ELECTROLYTE SOLUTIONS MILLIEQUIVALENTS MILLIMOLES AND MILLIOSMOLES SUMMER 2017


























- Slides: 28

ELECTROLYTE SOLUTIONS: MILLIEQUIVALENTS, MILLIMOLES AND MILLIOSMOLES SUMMER 2017 Augustina Kwevie Pharm. D Candidate 1

OBJECTIVES • By the end of this lecture, students should be able to: • Calculate the milliequivalent weight from an atomic or formula weight • Convert between milligrams and milliequivalents • Calculate problems involving millimoles and milliosmoles 2

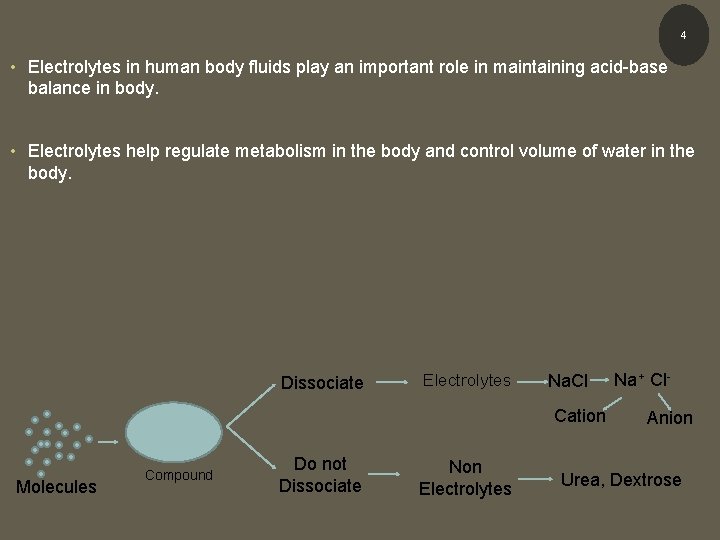
INTRODUCTION Electrolytes vs Non - electrolytes • Compounds in solution are often referred to as either electrolytes or non – electrolytes • Substances that do not dissociate are called non-electrolytes (urea, dextrose) – remain intact • Those with varying degrees of dissociation are called electrolytes (Na. Cl) • Electrolyte ions in blood plasma include cations (Na+, K+, Ca 2+, and Mg 2+) and anions (Cl-, HCO 3 -, HPO 42 -, SO 42 -) 3

4 • Electrolytes in human body fluids play an important role in maintaining acid-base balance in body. • Electrolytes help regulate metabolism in the body and control volume of water in the body. Dissociate Electrolytes Na. Cl Cation Molecules Compound Do not Dissociate Non Electrolytes Na+ Cl. Anion Urea, Dextrose

PHARMACEUTICAL APPLICATION • Electrolyte preparations are employed to treat fluid and electrolyte imbalances in the body. • Available as oral solutions, syrups, dry granules to be dissolved in water/juice, capsules, tablets and also intravenous infusions. 5

MILLIEQUIVALENTS • A chemical unit used by healthcare professionals across USA to express electrolyte concentration in solution • Internationally, molar concentrations (mmol/L or µmol/L) are employed. • A m. Eq represents the total number of ionic charges in solution, while taking into account valence of the ions. • A m. Eq expresses the chemical activity of an electrolyte 6

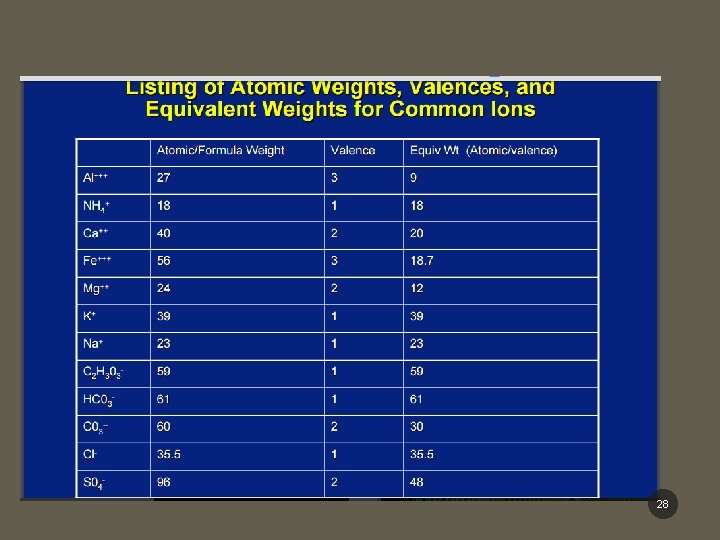
CALCULATIONS OF MILLIEQUIVALENTS • Equivalent weight (g) = atomic / molecular weight valence § Equivalent weight (Eq) = g § Milliequivalent weight (m. Eq) = mg Example: 1 equivalent KCl = 39 g K + 35. 5 g Cl = 74. 5 g /1 1 m. Eq = molecular weight (mg) valence Example: 1 m. Eq KCl = 39 mg K + 35. 5 mg Cl = 74. 5 mg/1 7

• To convert milligrams (mg) to milliequivalents (m. Eq) • m. Eq = mg × valence molecular weight • To convert m. Eq to mg • mg = m. Eq x molecular weight valence • m. Eq/m. L = mg/m. L × valence molecular weight • mg/m. L = m. Eq/m. L x molecular weight valence 8

PRACTICE PROBLEMS 9

EXAMPLE 1 • Convert 8 m. Eq of potassium to mg (MW of K+ = 39 g/mol) 10

EXAMPLE 2 • Calculate the concentration in mg/m. L, of a solution containing 3 m. Eq of KCl per m. L. (K+: 39 g/mol, Cl-: 35. 5 g/mol) 11

EXAMPLE 3 • What is the concentration in g/m. L of a solution containing 4 m. Eq of Calcium chloride (Ca. Cl 2. 2 H 2 O) per m. L? (Ca 2+: 40 g/mol, Cl-: 35. 5 g/mol, H: 1 g/mol, O: 16 g/mol) • 12

EXAMPLE 4: ESTIMATION OF % W/V FROM MEQ • What is the % w/v conc. of a 100 m. Eq/L solution of NH 4 Cl? (NH 4+: 18 g/mol, Cl-: 35. 5 g/mol) 13

EXAMPLE 5 • A solution has 10 mg / 100 m. L of K+ ions. Express the concentration in m. Eq/Lit (K+: 39 g/mol) 14

EXAMPLE 6 • A solution contains 10 mg/100 m. L of Ca 2+ ions. Express the concentration in m. Eq/Lit (Ca 2+: 40 g/mol) 15

EXAMPLE 7 • How many m. Eq of KCl is present in a 15 m. L dose of a 10% w/v KCl elixir? 16

EXAMPLE 8: CLINICAL APPLICATION • A person will receive 2 m. Eq of Na. Cl / kg of body weight. He weighs 132 lb. Calculate the ml of 0. 9% sterile Na. Cl that should be administered (Na+: 23 g/mol, Cl-: 35. 5 g/mol) 17

MILLIMOLES AND MICROMOLES Another way that drug amounts in solution can be expressed is as the number of particles in a given amount of solution • A mole is the molecular weight of substance in grams. • A millimole – one thousandth of a mole • A micromole – One millionth of a mole • SI expresses, electrolyte conc. in mmol/L § Conversions to remember: 1 mole = 1000 millimoles g/mole = mg/millimole 18

EXAMPLE 9 • How many mmol of monobasic sodium phosphate (m. w. 138 g/mol) are present in 100 g of the substance? 19

EXAMPLE 10 • Calculate the weight in mg of 1 mmol of HPO 4 - ( m. w. 96 g/mol) 20

EXAMPLE 11 • Convert plasma levels of 0. 5 µg/m. L of tobramycin (mw – 467. 52) to µmol/L? 21

OSMOLARITY • Osmotic pressure is important to biologic processes that involve the diffusion of solutes and the transfer of fluids through semi-permeable membranes • Osmotic pressure is proportional to the total number of particles in a solution • Unit of measurement is milliosmoles (m. Osmol) 22

OSMOLARITY • For non-electrolytes like dextrose, 1 mmol represents 1 mosmol • However for electrolytes, the total number of particles in solution depends on the degree of dissociation of a substance. • E. g. Assuming complete dissociation, 1 mmol Na. Cl represents 2 m. Osmol (Na+ + Cl-) of total particles • 1 mmol of Ca. Cl 2 represents 3 m. Osmol (Ca 2+ + 2 Cl-) • 1 mmol of sodium citrate (Na 3 C 6 H 5 O 7) represents 4 m. Osmol (3 Na+ + C 6 H 5 O 7 -) of total particles 23

• The milliosmolar value of the complete solution is equal to the sum of milliosmolar values of individual ions. • U. S. Pharmacopeia lists the following formula for calculation of ideal osmolar concentration: • Wt. of substance (g/L) • m. Osmol/L = --------------- × No. of Species × 1000 • Mol. Wt (g/mol) 24

EXAMPLE 12 • Calculate of ideal osmolarity for 0. 9% Sodium Chloride Solution? 25

OSMOLARITY VS. OSMOLALITY • Osmolarity -- “Milliosmoles of solute per liter of solution” • Osmolality – “Milliosmoles of solute per kilogram of solvent” • For dilute aqueous solutions – both terms are nearly identical • For more concentrated solutions – the two values are not identical • Pharmacist should make distinction between Osmolarity and Osmolality 26

EXAMPLE 13 • A solution has 5% anhydrous dextrose in water for injection. Represent the concentration in mosmol/lit? 27

28
 Millimoles to milliequivalents
Millimoles to milliequivalents Millimoles to milliosmoles
Millimoles to milliosmoles Milliequivalents practice problems
Milliequivalents practice problems Abbreviation for milliequivalents
Abbreviation for milliequivalents How many millimoles in a mole
How many millimoles in a mole Milliosmoles
Milliosmoles Fluid and electrolyte balance ppt
Fluid and electrolyte balance ppt Fluid and electrolyte balance ppt
Fluid and electrolyte balance ppt Water balance regulation
Water balance regulation An atypical accumulation of fluid in the interstitial space
An atypical accumulation of fluid in the interstitial space Chapter 25 fluid electrolyte and acid-base balance
Chapter 25 fluid electrolyte and acid-base balance Chapter 25 fluid electrolyte and acid-base balance
Chapter 25 fluid electrolyte and acid-base balance Water electrolyte imbalance
Water electrolyte imbalance Solubility
Solubility Hf electrolyte
Hf electrolyte Freddy santoso
Freddy santoso Signs and symptoms of hyperkalemia
Signs and symptoms of hyperkalemia Larox dm dual media electrolyte filter
Larox dm dual media electrolyte filter Degree of dissociation of electrolyte depends on
Degree of dissociation of electrolyte depends on What is electrolyte
What is electrolyte Electrolyte replacement therapy
Electrolyte replacement therapy Pltw summer training 2017
Pltw summer training 2017 Scout character description
Scout character description Astro quiz 2017 questions and answers
Astro quiz 2017 questions and answers Saasta astro quiz 2017 round 2 questions and answers
Saasta astro quiz 2017 round 2 questions and answers Pass andres astronomy quiz
Pass andres astronomy quiz Do no. 36 s. 2016
Do no. 36 s. 2016 Astro quiz round 2
Astro quiz round 2 Astro quiz 2019 round 1
Astro quiz 2019 round 1