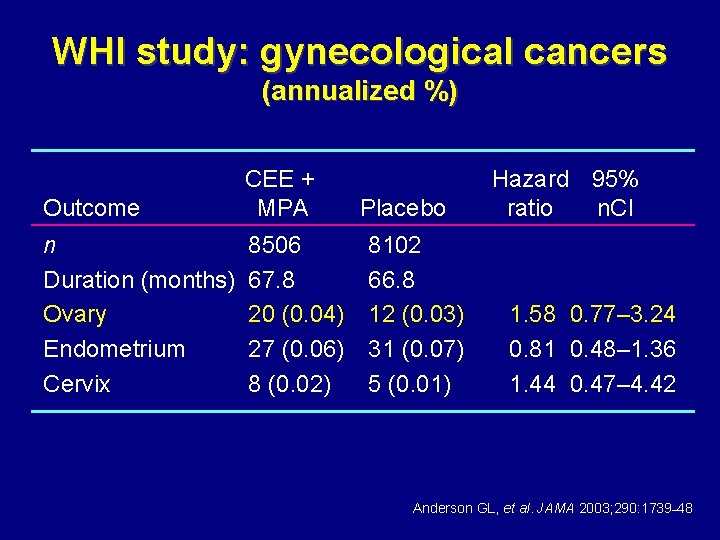
WHI study: gynecological cancers (annualized %) Outcome CEE + MPA n 8506 Duration (months) 67. 8 Ovary 20 (0. 04) Endometrium 27 (0. 06) Endometrioid (%) 14 (51. 9) Cervix 8 (0. 02) Placebo Hazard 95% CI ratio 8102 66. 8 12 (0. 03) 1. 58 0. 77– 3. 24 31 (0. 07) 0. 81 0. 48– 1. 36 17 (54. 8) 5 (0. 01) 1. 44 0. 47– 4. 42 Anderson GL, et al. JAMA 2003; 290: 1739– 48

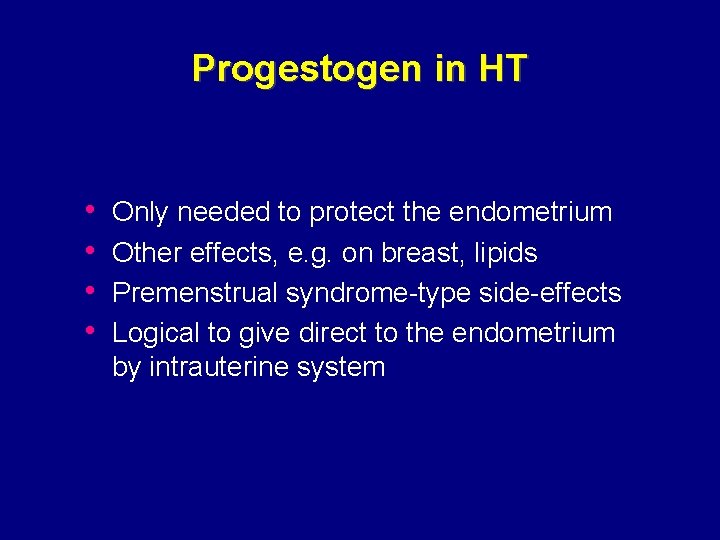
Progestogen in HT • • Only needed to protect the endometrium Other effects, e. g. on breast, lipids Premenstrual syndrome-type side-effects Logical to give direct to the endometrium by intrauterine system

MLS 10 µg LNG/day Mirena 20 µg LNG/day

Intrauterine application of progestins in hormone replacement therapy • Since 1991, 16 studies of intrauterine • • levonorgestrel in combination with different types and routes of estrogen (n = 809) Duration 6 months – 5 years IUS + oral CEE and E 2, patch, gel and implant New 10 µg system: 3 studies for 1– 2 years All 0% hyperplasia “Confirms endometrial safety for this application” Riphagen FE. Climacteric 2000; 3: 199– 21

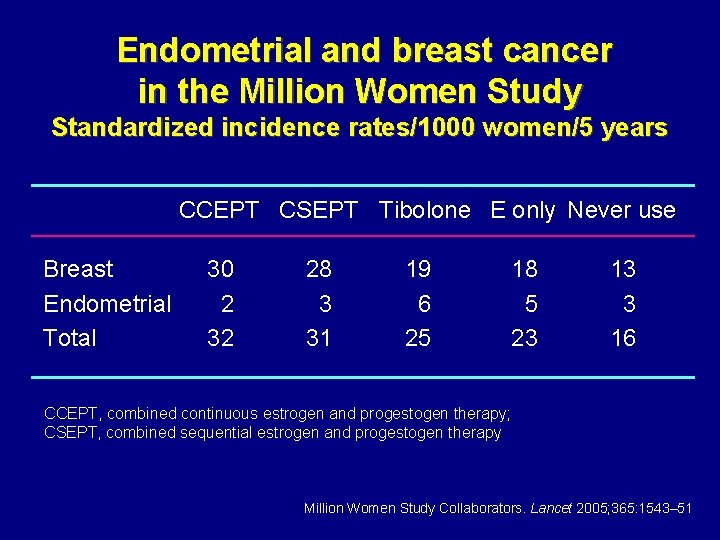
Endometrial and breast cancer in the Million Women Study Standardized incidence rates/1000 women/5 years CCEPT CSEPT Tibolone E only Never use Breast Endometrial Total 30 2 32 28 3 31 19 6 25 18 5 23 13 3 16 CCEPT, combined continuous estrogen and progestogen therapy; CSEPT, combined sequential estrogen and progestogen therapy Million Women Study Collaborators. Lancet 2005; 365: 1543– 51

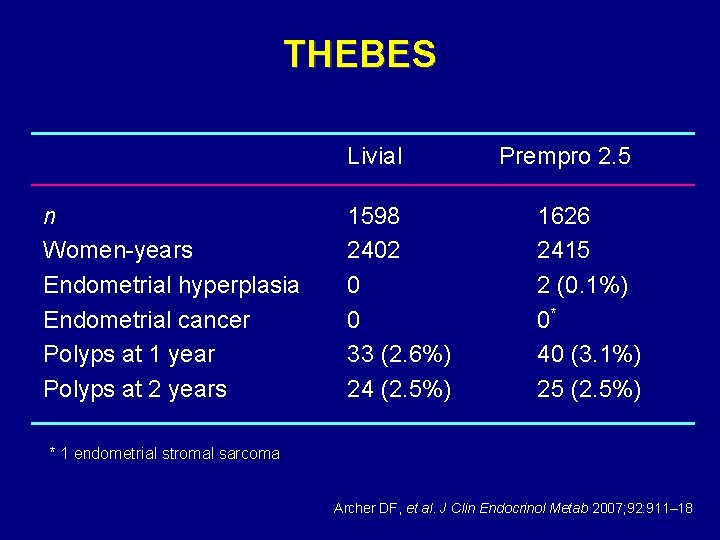
THEBES Tibolone Histology of the Endometrium and Breast Endpoint Study • Multicenter world-wide • Tibolone 1. 25 and 2. 5 mg vs. CEE/MPA • • 0. 625/2. 5 mg (Prempro) 24 months 3240/3000 subjects (1: 1: 2 randomization) Endometrial histology – hyperplasia and cancer Endometrial thickness, bleeding, breast pain, health-related quality of life

THEBES Livial n Women-years Endometrial hyperplasia Endometrial cancer Polyps at 1 year Polyps at 2 years 1598 2402 0 0 33 (2. 6%) 24 (2. 5%) Prempro 2. 5 1626 2415 2 (0. 1%) 0* 40 (3. 1%) 25 (2. 5%) * 1 endometrial stromal sarcoma Archer DF, et al. J Clin Endocrinol Metab 2007; 92: 911– 18

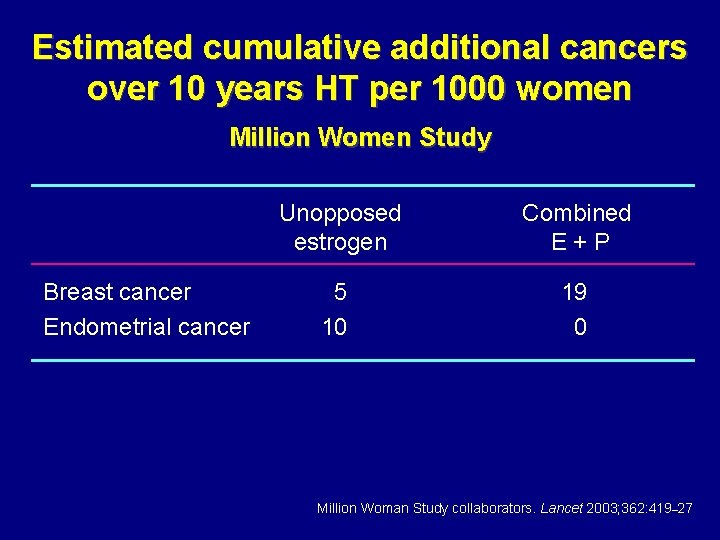
Estimated cumulative additional cancers over 10 years HT per 1000 women Million Women Study Breast cancer Endometrial cancer Unopposed estrogen Combined E+P 5 10 19 0 Million Woman Study collaborators. Lancet 2003; 362: 419– 27

Breast cancer and hormone therapy Million Women Study “…little advantage to using estrogen/progestogen in preference to estrogen-only HRT for women who still have a uterus” Million Women Study collaborators. Lancet 2003; 362: 419– 27

Is combined estrogen/progestogen therapy worth the risk? • Combined estrogen/progestogen therapy • • • associated with four-fold greater risk of breast cancer than unopposed estrogen therapy Risk of endometrial cancer from unopposed estrogen therapy much less After cessation of unopposed estrogen, risk of endometrial cancer persists for many years Other implications of unopposed estrogen Against perceived wisdom over last 25 years Hysterectomy rate

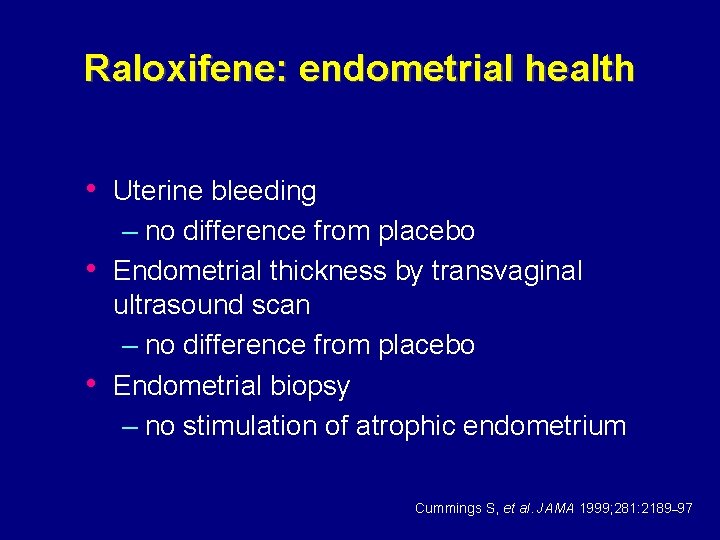
Raloxifene: endometrial health • Uterine bleeding – no difference from placebo • Endometrial thickness by transvaginal ultrasound scan – no difference from placebo • Endometrial biopsy – no stimulation of atrophic endometrium Cummings S, et al. JAMA 1999; 281: 2189– 97

Impact of recent reports and regulatory authorities • Lowest dose for shortest time • Lower dose regimens being produced • Is there a threshold dose/circulating level of • estrogen at which symptoms can be relieved and bone spared without causing endometrial stimulation? Is there a similar threshold for progestogen at which the endometrium will still be protected without affecting breast tissue?

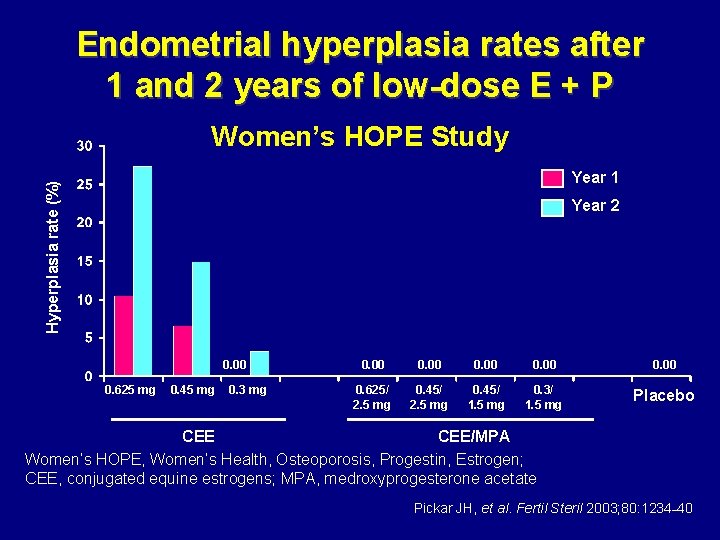
Endometrial hyperplasia rates after 1 and 2 years of low-dose E + P Women’s HOPE Study Hyperplasia rate (%) Year 1 Year 2 0. 00 0. 625 mg 0. 45 mg 0. 3 mg 0. 00 0. 625/ 2. 5 mg 0. 45/ 1. 5 mg 0. 3/ 1. 5 mg Placebo CEE/MPA Women’s HOPE, Women’s Health, Osteoporosis, Progestin, Estrogen; CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate Pickar JH, et al. Fertil Steril 2003; 80: 1234– 40

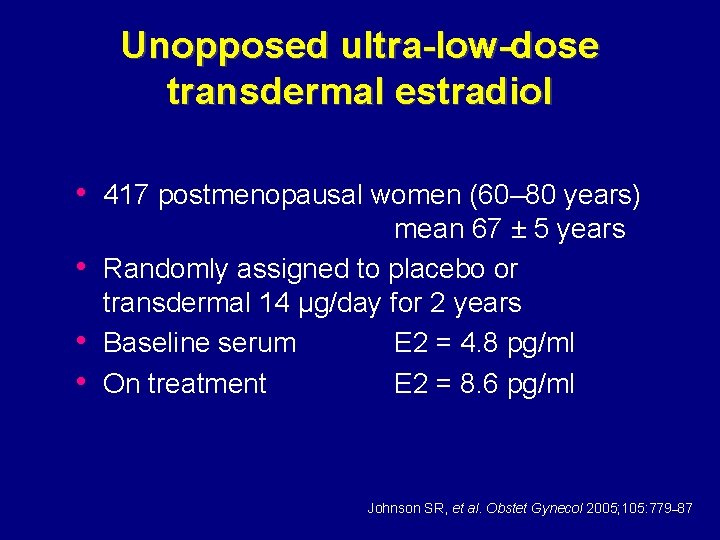
Unopposed ultra-low-dose transdermal estradiol • 417 postmenopausal women (60– 80 years) • • • mean 67 ± 5 years Randomly assigned to placebo or transdermal 14 µg/day for 2 years Baseline serum E 2 = 4. 8 pg/ml On treatment E 2 = 8. 6 pg/ml Johnson SR, et al. Obstet Gynecol 2005; 105: 779– 87

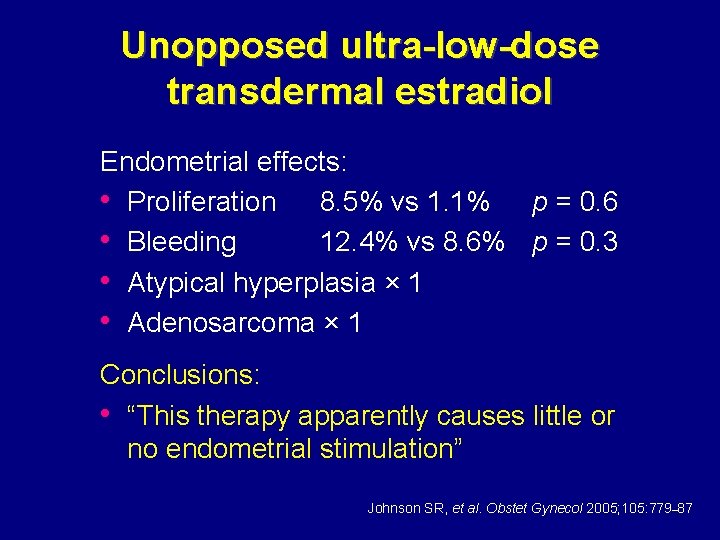
Unopposed ultra-low-dose transdermal estradiol Endometrial effects: • Proliferation 8. 5% vs 1. 1% p = 0. 6 • Bleeding 12. 4% vs 8. 6% p = 0. 3 • Atypical hyperplasia × 1 • Adenosarcoma × 1 Conclusions: • “This therapy apparently causes little or no endometrial stimulation” Johnson SR, et al. Obstet Gynecol 2005; 105: 779– 87

HT and endometrial safety • Endometrial cancer still a risk for • • • postmenopausal women taking HT Duration of progestogen important Change to CCEPT for long-term endometrial health ? Ultra-low-dose combination or unopposed estrogen

Hormone therapy and ovarian cancer David W. Sturdee

Ovarian cancer: background • Ovarian cancer is the second leading • • • cause of death from gynecologic cancers 1 Ovarian cancer is usually diagnosed in its advanced stage An estimated one woman in 70 will develop ovarian cancer in her lifetime, and one in 100 will die from the disease 2 Median age of diagnosis is 63 years 2 1 American Cancer Society. Cancer Facts and Figures 2003. Available at: www. cancer. org/docroot/STT/stt_0. asp. 2 Schneider HPG. Maturitas 2002; 43: S 35– 52

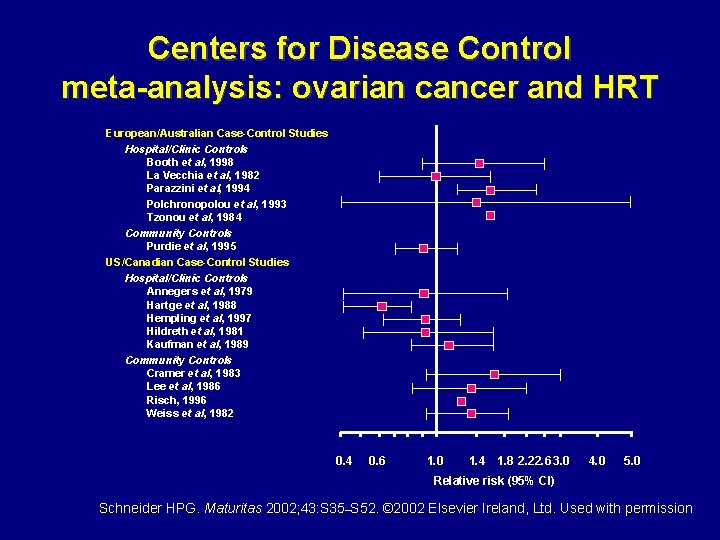
Centers for Disease Control meta-analysis: ovarian cancer and HRT European/Australian Case-Control Studies Hospital/Clinic Controls Booth et al, 1998 La Vecchia et al, 1982 Parazzini et al, 1994 Polchronopolou et al, 1993 Tzonou et al, 1984 Community Controls Purdie et al, 1995 US/Canadian Case-Control Studies Hospital/Clinic Controls Annegers et al, 1979 Hartge et al, 1988 Hempling et al, 1997 Hildreth et al, 1981 Kaufman et al, 1989 Community Controls Cramer et al, 1983 Lee et al, 1986 Risch, 1996 Weiss et al, 1982 0. 4 0. 6 1. 0 1. 4 1. 8 2. 22. 6 3. 0 4. 0 5. 0 Relative risk (95% CI) Schneider HPG. Maturitas 2002; 43: S 35–S 52. © 2002 Elsevier Ireland, Ltd. Used with permission

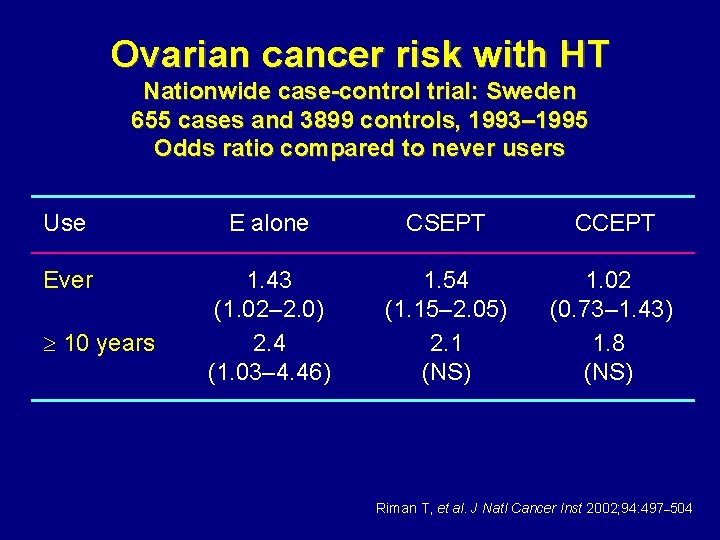
Ovarian cancer risk with HT Nationwide case-control trial: Sweden 655 cases and 3899 controls, 1993– 1995 Odds ratio compared to never users Use E alone CSEPT CCEPT Ever 1. 43 (1. 02– 2. 0) 2. 4 (1. 03– 4. 46) 1. 54 (1. 15– 2. 05) 2. 1 (NS) 1. 02 (0. 73– 1. 43) 1. 8 (NS) 10 years Riman T, et al. J Natl Cancer Inst 2002; 94: 497– 504

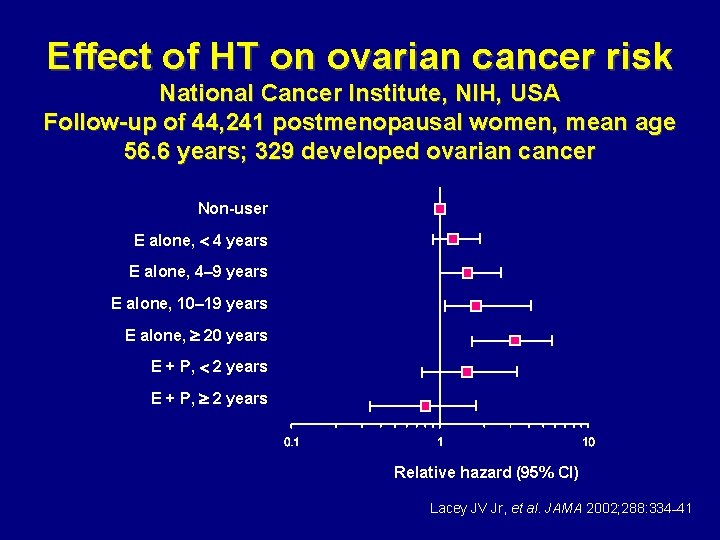
Effect of HT on ovarian cancer risk National Cancer Institute, NIH, USA Follow-up of 44, 241 postmenopausal women, mean age 56. 6 years; 329 developed ovarian cancer Non-user E alone, 4 years E alone, 4– 9 years E alone, 10– 19 years E alone, 20 years E + P, 2 years Relative hazard (95% CI) Lacey JV Jr, et al. JAMA 2002; 288: 334– 41

WHI study: gynecological cancers (annualized %) Outcome CEE + MPA n Duration (months) Ovary Endometrium Cervix 8506 67. 8 20 (0. 04) 27 (0. 06) 8 (0. 02) Placebo 8102 66. 8 12 (0. 03) 31 (0. 07) 5 (0. 01) Hazard 95% ratio n. CI 1. 58 0. 77– 3. 24 0. 81 0. 48– 1. 36 1. 44 0. 47– 4. 42 Anderson GL, et al. JAMA 2003; 290: 1739– 48

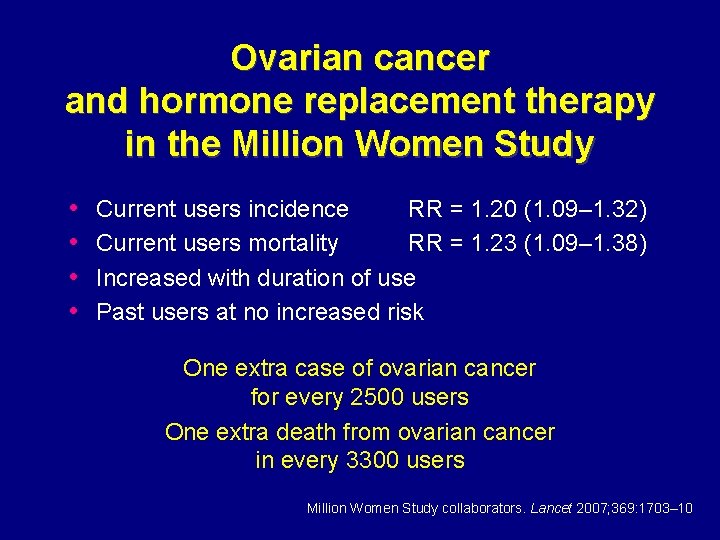
Ovarian cancer and hormone replacement therapy in the Million Women Study • • Current users incidence RR = 1. 20 (1. 09– 1. 32) Current users mortality RR = 1. 23 (1. 09– 1. 38) Increased with duration of use Past users at no increased risk One extra case of ovarian cancer for every 2500 users One extra death from ovarian cancer in every 3300 users Million Women Study collaborators. Lancet 2007; 369: 1703– 10

Hormone therapy and colorectal cancer David W. Sturdee

Colorectal cancer • Third most common cancer in US women, • • • and third most common cause of cancer death in women A woman’s lifetime risk of developing colorectal cancer is 5. 5%, with 91% of cases occurring after age 50 years Colon 72%, rectal 28% 5 -year survival for women with colorectal cancer is 63% American Cancer Society. Cancer Facts and Figures 2005 Available at: www. cancer. org/docroot/STT/stt_0. asp

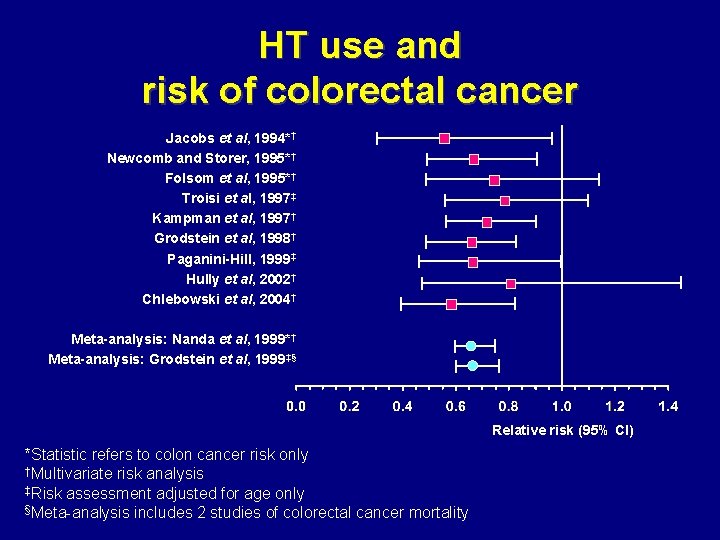
HT use and risk of colorectal cancer Jacobs et al, 1994*† Newcomb and Storer, 1995*† Folsom et al, 1995*† Troisi et al, 1997‡ Kampman et al, 1997† Grodstein et al, 1998† Paganini-Hill, 1999‡ Hully et al, 2002† Chlebowski et al, 2004† Meta-analysis: Nanda et al, 1999*† Meta-analysis: Grodstein et al, 1999‡§ Relative risk (95% CI) *Statistic refers to colon cancer risk only †Multivariate risk analysis ‡Risk assessment adjusted for age only §Meta-analysis includes 2 studies of colorectal cancer mortality

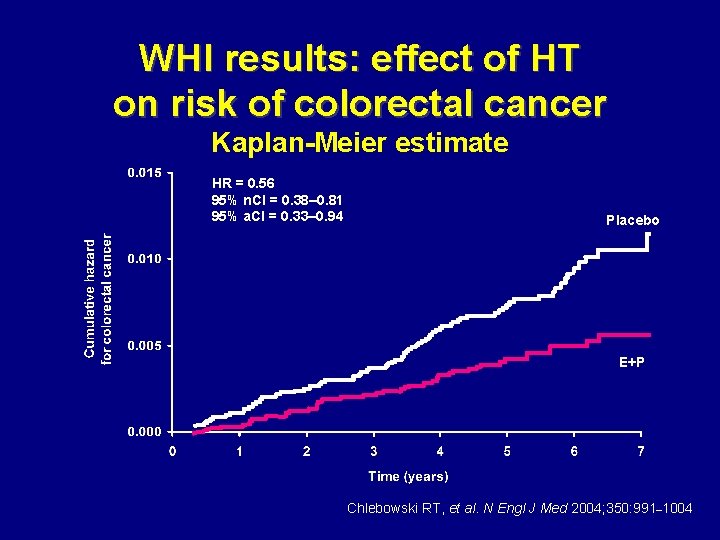
WHI results: effect of HT on risk of colorectal cancer Kaplan-Meier estimate HR = 0. 56 95% n. CI = 0. 38– 0. 81 95% a. CI = 0. 33– 0. 94 Placebo E+P Chlebowski RT, et al. N Engl J Med 2004; 350: 991– 1004

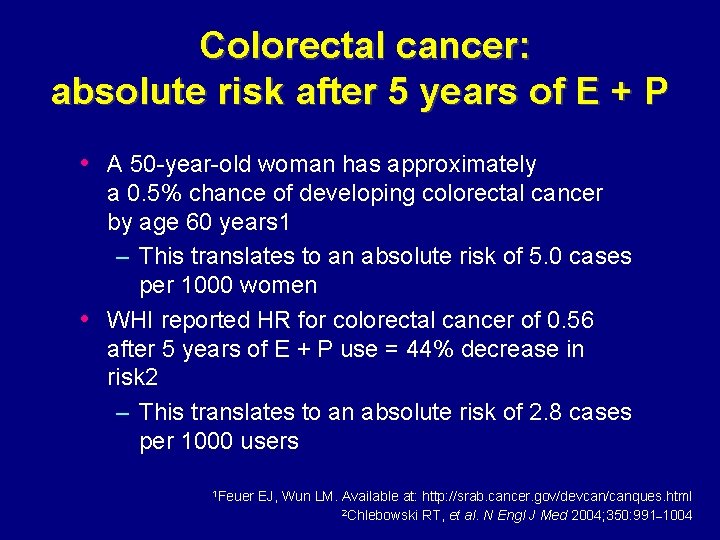
Colorectal cancer: absolute risk after 5 years of E + P • A 50 -year-old woman has approximately • a 0. 5% chance of developing colorectal cancer by age 60 years 1 – This translates to an absolute risk of 5. 0 cases per 1000 women WHI reported HR for colorectal cancer of 0. 56 after 5 years of E + P use = 44% decrease in risk 2 – This translates to an absolute risk of 2. 8 cases per 1000 users 1 Feuer EJ, Wun LM. Available at: http: //srab. cancer. gov/devcan/canques. html 2 Chlebowski RT, et al. N Engl J Med 2004; 350: 991– 1004

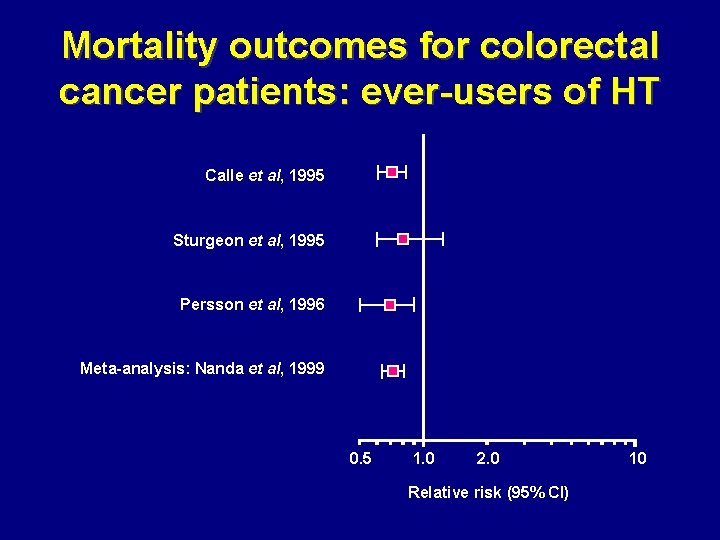
Mortality outcomes for colorectal cancer patients: ever-users of HT Calle et al, 1995 Sturgeon et al, 1995 Persson et al, 1996 Meta-analysis: Nanda et al, 1999 0. 5 1. 0 2. 0 Relative risk (95% CI) 10

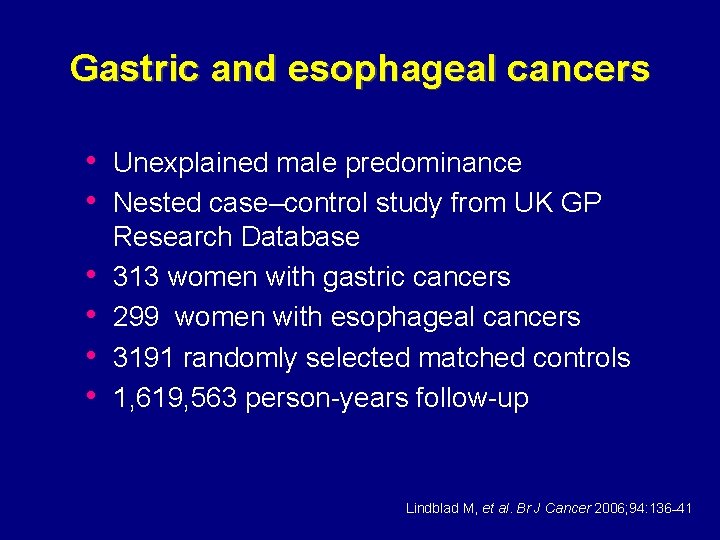
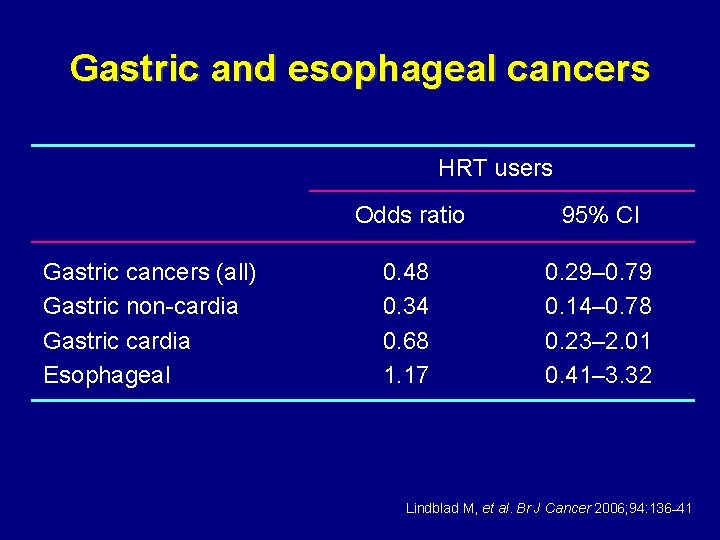
Gastric and esophageal cancers • Unexplained male predominance • Nested case–control study from UK GP • • Research Database 313 women with gastric cancers 299 women with esophageal cancers 3191 randomly selected matched controls 1, 619, 563 person-years follow-up Lindblad M, et al. Br J Cancer 2006; 94: 136– 41

Gastric and esophageal cancers HRT users Gastric cancers (all) Gastric non-cardia Gastric cardia Esophageal Odds ratio 95% CI 0. 48 0. 34 0. 68 1. 17 0. 29– 0. 79 0. 14– 0. 78 0. 23– 2. 01 0. 41– 3. 32 Lindblad M, et al. Br J Cancer 2006; 94: 136– 41

Effect of HRT on risk of endometrial and cancers other than breast Conclusions: • Estrogen alone and combined therapy with progestogen can affect the risk of endometrial and colorectal cancers in postmenopausal women but the data on ovarian and gastric cancer risks are less certain IMS, 2007

Acknowledgement Some of these slides have been adapted from those provided by the Council on Hormone Education
 David sturdee
David sturdee Female reproductive system
Female reproductive system Her2 positive cancers
Her2 positive cancers Eje hipotalamo hipofisis ovario utero
Eje hipotalamo hipofisis ovario utero Medidas del endometrio
Medidas del endometrio Postmenopausal endometrial thickness
Postmenopausal endometrial thickness Endometrial cells
Endometrial cells Endometrial hyperplasia
Endometrial hyperplasia Endometrial cancer
Endometrial cancer Endometrial cancer
Endometrial cancer Progesterone breakthrough bleeding
Progesterone breakthrough bleeding Uterus diagram
Uterus diagram Uterine ablation
Uterine ablation What is this organ
What is this organ Hiperplasia endometrial clasificación figo
Hiperplasia endometrial clasificación figo Endometrial histology menstrual cycle
Endometrial histology menstrual cycle Teratoma ovarium
Teratoma ovarium Self initiated other repair
Self initiated other repair Bipolar and other related disorders
Bipolar and other related disorders Bipolar and other related disorders
Bipolar and other related disorders Literary device
Literary device Solving square root and other radical equations
Solving square root and other radical equations Sociology is social science
Sociology is social science Respect other people's time and bandwidth.
Respect other people's time and bandwidth. What are two vocal styles of singing in pakistan
What are two vocal styles of singing in pakistan Internet etiquette or netiquette
Internet etiquette or netiquette Wrasse and bass symbiotic relationship
Wrasse and bass symbiotic relationship Intertext examples
Intertext examples Explain how currents and magnets exert forces on each other
Explain how currents and magnets exert forces on each other Alcohol is the most commonly used drug in our society
Alcohol is the most commonly used drug in our society Which type of plan shows the layout of the hvac system?
Which type of plan shows the layout of the hvac system? Process of removing food and other types of soil
Process of removing food and other types of soil Chapter 9 net present value and other investment criteria
Chapter 9 net present value and other investment criteria Chapter 31 schizophrenia and other psychoses
Chapter 31 schizophrenia and other psychoses Licensing franchising and other contractual strategies
Licensing franchising and other contractual strategies Topic 2 free enterprise and other economic systems
Topic 2 free enterprise and other economic systems Manaquinone
Manaquinone Solipsism and the problem of other minds
Solipsism and the problem of other minds Solving square root and other radical equations
Solving square root and other radical equations Taste and other tales a swim summary
Taste and other tales a swim summary Relationship between social work and other social sciences
Relationship between social work and other social sciences Teachers violating the code of ethics in the philippines
Teachers violating the code of ethics in the philippines Chapter 9 net present value and other investment criteria
Chapter 9 net present value and other investment criteria Topic 6 quadrilaterals and other polygons answers
Topic 6 quadrilaterals and other polygons answers Snmp uses two other protocols -------- and --------
Snmp uses two other protocols -------- and -------- Solving square root and other radical equations
Solving square root and other radical equations Short break line definition
Short break line definition Mi nombre sandra cisneros
Mi nombre sandra cisneros Kinds of intertextuality
Kinds of intertextuality My family and other animals characters
My family and other animals characters Bandwagon techniques examples
Bandwagon techniques examples